 |
J Circ Biomark 2024; 13: 14-22 ISSN 1849-4544 | DOI: 10.33393/jcb.2024.3149 ORIGINAL RESEARCH ARTICLE |
 |
Comparative evaluation of serum and gingival crevicular fluid levels of interleukin 21 in periodontally diseased and healthy patients
ABSTRACT
Background: Periodontitis is an inflammatory reaction to subgingival pathogenic microorganisms that causes gradual deterioration of the gingiva, periodontal ligament, and alveolar bone. Interleukin (IL)-21 is the most recently found member of type I cytokine family that is upregulated during inflammation. The current study aims to investigate the biological plausibility of IL-21 as a biomarker for chronic periodontitis.
Materials and methods: This cross-sectional clinico-biochemical investigation included 15 systemically healthy, 15 periodontally healthy, 15 chronic gingivitis, and 15 chronic periodontitis subjects aged 25 to 60 years. Following subject enrollment, gingival crevicular fluid (GCF) and blood samples were then taken from each subject. The concentration of IL-21 in all samples was determined using enzyme-linked immunosorbent assay (ELISA) kit. The data was examined using the Kruskal-Wallis test and the Spearman correlation test.
Results: Serum IL-21 levels in chronic periodontitis patients were substantially greater than in periodontally healthy individuals. GCF IL-21 levels were substantially greater in gingivitis and chronic periodontitis patients compared to periodontally healthy individuals. In terms of clinical indicators, serum IL-21 levels correlated significantly with bleeding index (BI) in the chronic periodontitis group. In chronic periodontitis group, disease severity as evaluated by probing pocket depth (PPD) and clinical attachment loss (CAL) did not correlate with serum or GCF IL-21 levels.
Conclusion: According to the current study’s findings, periodontally involved patients had higher IL-21 levels than periodontally healthy patients, suggesting it can be used as biomarker. Further studies with larger sample size can shed more light on the clinical advantage of IL-21 as a possible marker for disease activity and progression.
Keywords: Blood samples, Cytokines, Gingival crevicular fluid, Interleukin 21, Periodontal diseases, Periodontitis
Received: May 27, 2024
Accepted: August 20, 2024
Published online: September 20, 2024
Journal of Circulating Biomarkers - ISSN 1849-4544 - www.aboutscience.eu/jcb
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Host inflammatory response is triggered by bacterial biofilm, which is one of the factors contributing to multifactorial disease known as periodontitis (1). A prolonged exposure to bacteria and endotoxins is necessary for the development of gingival inflammation and progressive dysbiotic condition eventually leading to destruction, although it only accounts for 20% of the variation in the clinical manifestation of periodontitis (2). The primary cause of the soft and hard tissue destruction linked to periodontitis is the host immune system’s inflammatory response to the bacterial challenge. As a result, the inflammatory response plays a major role in determining the disease’s destructive nature (3). This includes the active expression of catabolic cytokines and inflammatory mediators like interleukin (IL)-1β and IL-6 through the activation of lymphocytes, fibroblasts, monocytes/macrophages, and other cellular components. Through tissue-derived matrix metalloproteinases, these cytokines and inflammatory mediators can act alone or in combination to promote collagen degradation and alveolar bone resorption (4).
Traditional methods for diagnosing periodontal diseases include clinical and radiographic examinations. These traditional measures provide information only about the existing disease and are incapable of predicting disease progression. As a result, advances in oral and periodontal disease diagnostic research are shifting toward methods for identifying and quantifying periodontal risk using objective measures such as biomarkers. One of these biomarkers that can be detected in several inflammatory conditions is IL-21 (5,6).
Gingival crevicular fluid (GCF) is produced by the blood vessel gingival plexus, which is located next to the epithelial lining of the dentogingival space. Several molecular components originating from the host response are carried by GCF, which is regarded as a major defense against periodontal infection. GCF-present host response mediators can help with both the status of disease activity and the identification of different periodontal diseases (7). Numerous cytokines, including IL-1, IL-6, IL-8, and IL-12, have been thoroughly examined in relation to chronic periodontitis, and research has been done on their role in the breakdown of periodontal tissues (8-11). The research of IL-21, a recently discovered cytokine, has received more attention in recent years.
IL-21, an inflammatory cytokine mostly expressed by activated T-helper (Th)1 and Th17 cells, two distinct proinflammatory lineages, is not expressed by Th2 cells in humans (12,13). It operates by binding to the type I cytokine receptor IL-21R. Because it has been linked to the pathophysiology of inflammatory breakdown in a number of systemic disorders, including rheumatoid arthritis, colitis, and inflammatory bowel disease, IL-21 has recently attracted attention (14-16). Given the critical function that IL-21 plays in inflammation (17), excessive IL-21 production amplifies local inflammation and exacerbates tissue damage and destruction (18).
The levels of IL-21 may rise as a result of periodontal diseases’ propensity for chronic inflammation. Few studies have been conducted on IL-21 in patients with chronic periodontitis (14,19). However, no research has been done to compare GCF and serum IL-21 levels in healthy individuals, gingivitis patients, or periodontitis patients (19). Thus, the current study aims to measure the levels of IL-21 in the blood and GCF of patients with chronic periodontitis, compare them with those of gingivitis and healthy individuals, and assess the usefulness of this information as a diagnostic tool for periodontal disease.
Materials and methods
This cross-sectional study included 45 patients, ages 25 to 60, who were patients at Sri Sai College of Dental Surgery’s Outpatient Department of Periodontics in Vikarabad, Telangana. The Institutional Ethical Committee gave its approval before the trial started. This study included three groups: Group A, or the healthy group, consisted of 15 participants who were 25-60 years old and had a systemically healthy periodontium with probing pocket depth (PPD) of less than 3 mm. Group B, which consisted of 15 individuals aged 25-60 years who were systemically healthy, had gingivitis. They exhibited clinical symptoms of inflammation and had a score of less than 2, using Modified Gingival Index (MGI). Group C, or the periodontitis group, consisted of 15 individuals, aged 25 to 60, who were in general health but had moderate to severe chronic periodontitis. At least four of their teeth had PPDs or clinical attachment loss (CAL) of at least 5 mm, bleeding when probed, and radiographic signs of bone loss. All the parameters, such as plaque index (PI), MGI, Saxton’s bleeding index (BI), PPD, and CAL, were recorded in all the groups.
Inclusion criteria
Patients between the ages of 25 and 50, patients in general good health, subjects with at least 18 fully erupted teeth, subjects with bleeding during probing, PPD of at least 5 mm, and clinical attachment level of at least 6 mm were included.
Exclusion criteria
Atherosclerotic vascular disease (including peripheral artery disease, stroke, and cardiovascular diseases), immunological disorders, osteoporosis, arthritis, a history of periodontal intervention within the previous 6 months, anti-inflammatory and nonsteroidal anti-inflammatory medication use within 3 months of the periodontal assessment, and pregnancy or lactation were the exclusion criteria.
The Ethics Committee for Human Trials of Sri Sai College of Dental Surgery approved the research, which was carried out in Sri Sai College of Dental Surgery in compliance with the World Medical Association’s 2008 Declaration of Helsinki (reference no: 615/SSCDS/IRB-E/2017). Each patient or their family member gave their informed consent after the periodontal exam, GCF, and blood sample removal were fully explained.
GCF and serum sample collection for IL-21
After instructing the subjects to sit comfortably upright in the dentist chair, the selected test site was air-dried and isolated with cotton rolls. To avoid contamination and obstruction of the microcapillary pipette, supragingival plaque was removed with an ultrasonic scaler while avoiding contact with the marginal gingiva.
The following day, GCF was collected using a calibrated volumetric microcapillary pipette (Sigma Aldrich Chemical Company, USA, Catalog No. p0549) with a black color code. The pipette was used to insert the tip at the entrance of the gingival crevice (unstimulated) and leave it there for 5 to 20 minutes. A 3 μL GCF standard volume was obtained. Adequate GCF was difficult to get from periodontally healthy persons (Fig. 1) and gingivitis subjects; therefore, pooled samples were collected from numerous sites to ensure that each subject received the minimal amount required (3 µL).
FIGURE 1 - Method of gingival crevicular fluid collection.
When chronic periodontitis is present, gathering GCF takes less time and is less laborious since the site with the highest PPD can provide the necessary amount. The collected samples were wrapped in aluminum foil and kept at 80°C until the assay time. Samples tainted with blood or saliva and those with air bubbles were rejected.
For serum samples, after disinfecting the skin over the antecubital fossa, a 20-gauge needle was used to venipuncture 3 mL of blood into a 5-mL syringe. A sample of collected blood was left to coagulate for approximately half an hour at room temperature (Fig. 2). After separating the serum component, the sample was centrifuged for 10 minutes at 3000 rpm. It was then moved to a polycarbonate container and kept at −80°C until the test was scheduled.
FIGURE 2 - Collection of blood samples.
Estimation of interleukin 21 levels
Using a commercially available human IL-21, or IL-21 enzyme-linked immunosorbent assay (ELISA) kit (BioAssay Technology Laboratory affiliated to Shanghai Korain Biotech Co., Ltd., Shanghai, China) (Fig. 3), the levels of GCF and serum IL-21 were determined. Phosphate-buffered saline was used to dilute GCF samples to a level of 100 µL.

FIGURE 3 - Enzyme-linked immunosorbent assay kit.
Assay principle
An ELISA kit is intended to detect various biomarkers in bodily fluid samples. Human IL-21 antibody was precoated onto the plate. The sample’s IL-21 was introduced, and it binds to the antibodies coated on the wells. After that, biotinylated human IL-21 antibody was added, and it binds to the sample’s IL-21.
Next, streptavidin-horseradish peroxidase (HRP), which binds to the IL-21 antibody that has been biotinylated, was added. During a washing phase, unbound streptavidin-HRP is removed following incubation. After that, the substrate solution was added, and color changed proportionately to the concentration of human IL-21. The addition of an acidic stop solution ends the process, and the absorbance is measured at 450 nm.
Sensitivity
The sensitivity or minimum detectable dose of human IL-21 is reported to be 2.46 ng/L.
Assay procedure
- Every reagent, standard solution, and sample was made in accordance with the directions. Prior to use, all reagents were brought to room temperature, and the experiment was conducted.
- After figuring out how many strips were needed for the assay, the strips were put into the frames and used. The leftover strips were kept between 2 and 8°C.
- The standard well was later filled with 50 μL of standard solution.
- In addition, 40 μL of the sample and 10 μL of the anti-IL-21 antibody were added to the sample wells. Following a thorough mixing of 50 μL streptavidin-HRP into both the standard and sample wells, the plate was sealed with a sealer and allowed to sit at 37°C for 60 minutes (Fig. 4).
FIGURE 4 - After addition of samples in enzyme-linked immunosorbent assay plates.
- All of the wells were aspirated, filled to the brim with wash buffer, and automatically cleaned five times. The plate was blotted onto paper towels or other absorbent material.
- Subsequently, 50 μL of substrate solution A and 50 μL of substrate solution B were applied to each well. The plate was sealed with a fresh sealer and left in the dark at 37°C for 10 minutes.
- The last step involved adding 50 μL of Stop Solution to each well, which instantly caused the color to shift to yellow (Fig. 5).
FIGURE 5 - Enzyme-linked immunosorbent assay microplates after addition of stop solution.
- Within 10 minutes of administering the stop solution, each well’s optical density (OD value) was ascertained using a microplate reader set to 450 nm (Tab. 1).
| Components | Quantity |
|---|---|
| Standard solution (1600 ng/L) | 0.5 mL ×1 |
| Precoated ELISA plate | 12 × 8 well strips ×1 |
| Standard diluent | 3 mL ×1 |
| Streptavidin-HRP | 6 mL ×1 |
| Stop solution | 6 mL ×1 |
| Substrate solution A | 6 mL ×1 |
| Substrate solution B | 6 mL ×1 |
| Wash buffer concentrate (30×) | 20 mL ×1 |
| Biotinylated human IL-21 antibody | 1 mL ×1 |
| User instruction | 1 |
| Plate sealer | 2 |
| Zipper bag | 1 |
ELISA = enzyme-linked immunosorbent assay; HRP = horseradish peroxidase; IL = interleukin; NS = no statistical significance; S = statistical significance (p≤0.05).
Statistical analysis
Statistical Package for the Social Sciences (SPSS) for Windows (Version 22.0, Released 2013; Armonk, NY: IBM Corp.) was used for providing statistical analysis.
The data was presented as mean ± standard deviation (SD). Using SPSS version 20.0 software, the following statistical tests were used to analyze the parameters.
- The Kruskal-Wallis test was used to do intergroup analysis for the following variables: serum, GCF IL-21, MGI, PI, PPD, CAL, age, and gender.
- In group A, the relationship between age and gender and serum and GCF IL-21 levels was assessed. In group B, there was a correlation between the levels of serum and GCF IL-21 and the clinical indices MGI, PI, and BI, as well as age and gender. In group C, the levels of serum and GCF IL-21 were correlated with age, gender, and clinical indices and parameters, such as MGI, PI, BI, PPD, and CAL. The Spearman correlation coefficient test was used to perform all of these group-specific correlations.
Results
Of the 15 individuals in group A, 9 (60%) were male and 6 (40%) were female; of the 15 patients in group B, 8 (55%) were male and 7 (45%) were female; of the 15 patients in group C, 7 (45%) were male and 8 (55%) were female (Tab. 2). According to Table 2, the participants in groups A, B, and C had mean ages of 34.07 ± 2.99, 33.93 ± 2.79, and 34.67 ± 2.35, respectively.
Plaque index
Groups A, B, and C had mean PI scores of 0.73 ± 0.353, 1.09 ± 0.171, and 1.97 ± 0.395, respectively. Group C had the highest mean PI score, followed by group B, and group A had the lowest value, according to the intergroup comparison of mean PI scores. The results showed that there was no statistically significant difference between groups A and B, but there was between groups B and C and between groups A and C (Tab. 2).
Modified gingival index
MGI was used to determine the gingival status, and the mean values for groups A, B, and C were 0.39 ±0.195, 1.08 ± 0.258, and 1.76 ± 0.509 (Fig. 6). The mean gingival index intergroup comparison showed that group C had high values, whereas group B had moderate values that were greater than those of group A. There is statistical significance in the outcomes for each of the three groups (Tab. 2).
Bleeding index
Saxton’s BI was used to measure bleeding, and the mean values for groups A, B, and C were 0.32 ± 0.315, 0.77 ± 0.277, and 1.33 ± 0.132, respectively. According to the intergroup comparison of mean BI values, group C had the highest bleeding scores, group B had moderate scores, and group A had the lowest or minimum value when compared to the other groups (Fig. 6). The findings between groups A and C, as well as between groups B and C, are statistically significant; however, the results between groups A and B are not (Tab. 2).
| Group | p-Value | |||
|---|---|---|---|---|
| A | B | C | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (years) | 34.07±2.99 | 33.93±2.79 | 34.67±2.35 | 0.736 (NS) |
| Sex – Male | 60.0% | 55.0% | 45.0% | |
| Female | 40.0% | 45.0% | 55.0% | |
| PI | 0.73 ± 0.353 | 1.09 ± 0.171 | 1.97 ± 0.395 | 0.000
A vs. B (NS) B vs. C (S) A vs. C (S) |
| MGI | 0.39 ± 0.195 | 1.08 ± 0.258 | 1.76 ± 0.509 | 0.000
A vs. B (S) B vs. C (S) A vs. C (S) |
| BI | 0.32 ± 0.315 | 0.77 ± 0.277 | 1.33 ± 0.132 | 0.000
A vs. B (NS) B vs. C (S) A vs. C (S) |
Mean (mm) PPD |
1.27 ± 0.346 | 1.82 ± 0.568 | 4.22 ± 0.538 | 0.000
A vs. B (NS) B vs. C (S) A vs. C (S) |
Mean (mm) CAL |
0 | 0 | 4.55 ± 0.864 | 0.000
A vs. B (NS) B vs. C (S) A vs. C (S) |
BI = bleeding index; CAL = clinical attachment loss; MGI = Modified Gingival Index; NS = no statistical significance; PI = plaque index; PPD = probing pocket depth; S = statistical significance (p≤0.05); SD = standard deviation.
Probing pocket depth
The mean PPD was 1.27 ± 0.346 mm in group A, 1.82 ± 0.568 mm in group B, and 4.22 ± 0.538 (Fig. 6) in group C; the intergroup comparison of mean PPD scores of groups A and B revealed statistically significant high score for group C (Tab. 2)
Clinical attachment loss
Mean CAL was 0.00 mm in groups A and B and 4.55 ± 0.864 mm (Fig. 6) in group C. There was a statistically significant difference of CAL values in group C vs. other groups (Tab. 2).
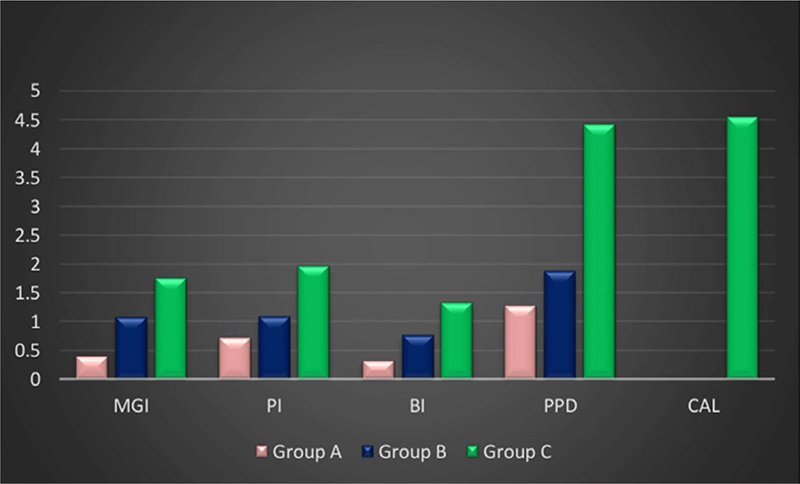
FIGURE 6 - Intergroup comparison of clinical parameters. BI = bleeding index; CAL = clinical attachment loss; MGI = Modified Gingival Index; PI = plaque index; PPD = probing pocket depth.
Concentration of IL-21 in serum
The amount of IL-21 in the serum was reported in nanograms per milliliter. All of the participants in groups A, B, and C had measurable amounts of IL-21 in their serum. Group A patients had the highest observed level of IL-21 in their serum at 57 ng/mL, whereas group B had 100 ng/mL and group C 545 ng/mL. Group A subjects’ serum had a minimum IL-21 level of 30 ng/mL, group B’s was 66 ng/mL, and group C’s was 83 ng/mL. The mean serum concentrations in groups A, B, and C were 44.866 ± 8.9, 87.33 ± 10.14, and 139.3 ± 113.5 ng/mL, in that order. Group C had greater values than group A in this statistically significant comparison of serum IL-21 levels. Between groups A and B and between groups B and C, however, there was no discernible change (Tabs. 3 and 4).
| Group | IL-21 levels | Mean ± SD | Minimum (ng/mL) | Maximum (ng/mL) |
|---|---|---|---|---|
| A | Serum | 44.866 ± 8.9 | 30 | 57 |
| GCF | 21.933 ± 9.1 | 10 | 40 | |
| B | Serum | 87.33 ± 10.14 | 66 | 100 |
| GCF | 66.400 ± 9.47 | 55 | 80 | |
| C | Serum | 139.3 ± 113.5 | 83 | 545 |
| GCF | 66.400 ± 7.28 | 59 | 82 |
GCF = gingival crevicular fluid; IL = interleukin; NS = no statistical significance; S = statistical significance (p≤0.05); SD = standard deviation.
| Group | p-Value | |||
|---|---|---|---|---|
| A | B | C | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Serum IL-21 concentration (ng/mL) | 44.866 ± 8.9 | 87.33 ± 10.1 | 139.3 ± 11.3 | ≤0.001
A vs. B = (NS) A vs. C = (S) B vs. C = (NS) |
| GCF IL-21 concentration (ng/mL) | 21.933 ± 9.1 | 66.400 ± 9.47 | 66.400 ± 7.82 | ≤0.001
A vs. B = (S) A vs. C = (S) B vs. C = (NS) |
GCF = gingival crevicular fluid; IL = interleukin; NS = no statistical significance; S = statistical significance (p≤0.05); SD = standard deviation.
In GCF, the highest levels of IL-21 were 40 ng/mL in the GCF of patients in group A, 80 ng/mL in group B, and 82 ng/mL in group C. In group A, the minimum IL-21 levels were 10 ng/mL; in groups B and C, the levels were 55 and 59 ng/ml, respectively, in the GCF. In groups A, B, and C, the corresponding mean GCF concentrations were 21.933 ± 9.1, 66.400 ± 9.47, and 66.400 ± 7.28 ng/mL. Compared to group A, groups B and C had statistically significant higher IL-21 levels (Tabs. 2 and 4).
Regarding the correlation between IL-21 and demographics, there was statistically significant correlation between age for each of the three groups and serum IL-21 levels and GCF. In other groups, there was no statistically significant relationship between age and serum and GCF IL-21 levels (Tab. 5).
When the correlation between IL-21 and clinical parameters was estimated, no statistically significant association was seen in group B between serum IL-21 levels and GCF with clinical indicators, such as PI, MGI, and BI (Tab. 6). With the exception of BI, which is substantially correlated with serum IL-21 levels, group C’s GCF and serum IL-21 concentration did not significantly correlate with any of the clinical indicators examined. There were no discernible relationships found between the levels of PPD, CAL, and IL-21 (Tab. 7).
Discussion
Periodontitis is the sixth most common disease in humans, affecting 740 million people worldwide. Periodontitis is a bacterially induced chronic tissue destructive inflammation of the teeth. This periodontal microbiota causes the release of proinflammatory mediators both locally and systemically. As the paradigm of chronic infection in dental pathology, periodontal disease shares several pathogenic pathways with cardiovascular diseases as a result of the low-grade state of systemic inflammation posed by periodontitis (20).
Early diagnosis is essential since disease progression is unpredictable and irreversible. The diagnostic and prognostic efficacy of traditional clinical diagnostic parameters, such as bleeding on probing, PPD, and CAL, is limited (21). Therefore, developments in the diagnosis of periodontal disease are moving in the direction of techniques that allow periodontal risk to be recognized and measured using objective metrics like biomarkers. Diagnostic evidence of the infection/host immune response axis can be obtained through site-specific examination of GCF and subject-based data based on serum assays (6).
| Serum IL-21 | GCF IL-21 | |||
|---|---|---|---|---|
| Group A | Age | Spearman correlation coefficient | −0.287 | −0.058 |
| p-value | 0.2990 | 0.837 | ||
| Group B | Age | Spearman correlation coefficient | −0.151 | −0.333 |
| p-value | 0.590 | −0.226 | ||
| Group C | Age | Spearman correlation coefficient | −0.391 | −0.292 |
| p-value | 0.149 | 0.290 |
GCF = gingival crevicular fluid; IL = interleukin; NS = no statistical significance; S = statistical significance (p≤0.05).
| PI | MGI | BI | ||
|---|---|---|---|---|
| Serum IL-21 | Spearman correlation coefficient | −0.259 | −0.298 | 0.095 |
| p-value | 0.35 | 0.28 | 0.73 | |
| GCF IL-21 | Spearman correlation coefficient | 0.10 | 0.25 | −0.291 |
| p-value | 0.97 | 0.36 | 0.29 | |
BI = bleeding index; GCF = gingival crevicular fluid; IL = interleukin; MGI = Modified Gingival Index; NS = no statistical significance; PI = plaque index; S = statistical significance (p≤0.05).
| Mean PPD | Mean CAL | MGI | PI | BI | ||
|---|---|---|---|---|---|---|
| Serum IL-21 | Spearman correlation coefficient | 0.246 | 0.136 | 0.199 | −0.169 | −0.621 |
| p-value | 0.378 | 0.629 | 0.478 | 0.546 | 0.013 | |
| GCF IL-21 | Spearman correlation coefficient | 0.473 | 0.361 | −0.474 | −0.189 | 0.097 |
| p-value | 0.075 | 0.186 | 0.074 | 0.500 | 0.730 | |
BI = bleeding index; CAL = clinical attachment loss; GCF = gingival crevicular fluid; IL = interleukin; MGI = Modified Gingival Index; NS = no Statistical significance; PI = plaque index; PPD = probing pocket depth; S = statistical significance (p≤0.05).
Although there is an array of evidence and research about biomarkers for periodontal disease in the literature, appropriate molecular indicators of destruction of both soft and hard tissues that can take the place of clinical gold standards are still missing. However, scientists have been aggressively looking for clear indicators of periodontitis (22).
IL-21 is a pleiotropic cytokine that influences immunological responses. IL-21 promotes the growth of Th17 cells, which contribute significantly to periodontitis. In addition to periodontitis, it has been linked to inflammatory disorders such as rheumatoid arthritis and systemic lupus erythematosus (23). The available information on the connection of IL-21 with chronic periodontitis is limited. This study aims to assess IL-21 levels in serum and GCF of chronic periodontitis patients, correlate them with clinical indicators, and compare them to levels in gingivitis patients and healthy people. IL-21 increases the host’s immunological response and local inflammation. This study suggests that IL-21 plays a significant role in the etiology of periodontal disease (24).
In this investigation, group C had significantly higher mean serum IL-21 concentrations (139.3 ± 113.5 ng/mL) than group A (44.866 ± 8.9 ng/mL), but not group B (87.33 ± 10.1 ng/mL). Lokhande et al (25) found that the periodontitis group had considerably higher serum levels of IL-21 (497.78 ± 297.06) compared to the healthy group (65.34 ± 42.66), which is in accordance with current investigation, where serum levels showed no statistically significant difference between groups B and C.
The current study is the first of its type to measure GCF levels of IL-21 in distinct degrees of periodontal inflammation, including gingivitis and periodontitis, when compared to a healthy cohort. In this investigation, groups C and B had considerably higher mean GCF concentrations of IL-21 (66.400 ± 7.82 and 66.400 ± 9.47 ng/mL, respectively) compared to group A (21.933±9.1 ng/mL). There was no significant difference between groups B and C, demonstrating that IL-21 is a generic inflammatory marker.
The current study suggests that IL-21 may play a role in periodontitis by causing inflammation and tissue damage. The current investigation found no significant correlation between illness severity (defined by PPD and CAL) and IL-21 levels in GCF or serum in group C. Dutzan et al (23) found a substantial positive association between GCF levels of IL-21 and PPD and CAL, which contradicts our findings. Lokhande et al (25) found a strong association between PPD and serum IL-21 levels, but no correlation between IL-21 levels in saliva or serum with CAL.
Zhao et al (26) found that nonsurgical periodontal therapy decreased Th17-related cytokines (IL-17, IL-21) in GCF of chronic periodontitis patients. Reduced IL-21 levels suggest a link between periodontal inflammation and disease activity.
Napimoga et al (27) conducted a study on the local levels of IL-21 and IL-21 receptor in gingival tissues of chronic periodontitis and periodontally healthy subjects, as well as their relationship with salivary immunoglobulin A (IgA) levels. The study found that chronic periodontitis patients had considerably greater mean IgA levels and messenger ribonucleic acid (mRNA) levels for IL-21 compared to those who were periodontally healthy, which has consistent results with our study. Elevated IgA levels in the study suggest that IL-21 plays a considerable role in periodontal damage, sometimes even more than periodontal bacteria themselves.
Vahabi et al (28) conducted a study that compared salivary concentrations of IL-17 and IL-18 in patients with chronic periodontitis and healthy individuals. They concluded that an elevated salivary IL-18 level in chronic periodontitis patients has the potential to be a biomarker for periodontal tissue destruction, which was in accordance with our study where we concluded that elevated levels of IL-21 were found in chronic periodontitis individuals.
The current study’s lack of association could be attributed to the small sample size, disease progression, and site specificity. Although clinical parameters indicate cumulative damage, it is impossible to assess the current state of disease activity within tissues. It has been proposed that disease activity is not continuous, but rather consists of active and remission states. If a sample is collected from a remission site that was previously active, the levels may not correlate with clinical criteria. More research is needed with bigger patient samples with different levels of active and inactive areas.
GCF collection in periodontitis locations may not produce comparable amounts due to variations in production and flow rates based on the periodontium’s inflammatory condition. Our study found a strong positive connection between serum levels of IL-21 and BI in group C. Gingival bleeding is an early and clear indicator of gingival irritation. Since IL-21 is an inflammatory biomarker, there may be a positive link between these two traits.
The study found no significant association between age and GCF or serum IL-21 levels across three groups. Age is a variable that requires a bigger sample size for evaluation, and no attributes can be made at this time. Chronic periodontitis is a polymicrobial disease with multiple causes. The host’s susceptibility is determined by interactions between bacteria, the immune system, and the environment, with the host factor playing a significant influence. Estimating host response indicators may aid in monitoring disease progression in advanced gingivitis or early periodontitis patients, as early tissue damage can be challenging to diagnose (29). Estimating host response indicators may aid in assessing disease progression. More cross-sectional and interventional studies with higher sample sizes are needed to determine the efficacy of IL-21 as a biomarker in diagnosis and treatment.
Conclusion
To the best of our knowledge, the current study is the first of its kind to quantify GCF and serum levels of IL-21 in various stages of periodontal inflammation, including gingivitis and periodontitis, when compared to a healthy cohort. Despite the limitations of this study, IL-21 could be a viable biomarker for detecting periodontitis. Further cross-sectional and interventional research with bigger sample sizes are needed to compare the association of IL-21 levels in various types of periodontal disease and consider it as a potential diagnostic biomarker.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contribution: All authors contributed equally to this manuscript.
Compliance with ethics guidelines: The study protocol is approved by the University Ethics Committee for Human Trials of Sri Sai College of Dental Surgery, Vikarabad, Telangana (Ref no. 615/SSCDS/IRB-E/2017). Written informed consent has been obtained from the participants.
Availability of data and materials: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
- 1. Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32(s6)(suppl 6):108-129. CrossRef PubMed
- 2. Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65(3):260-267. CrossRef PubMed
- 3. Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Biol Med. 2002;13(1):17-34. CrossRef PubMed
- 4. Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1(1):821-878. CrossRef PubMed
- 5. Taba M Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49(3):551-571, vi. CrossRef PubMed
- 6. Fazal I, Shetty B, Yadalam U, Khan SF, Nambiar M. Effectiveness of periodontal intervention on the levels of N-terminal pro-brain natriuretic peptide in chronic periodontitis patients. J Circ Biomark. 2022;11:48-56. CrossRef PubMed
- 7. AlRowis R, AlMoharib HS, AlMubarak A, Bhaskardoss J, Preethanath RS, Anil S. Oral fluid-based biomarkers in periodontal disease – part 2. Gingival crevicular fluid. J Int Oral Health. 2014;6(5):126-135. PubMed
- 8. Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL-1beta profiles in periodontal disease. J Clin Periodontol. 2002;29(1):48-53. CrossRef PubMed
- 9. Irwin CR, Myrillas TT. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998;4(1):43-47. CrossRef PubMed
- 10. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559-564. CrossRef PubMed
- 11. Tsai IS, Tsai CC, Ho YP, Ho KY, Wu Y-M, Hung CC. Interleukin-12 and interleukin-16 in periodontal disease. Cytokine. 2005;31(1):34-40. CrossRef PubMed
- 12. de Rham C, Ferrari-Lacraz S, Jendly S, Schneiter G, Dayer JM, Villard J. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res Ther. 2007;9(6):R125. CrossRef
- 13. Sarra M, Pallone F, Monteleone G. Interleukin-21 in chronic inflammatory diseases. Biofactors. 2013;39(4):368-373. CrossRef PubMed
- 14. Rasmussen TK, Andersen T, Hvid M, et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol. 2010;37(10):2014-2020. CrossRef PubMed
- 15. Gerlach K, Daniel C, Lehr HA, et al. Transcription factor NFATc2 controls the emergence of colon cancer associated with IL-6-dependent colitis. Cancer Res. 2012;72(17):4340-4350. CrossRef PubMed
- 16. Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(7):1090-1100. CrossRef PubMed
- 17. Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480-483. CrossRef PubMed
- 18. Onoda T, Rahman M, Nara H, et al. Human CD4+ central and effector memory T cells produce IL-21: effect on cytokine-driven proliferation of CD4+ T cell subsets. Int Immunol. 2007;19(10):1191-1199. CrossRef PubMed
- 19. Pelletier M, Girard D. Biological functions of interleukin-21 and its role in inflammation. ScientificWorldJournal. 2007;7:1715-1735. CrossRef PubMed
- 20. Shetty B, Fazal I, Khan SF, et al. Association between cardiovascular diseases and periodontal disease: more than what meets the eye. Drug Target Insights. 2023;17:31-38. CrossRef PubMed
- 21. Persson GR. Site-based versus subject-based periodontal diagnosis. Periodontol 2000. 2005;39(1):145-163. CrossRef PubMed
- 22. Loos BG, Tjoa S. Host-derived diagnostic markers for periodontitis: do they exist in gingival crevice fluid? Periodontol 2000. 2005;39(1):53-72. CrossRef PubMed
- 23. Dutzan N, Rivas C, García-Sesnich J, et al. Levels of interleukin-21 in patients with untreated chronic periodontitis. J Periodontol. 2011 Oct;82(10):1483-1489. CrossRef. PubMed.
- 24. Dutzan N, Vernal R, Vaque JP, et al. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol. 2012;83(7):948-954. CrossRef PubMed
- 25. Lokhande RV, Ambekar JG, Bhat KG, Dongre NN. Interleukin-21 and its association with chronic periodontitis. J Indian Soc Periodontol. 2019;23(1):21-24. CrossRef PubMed
- 26. Zhao L, Zhou Y, Xu Y, Sun Y, Li L, Chen W. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J Clin Periodontol. 2011;38(6):509-516. CrossRef PubMed
- 27. Napimoga MH, Nunes LH, Maciel AA, et al. Possible involvement of IL-21 and IL-10 on salivary IgA levels in chronic periodontitis subjects. Scand J Immunol. 2011;74(6):596-602. CrossRef PubMed
- 28. Vahabi S, Yadegari Z, Pournaghi S. The comparison of the salivary concentration of interleukin-17 and interleukin-18 in patients with chronic periodontitis and healthy individuals. Dent Res J (Isfahan). 2020;17(4):280-286. CrossRef PubMed
- 29. Khiste SV, Ranganath V, Nichani AS, Rajani V. Critical analysis of biomarkers in the current periodontal practice. J Indian Soc Periodontol. 2011;15(2):104-110. CrossRef PubMed