 |
J Circ Biomark 2024; 13: 7-13 ISSN 1849-4544 | DOI: 10.33393/jcb.2024.2689 ORIGINAL RESEARCH ARTICLE |
 |
Relation between interleukin-13 and annexin-V levels and carotid intima-media thickness in nephrotic syndrome
ABSTRACT
Background and aim: The aim of the current study is to assess the relation between carotid intima-media thickness (CIMT) measurements, renal Doppler resistive index (RI) and serum levels of interleukin-13 (IL-13) and annexin-V (An-V) in children with idiopathic nephrotic syndrome (INS).
Materials and methods: The present case-control study was conducted on 60 children with INS and 60 age- and sex-matched healthy children. All participants were subjected to evaluation of serum levels of IL-13 and An-V and ultrasound Doppler measurement of CIMT and renal RI.
Results: Patients expressed significantly higher An-V (5.9 ± 2.6 vs. 2.1 ± 0.8 ng/mL, p<0.001) and IL-13 (19.2 ± 7.6 vs. 3.4 ± 1.4 ng/L) levels when compared with healthy counterparts. Moreover, it was shown that patients had significantly higher CIMT (0.49 ± 0.06 vs. 0.35 ± 0.03, p<0.001) as compared to controls. No significant differences were noted between the studied groups regarding right or left RIs. Correlation analysis identified significant direct correlation between serum An-V levels and albumin/creatinine ratio (ACR) (r = 0.55), cholesterol (r = 0.48), triglycerides (r = 0.36), IL-13 (r = 0.92) and CIMT (r = 0.53). Similar correlations could be found between serum IL-13 levels and CIMT measurements and the corresponding parameters.
Conclusions: The present study suggests an association between higher early atherosclerosis expressed as elevated CIMT measurements in children with INS and elevated serum levels of An-V and IL-13.
Keywords: Annexin-V, Carotid intima-media thickness, Idiopathic nephrotic syndrome, Interleukin-13
Received: October 22, 2023
Accepted: May 16, 2024
Published online: June 18, 2024
Journal of Circulating Biomarkers - ISSN 1849-4544 - www.aboutscience.eu/jcb
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Idiopathic nephrotic syndrome (INS) is a common childhood disease with average global incidence of 4.7 per 100,000 persons (1). According to one report, most affected children in African countries including Egypt are steroid sensitive (2). Fortunately, the condition has generally favorable prognosis with the majority of patients achieving complete remission (3). However, about 1% of patients may develop end-stage renal disease by the age of 18 (4). INS is characterized by significant proteinuria as well as other pathological alterations. It results from enhanced plasma protein permeability of the glomerular filtration membrane. Although most patients benefit from steroid treatment, 10%-20% of affected children have steroid-resistant nephrotic syndrome (SRNS) (5,6).
While the exact cause of INS is unknown, an imbalance between T helper (Th)1 and Th2 cells is most likely to blame. It is suggested that activation of Th2 cells is a key factor in the pathophysiology of minimal change disease (MCD). Th2-released cytokines interleukin-4 (IL-4), IL-10 and especially IL-13 may play a part in the induction of proteinuria (7). IL-13 is a pleiotropic type 2 cytokine involved in the pathogenesis of many allergic (8), inflammatory (9) and malignant conditions (10). In MCD patients, IL-13 may change glomerular permeability and cause proteinuria (7). Annexin-V (An-V) is a cytoplasmic calcium-binding protein with pleotropic functions (11,12). The cells of the distal tubules and the glomerular epithelium contain considerable levels of An-V. It is frequently used as an apoptotic marker in many pathological conditions including systemic sclerosis (13), diabetes (14) and atherosclerosis (15). It helped to explain a variety of kidney-related events, such as acute kidney damage and diabetic nephropathy. It has also been utilized as a biochemical indicator of atherosclerosis in patients with chronic kidney disease (16).
The carotid intima-media thickness (CIMT) measured by ultrasound is used as a marker of atherosclerotic changes (17). In children with INS, atherosclerotic changes may accelerate progression to chronic kidney disease and are mainly related to dyslipidemia (18,19). Intrarenal hemodynamics was found to be a potential indicator of systemic vascular injury and a helpful tool for better stratifying short- and long-term cardiovascular risk in various clinical subgroups. Particularly, renal Doppler resistive index (RI) and renal pulsatility index (PI) are closely associated with well-known markers of subclinical organ damage, such as CIMT, arterial stiffness, and left ventricular mass (20).
Renal RI has been shown as a noninvasive prognostic marker for assessing the renal disease progression, especially in hypertension, as well as proteinuria (21-24). Additionally, numerous studies have shown that intrarenal hemodynamic dysfunction directly contributes to unfavorable cardiovascular events and even death across a variety of populations, particularly in those with chronic kidney disease (12). The present study hypothesized that there is a relation between subclinical atherosclerosis and inflammatory markers in children with INS. The aim of the current study is to assess the relation between CIMT, renal Doppler RI and serum levels of IL-13 and An-V in children with INS.
Materials and methods
Study design
The present cross-sectional case-control study was conducted during the period from August 2021 to May 2022. The study protocol was approved by the ethical committee and the legal guardians of included children proved written informed consent before participation.
Participants
The study included 60 children with INS diagnosed on the basis of the International Study of Kidney Disease in Children criteria (25). In addition, there were 60 age- and sex-matched healthy children who served as the control group. Children with acquired or congenital heart disease, active systemic vasculitis, chronic kidney disease and severe edema at the time of examination or taking lipid-lowering drugs were excluded from the study.
Variables
All participants underwent thorough clinical evaluations, including measurements of weight, height, body mass index (BMI), arterial blood pressure (BP), examination for edema and cardiac examination to exclude congenital or acquired heart disease. All patients completed a thorough clinical history that included past medical history, age at diagnosis, disease duration, responsiveness to steroid treatment, frequency of relapses and use of immunosuppressive drugs. Steroid sensitivity was defined as complete remission at the standard dose of prednisone or prednisolone (PDN) (60 mg/m2/day or 2 mg/kg/day, maximum 60 mg/day) within 4 weeks, while steroid resistance was defined as failure to achieve remission after 4-8 weeks of daily oral PDN therapy at a dose of 2 mg/kg/day (26). In SRNS patients, 19 patients received calcineurin inhibitors, while 3 patients received mycophenolate mofetil.
For measurement of BP, the patient was seated correctly; BP readings for the systolic and diastolic phases were taken using a mercury sphygmomanometer with an appropriate cuff size. At 2-minute intervals, three measurements were collected from each participant, and the average of the last two measurements was recorded. Values >95th percentile are regarded as elevated by the American Academy of Pediatrics for systolic BP and diastolic BP in the pediatrics population (27).
For laboratory assessment, 6 mL of venous blood was withdrawn after 10 hours of fasting and divided into three aliquots; 2 mL was evacuated in ethylenediaminetetraacetic acid (EDTA) tube for complete blood count (Sysmex KX21N, Kobe, Japan). The remaining 4 mL was evacuated in two serum separator tubes, centrifuged at 3,500 rpm for 10 minutes; two mL serum was used for measurement of kidney function tests (urea and creatinine), lipid profile (total cholesterol, triglycerides) and serum albumin; and the other 2 mL was separated and frozen at −20°C for later analysis of IL-13 and An-V. An early morning urine sample was used for albumin/creatinine ratio (ACR) measurement. Measurement of serum IL-13 and An-V was done using the commercial enzyme-linked immunosorbent assay (ELISA) kits (Bioassay Technology Laboratory, China; Cat. No. 202208016). The assay ranges of IL-13 and An-V were from 0.5 to 100 ng L and 0.1 to 35 ng mL, respectively.
Measurement of CIMT was done using the ultrasound machine (Philips 50G ultrasound machine) with high-frequency linear probe (7.5 MHz) (Fig. 1). The examination was done in supine position; the neck is extended and slightly turned to the contralateral side. Prior to measurement, the patients had rest for 10 minutes. The arterial wall of the common carotid artery was assessed bilaterally in a longitudinal view; both walls were clearly visualized to obtain proper measurements. Zoomed frozen images were taken clearly demonstrating the intima-media thickness. Measurements were taken from the far wall of each common carotid artery 1 cm proximal to bifurcation. The measurements were made three times on each side and the average of the measurements was taken. The mean of measurements of the left and right common carotid arteries was calculated.
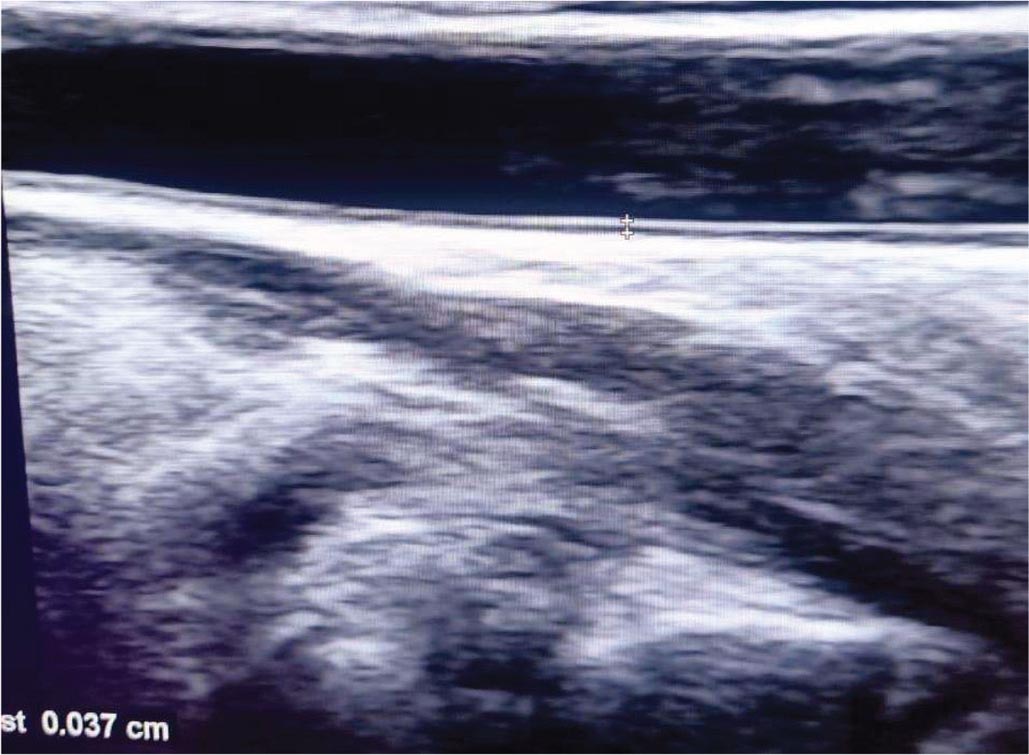
FIGURE 1 - B mode ultrasound of carotid intima-media thickness measurement.
Renal Doppler ultrasound evaluation was done in the lateral oblique position or oblique position; however, the left kidney was better examined in a right lateral decubitus. The examination was done using Philips 50G ultrasound machine with low-frequency convex probe (5 MHz) (Fig. 2). Firstly, both kidneys were scanned using B mode for detection of any gross abnormalities such as stones, backpressure changes, echogenicity, cortical thickness or focal lesions. Color Doppler was applied to see interlobar arteries, and then the sample volume was applied to cover only the arterial diameter of interest. Optimum waveform was obtained by adjustment of both the pulse repetition frequency (PRF) and the wall filter to avoid aliasing. Measurement of Doppler parameters was taken at breath holds in cooperative children and in younger children at quiet respiration. For each kidney, RIs of renal interlobar arteries at the upper, middle and lower poles were assessed three times, then the average of the measurements was taken. The arterial RI was calculated by the equation (peak systolic velocity − end diastolic velocity) divided by (peak systolic velocity), and its value is derived via the computer algorithm in the ultrasound machine.
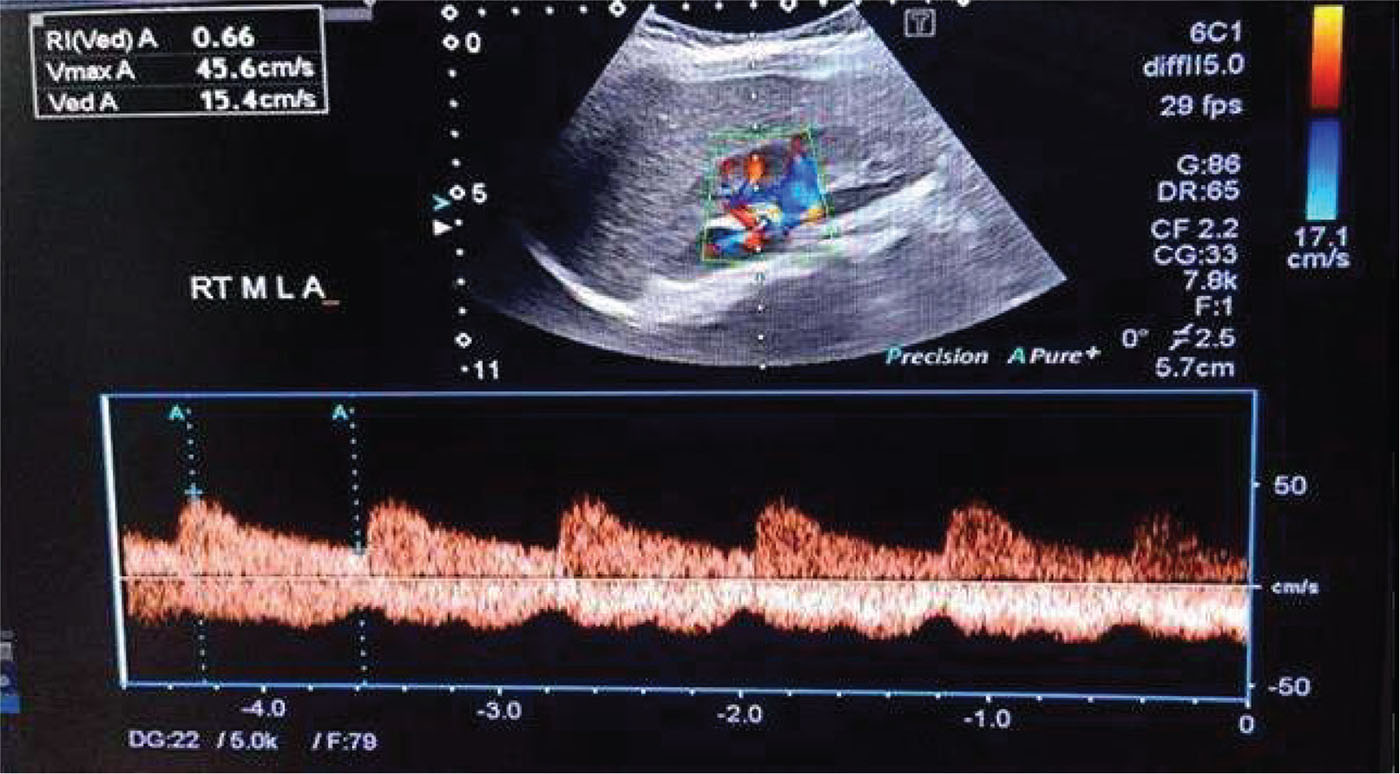
FIGURE 2 - Color duplex ultrasound measurements of the right and middle pole interlobar artery resistive index.
Statistical methods
Results were reviewed, given codes and enrolled into the Statistical Package for Social Sciences (IBM SPSS) version 23. Normality of data distribution was assessed using Kolmogorov-Smirnov test. Quantitative data were displayed as means and standard deviation (SD) or median and interquartile range (IQR). Qualitative data were displayed as numbers and percentages. Comparison of non-quantitative data was done by Chi-square test. Comparison of quantitative data with the parametric patterns was done by independent t-test, while non-parametric data were compared using Mann-Whitney U-test. Correlation between various variables was done using Pearson’s correlation coefficient for parametric data and Spearman’s correlation coefficient for non-parametric data. A p-value was considered to be significant if less than 0.05.
Results
The present study included 60 children with INS and 60 age- and sex-matched healthy controls. Patients had disease duration of 2.9 ± 2.6 years. Comparison between the studied groups regarding clinical, laboratory and ultrasonic parameters revealed that patients had significantly lower serum albumin levels (1.7 ± 0.4 vs. 3.5 ± 0.3 g/dL, p<0.001) and significantly higher platelet count (381.4 ± 105.2 vs. 332.4 ± 94.2, p = 0.008), ACR (5,945.5 ± 2,866.6 vs. 75.4 ± 122.4 mg/g, p<0.001), cholesterol (456.3 ± 139.5 vs. 163.8 ± 126.3 mg/dL, p<0.001) and triglyceride (214.6 ± 112.0 vs. 123.6 ± 38.7 mg/dL, p<0.001) levels when compared with controls. No statistically significant differences were found between the studied groups regarding diastolic and systolic BP measurements (Tab. 1).
| Patients
n = 60 |
Controls
n = 60 |
p value | |
|---|---|---|---|
| Age (years) | 9.6 ± 3.6 | 9.7 ± 3.7 | 0.83 |
| Male/female n | 39/21 | 43/17 | 0.43 |
| BMI (kg/m2) | 21.3 ± 5.6 | 20.1 ± 3.8 | 0.19 |
| SBP (mm Hg) | 102.1 ± 10.3 | 103.5 ± 8.4 | 0.41 |
| DBP (mm Hg) | 65.2 ± 9.0 | 64.9 ± 8.2 | 0.83 |
| Laboratory findings | |||
| WBCs (109/L) | 5.6 (4.9-6.6) | 5.1 (4.7-6.2) | 0.21 |
| Hemoglobin (mg/dL) | 13.0 ± 1.3 | 12.9 ± 1.4 | 0.9 |
| Platelets (109/L) | 381.4 ± 105.2 | 332.4 ± 94.2 | 0.008 |
| Albumin (g/dL) | 1.7 ± 0.4 | 3.5 ± 0.3 | <0.001 |
| Urea (mg/dL) | 19.5 ± 5.3 | 18.0 ± 4.6 | 0.1 |
| Creatinine (mg/dL) | 0.37 ± 0.22 | 0.33 ± 0.22 | 0.27 |
| ACR (mg/g) | 5945.5 ± 2866.6 | 75.4 ± 122.4 | <0.001 |
| Cholesterol (mg/dL) | 456.3 ± 139.5 | 163.8 ± 126.3 | <0.001 |
| Triglycerides (mg/dL) | 214.6 ± 112.0 | 123.6 ± 38.7 | <0.001 |
| Annexin-V (ng/mL) | 5.9 ± 2.6 | 2.1 ± 0.8 | <0.001 |
| IL-13 (ng/L) | 19.2 ± 7.6 | 3.4 ± 1.4 | <0.001 |
| Ultrasound findings | |||
| CIMT (mm) | 0.49 ± 0.06 | 0.35 ± 0.03 | <0.001 |
| Right RI | 0.61 ± 0.02 | 0.60 ± 0.02 | 0.5 |
| Left RI | 0.60 ± 0.02 | 0.60 ± 0.02 | 0.73 |
ACR = albumin/creatinine ratio; BMI = body mass index; CIMT = carotid intima-media thickness; DBP = diastolic blood pressure; IL-13 = interleukin-13; RI = resistive index; SBP = systolic blood pressure; WBCs = white blood cells.
In addition, they expressed significantly higher An-V (5.9 ± 2.6 vs. 2.1 ± 0.8 ng/mL, p<0.001) and IL-13 (19.2 ± 7.6 vs. 3.4 ± 1.4 ng/L) levels when compared with healthy counterparts. Moreover, it was shown that patients had significantly higher CIMT (0.49 ± 0.06 vs. 0.35 ± 0.03, p<0.001) as compared to controls. No significant differences were noted between the studied groups regarding right or left RIs (Tab. 1).
Correlation analysis identified significant direct correlation between serum An-V levels and ACR (r = 0.55), cholesterol (r = 0.48), triglycerides (r = 0.36), IL-13 (r = 0.92) and CIMT (r = 0.53). Similar correlations could be found between serum IL-13 levels and CIMT measurements and the corresponding parameters (ACR, cholesterol, triglycerides) (Tab. 2).
| CIMT | Annexin-V | IL-13 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | 0.003 | 0.98 | −0.034 | 0.71 | −0.027 | 0.77 |
| BMI | −0.02 | 0.88 | −0.01 | 0.88 | −0.04 | 0.7 |
| SBP | −0.06 | 0.67 | −0.13 | 0.15 | −0.11 | 0.25 |
| DBP | −0.14 | 0.3 | −0.09 | 0.32 | −0.04 | 0.68 |
| Disease duration | −0.05 | 0.73 | −0.14 | 0.29 | −0.16 | 0.23 |
| WBCs | 0.006 | 0.96 | −0.005 | 0.97 | 0.01 | 0.19 |
| Hemoglobin | 0.08 | 0.56 | −0.1 | 0.3 | −0.06 | 0.51 |
| Platelets | 0.06 | 0.67 | 0.13 | 0.15 | 0.19 | 0.043 |
| Albumin | −0.77 | <0.001 | 0.55 | <0.001 | 0.65 | <0.001 |
| Urea | −0.2 | 0.12 | 0.13 | 0.16 | 0.16 | 0.1 |
| Creatinine | −0.09 | 0.49 | 0.13 | 0.15 | 0.12 | 0.18 |
| ACR | 0.77 | <0.001 | 0.55 | <0.001 | 0.65 | <0.001 |
| Cholesterol | 0.7 | <0.001 | 0.48 | <0.001 | 0.58 | <0.001 |
| Triglycerides | 0.31 | 0.017 | 0.36 | <0.001 | 0.39 | <0.001 |
| Annexin-V | 0.53 | <0.001 | – | – | 0.92 | <0.001 |
| IL-13 | 0.64 | <0.001 | 0.92 | <0.001 | – | – |
| CIMT | – | – | 0.53 | <0.001 | 0.64 | <0.001 |
| Right RI | −0.08 | 0.54 | 0.08 | 0.37 | 0.06 | 0.53 |
| Left RI | −0.06 | 0.67 | 0.04 | 0.69 | −0.05 | 0.56 |
ACR = albumin/creatinine ratio; BMI = body mass index; CIMT = carotid intima-media thickness; DBP = diastolic blood pressure; IL-13 = interleukin-13; RI = resistive index; SBP = systolic blood pressure; WBCs = white blood cells.
The present study included 38 (63.3%) steroid-sensitive patients. Comparison between steroid-sensitive and steroid-resistant patients regarding An-V and IL-13 levels, CIMT measurements and other clinical parameters revealed no statistically significant differences (Tab. 3).
Discussion
The present study found an association between increased CIMT levels and higher levels of serum An-V and IL-13 levels, which is a novel finding to the best of our knowledge. The study compared clinical, laboratory and Doppler ultrasound parameters in children with INS and healthy controls. The study found that patients exhibited significantly higher CIMT measurements as compared to healthy controls. Similar results were reported by previous studies (17,28-32), while Kniazewska et al (33) and Rahul et al (34) found no significant differences in CIMT measurements between children with INS and healthy counterparts. This may be explained by the different clinical profile of the studied groups. Elevated CIMT in INS children is usually explained by concurrent hypertension and dyslipidemia. Notably, our patients had significantly higher serum cholesterol and triglyceride levels as compared to controls while both groups expressed comparable systolic and diastolic BP values.
| Steroid sensitive
n = 38 |
Steroid resistant
n = 22 |
p value | |
|---|---|---|---|
| Age (years) | 9.8 ± 3.7 | 9.3 ± 3.4 | 0.56 |
| Male/female n | 23/15 | 16/6 | 0.34 |
| BMI (kg/m2) | 20.5 ± 4.6 | 22.7 ± 7.1 | 0.16 |
| SBP (mm Hg) | 102.1 ± 8.5 | 102.0 ± 12.8 | 0.99 |
| DBP (mm Hg) | 63.2 ± 6.4 | 68.6 ± 11.6 | 0.05 |
| Laboratory findings | |||
| WBCs (109/L) | 5.5 (4.9-6.1) | 5.7 (4.9-9.6) | 0.42 |
| Hemoglobin (mg/dL) | 12.9 ± 1.4 | 13.3 ± 1.2 | 0.29 |
| Platelets (109/L) | 380.3 ± 95.4 | 383.2 ± 122.7 | 0.92 |
| Albumin (g/dL) | 1.7 ± 0.4 | 1.8 ± 0.3 | 0.81 |
| Urea (mg/dL) | 20.3 ± 5.9 | 18.2 ± 4.0 | 0.14 |
| Creatinine (mg/dL) | 0.40 ± 0.21 | 0.31 ± 0.21 | 0.11 |
| Cholesterol (mg/dL) | 464.3 ± 153.9 | 442.5 ± 112.6 | 0.56 |
| Triglycerides (mg/dL) | 210.0 ± 110.4 | 222.6 ± 117.0 | 0.68 |
| Annexin-V (ng/mL) | 19.8 ± 8.0 | 18.3 ± 7.1 | 0.55 |
| IL-13 (ng/L) | 6.3 ± 2.6 | 5.2 ± 2.4 | 0.55 |
| Ultrasound findings | |||
| CIMT (mm) | 0.493 ± 0.061 | 0.488 ± 0.056 | 0.76 |
| Right RI | 0.611 ± 0.024 | 0.609 ± 0.026 | 0.79 |
| Left RI | 0.608 ± 0.019 | 0.607 ± 0.024 | 0.85 |
BMI = body mass index; CIMT = carotid intima-media thickness; DBP = diastolic blood pressure; IL-13 = interleukin-13; RI = resistive index; SBP = systolic blood pressure; WBCs = white blood cells.
The present study proposed to assess if serum An-V and IL-13 levels are related to dyslipidemia and CIMT measurements in those patients. The study showed significantly higher concentrations of An-V in children with INS in comparison with healthy controls. Jakubowska and Kiliś-Pstrusińska (16) also reported higher concentrations of plasma An-V in children with INS. Moreover, we detected a positive correlation between serum An-V with ACR in line with the conclusions of Simsek and colleagues (35). The cause of elevated plasma An-V concentrations in INS is still unknown. It is well recognized that An-V pathophysiological function is linked to cell apoptosis, and increased circulating lymphocyte apoptosis have been observed in children with nephrotic syndrome (35). Interestingly, Ye and colleagues (36) had demonstrated higher level of anti-annexin antibodies in children with INS, which significantly correlated with urine protein level. In addition, An-V levels were well correlated with dyslipidemia and CIMT in our study. To the best of our knowledge, this is a new finding in children with INS. In support of this result, one study on adult diabetic population observed positive correlation between serum An-V levels and CIMT (37).
Moreover, the present study detected a significantly higher concentration of IL-13 in INS patients in comparison with healthy controls. The overexpression of IL-13 is probably related to downregulation of podocin, nephrin and dystroglycan (essential molecules in preserving the slit diaphragm integrity of the glomeruli) (38).
Our findings are also supported by the studies of Kimata et al (39) who reported increased levels of urinary IL-13 in untreated children with INS. The present study also documented a significant positive correlation between serum IL-13 levels and ACR, cholesterol and triglyceride levels in INS patients. The relation between IL-13 levels in dyslipidemia observed by the present study may be explained by the interesting experimental work of Low et al (40). In their study on cytokine-induced rat MCD model, they showed that IL-13 induced changes in hepatic handling of cholesterol that resulted in hypercholesterolemia even before the onset of proteinuria. Our results may have therapeutic implications. Possible blockade of IL-13 pathways was recently studied in many inflammatory conditions (41-43). Use of similar approach in INS may be tried in the forthcoming future.
Notably, no significant differences were found between patients and controls regarding renal hemodynamic parameters. This reflects the fact that in spite of the increased CIMT in the studied patients, the renal hemodynamics remained unaffected. Also, the study showed no significant difference in CIMT between steroid response and SRNS and this came in agreement with Ahmed et al (17) who reported no significant difference in the CIMT between different steroid response and resistant groups. Also Rahul et al (34) found no significant difference in CIMT between steroid-sensitive nephrotic syndrome (SSNS) and SRNS patients.
Finally, we noticed that our patients expressed significantly higher platelet count. This comes in agreement with Gulleroglu et al (44) who reported increased platelet count and platelet volume in children with INS. The precise mechanism of this finding is still unknown. However, it has been noted that platelet hyper-aggregation and high platelet numbers are associated with hypoalbuminemia and hypercholesterolemia. The contribution of these cofactors to the development and clinical presentation of INS remains to be elucidated. Findings of the present study are limited by its cross-sectional design and the relatively small sample size. In conclusion, the present study suggests an association between early atherosclerosis expressed as elevated CIMT measurements in children with INS and elevated serum levels of An-V and IL-13. Further studies are recommended to confirm these findings and to explore their practical implications.
Acknowledgment
The authors are grateful to the patients’ families for their participation in this work.
Disclosures
Conflict of interest: The authors have no conflicts of interest in this work.
Financial support: This research received no external funding.
Ethical approval: Written informed consent was completed by each child’s parents. The study protocol was approved by Al-Azhar University local Ethics Committee, Faculty of Medicine (for girls), the council number is 202107919, and all procedures were in accordance with the Helsinki Declaration.
Consent of publication: Not applicable.
Data availability statement: Data of this study will be available from the corresponding author upon reasonable request.
Authors’ Contributions: All authors contributed significantly to the work reported, whether that is in the conception, study design, implementation, data collection, analysis and interpretation, or in each of these areas. They also participated in writing, revising or critically evaluating the article, gave their final approval for the version that would be published, decided on the journal to which the article would be submitted and agreed to be held accountable for all aspects of the work.
References
- 1. Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:39. CrossRef PubMed
- 2. Wine R, Vasilevska-Ristovska J, Banh T, et al; H3 Africa Kidney Disease Research Network. Trends in the epidemiology of childhood nephrotic syndrome in Africa: a systematic review. Glob Epidemiol. 2021;3:100061. CrossRef PubMed
- 3. Özlü SG, Demircin G, Tökmeci N, et al. Long-term prognosis of idiopathic nephrotic syndrome in children. Ren Fail. 2015;37(4):672-677. CrossRef PubMed
- 4. Carter SA, Mistry S, Fitzpatrick J, et al. Prediction of short- and long-term outcomes in childhood nephrotic syndrome. Kidney Int Rep. 2019;5(4):426-434. CrossRef PubMed
- 5. Kitsou K, Askiti V, Mitsioni A, Spoulou V. The immunopathogenesis of idiopathic nephrotic syndrome: a narrative review of the literature. Eur J Pediatr. 2022;181(4):1395-1404. CrossRef PubMed
- 6. Guo HL, Li L, Xu ZY, et al. Steroid-resistant nephrotic syndrome in children: a mini-review on genetic mechanisms, predictive biomarkers and pharmacotherapy strategies. Curr Pharm Des. 2021;27(2):319-329. CrossRef PubMed
- 7. da Silva Filha R, Burini K, Pires LG, Brant Pinheiro SV, Simões E Silva AC. Idiopathic nephrotic syndrome in pediatrics: an up-to-date. Curr Pediatr Rev. 2022;18(4):251-264. CrossRef PubMed
- 8. Matera MG, Ora J, Calzetta L, Rogliani P, Cazzola M. Investigational anti IL-13 asthma treatments: a 2023 update. Expert Opin Investig Drugs. 2023;32(5):373-386. CrossRef PubMed
- 9. Iwaszko M, Biały S, Bogunia-Kubik K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells. 2021;10(11):3000. CrossRef PubMed
- 10. Shi J, Song X, Traub B, Luxenhofer M, Kornmann M. Involvement of IL-4, IL-13 and their receptors in pancreatic cancer. Int J Mol Sci. 2021;22(6):2998. CrossRef PubMed
- 11. Shobeiri SS, Sankian M. Polyvinyl alcohol can stabilize FITC conjugated recombinant annexin V for apoptotic cells detection. Protein Pept Lett. 2022;29(9):806-814. CrossRef PubMed
- 12. Simonsen AC, Boye TL, Nylandsted J. Annexins bend wound edges during plasma membrane repair. Curr Med Chem. 2020;27(22):3600-3610. CrossRef PubMed
- 13. Horimoto AMC, de Jesus LG, de Souza AS, Rodrigues SH, Kayser C. Anti-annexin V autoantibodies and vascular abnormalities in systemic sclerosis: a longitudinal study. Adv Rheumatol. 2020;60(1):38. CrossRef PubMed
- 14. Bratseth V, Margeirsdottir HD, Chiva-Blanch G, et al. Annexin V+ microvesicles in children and adolescents with type 1 diabetes: a prospective cohort study. J Diabetes Res. 2020;2020:7216863. CrossRef PubMed
- 15. Hu Y, Liu G, Zhang H, et al. A comparison of [99mTc]duramycin and [99mTc]annexin V in SPECT/CT imaging atherosclerotic plaques. Mol Imaging Biol. 2018;20(2):249-259. CrossRef PubMed
- 16. Jakubowska A, Kiliś-Pstrusińska K. Annexin V in children with idiopathic nephrotic syndrome treated with cyclosporine A. Adv Clin Exp Med. 2020;29(5):603-609. CrossRef PubMed
- 17. Ahmed HM, Ameen EE, Awad MS, Botrous OE. Assessment of carotid intima media thickness and left ventricular mass index in children with idiopathic nephrotic syndrome. Vasc Health Risk Manag. 2021;17:349-356. CrossRef PubMed
- 18. Hyla-Klekot L, Bryniarski P, Pulcer B, Ziora K, Paradysz A. Dimethylarginines as risk markers of atherosclerosis and chronic kidney disease in children with nephrotic syndrome. Adv Clin Exp Med. 2015;24(2):307-314. CrossRef PubMed
- 19. Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12(1):37-47. CrossRef PubMed
- 20. Geraci G, Buccheri D, Zanoli L, et al. Renal haemodynamics and coronary atherosclerotic burden are associated in patients with hypertension and mild coronary artery disease. Exp Ther Med. 2019;17(4):3255-3263. CrossRef PubMed
- 21. Youssef DM, Fawzy FM. Value of renal resistive index as an early marker of diabetic nephropathy in children with type-1 diabetes mellitus. Saudi J Kidney Dis Transpl. 2012;23(5):985-992. CrossRef PubMed
- 22. Moriconi D, Mengozzi A, Duranti E, et al. The renal resistive index is associated with microvascular remodeling in patients with severe obesity. J Hypertens. 2023;41(7):1092-1099. CrossRef PubMed
- 23. Ghafori M, Rashedi A, Montazeri M, Amirkhanlou S. The relationship between Renal Arterial Resistive Index (RRI) and renal outcomes in patients with resistant hypertension. Iran J Kidney Dis. 2020;14(6):448-453. PubMed
- 24. Provenzano M, Rivoli L, Garofalo C, et al. Renal resistive index in chronic kidney disease patients: possible determinants and risk profile. PLoS One. 2020;15(4):e0230020. CrossRef PubMed
- 25. Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20(6):765-771. CrossRef PubMed
- 26. Trautmann A, Vivarelli M, Samuel S, et al; International Pediatric Nephrology Association. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1529-1561. CrossRef PubMed
- 27. Flynn JT, Kaelber DC, Baker-Smith CM, et al; SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. CrossRef PubMed
- 28. Hooman N, Isa-Tafreshi R, Otukesh H, Mostafavi SH, Hallaji F. Carotid artery function in children with idiopathic nephrotic syndrome. Nefrologia. 2013;33(5):650-656. CrossRef PubMed
- 29. Youssef DM, Gomaa MA, El-Akhras A, et al. Brachial artery flow-mediated dilatation and carotid intima-media thickness in children with idiopathic nephrotic syndrome. Iran J Kidney Dis. 2018;12(6):331-340. PubMed
- 30. Skrzypczyk P, Kuźma-Mroczkowska E, Kułagowska J, Brzewski M, Okarska-Napierała M, Pańczyk-Tomaszewska M. Carotid intima-media thickness in children with idiopathic nephrotic syndrome: a single center cross-sectional study. Clin Nephrol. 2019;91(6):353-362. CrossRef PubMed
- 31. Paripović A, Stajić N, Putnik J, Gazikalović A, Bogdanović R, Vladislav V. Evaluation of carotid intima media thickness in children with idiopathic nephrotic syndrome. Nephrol Ther. 2020;16(7):420-423. CrossRef PubMed
- 32. Kamel AS, AlGhawass MME, Sayed MA, Roby SA. Evaluation of carotid intima media thickness in children with idiopathic nephrotic syndrome. Ital J Pediatr. 2022;48(1):195. CrossRef PubMed
- 33. Kniazewska MH, Obuchowicz AK, Wielkoszyński T, et al. Atherosclerosis risk factors in young patients formerly treated for idiopathic nephrotic syndrome. Pediatr Nephrol. 2009;24(3):549-554. CrossRef PubMed
- 34. Rahul I, Krishnamurthy S, Satheesh S, Biswal N, Bobby Z, Lakshminarayanan S. Brachial artery flow-mediated dilatation and carotid intima medial thickness in pediatric nephrotic syndrome: a cross-sectional case-control study. Clin Exp Nephrol. 2015;19(1):125-132. CrossRef PubMed
- 35. Simsek B, Buyukcelik M, Soran M, et al. Urinary annexin V in children with nephrotic syndrome: a new prognostic marker? Pediatr Nephrol. 2008;23(1):79-82. CrossRef PubMed
- 36. Ye Q, Zhang Y, Zhuang J, et al. The important roles and molecular mechanisms of annexin A2 autoantibody in children with nephrotic syndrome. Ann Transl Med. 2021;9(18):1452. CrossRef PubMed
- 37. Bilgir O, Vural HA, Bilgir F, Akan OY, Demir I. Serum annexin V and anti-annexin V levels and their relationship with metabolic parameters in patients with type 2 diabetes. Rev Assoc Med Bras (1992). 2019 Sep 12;65(8):1042-1047. CrossRef
- 38. Lai KW, Wei CL, Tan LK, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18(5):1476-1485. CrossRef PubMed
- 39. Kimata H, Fujimoto M, Furusho K. Involvement of interleukin (IL)-13, but not IL-4, in spontaneous IgE and IgG4 production in nephrotic syndrome. Eur J Immunol. 1995;25(6):1497-1501. CrossRef PubMed
- 40. Low LD, Lu L, Chan CY, et al. IL-13-driven alterations in hepatic cholesterol handling contributes to hypercholesterolemia in a rat model of minimal change disease. Clin Sci (Lond). 2020;134(2):225-237. CrossRef PubMed
- 41. Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188-1204. CrossRef PubMed
- 42. Pelaia C, Pelaia G, Crimi C, et al. Biological therapy of severe asthma with dupilumab, a dual receptor antagonist of interleukins 4 and 13. Vaccines (Basel). 2022;10(6):974. CrossRef PubMed
- 43. Kariyawasam HH. Chronic rhinosinusitis with nasal polyps: mechanistic insights from targeting IL-4 and IL-13 via IL-4Rα inhibition with dupilumab. Expert Rev Clin Immunol. 2020;16(12):1115-1125. CrossRef PubMed
- 44. Gulleroglu K, Yazar B, Sakalli H, Ozdemir H, Baskin E. Clinical importance of mean platelet volume in children with nephrotic syndrome. Ren Fail. 2014;36(5):663-665. CrossRef PubMed