 |
J Circ Biomark 2022; 11: 48-56 ISSN 1849-4544 | DOI: 10.33393/jcb.2022.2454 ORIGINAL RESEARCH ARTICLE |
 |
Effectiveness of periodontal intervention on the levels of N-terminal pro-brain natriuretic peptide in chronic periodontitis patients
ABSTRACT
Background: N-terminal-pro-brain natriuretic peptide (NT-proBNP) is an inactive hormone that is seen during inflammation and is a known biomarker of cardiovascular disease (CVD). Evidence suggests that periodontitis has a bidirectional relationship with CVD and NT-proBNP has a potential role in periodontal disease. However, there is no evidence on the impact of nonsurgical periodontal therapy (NSPT) on the levels of NT-proBNP in gingival crevicular fluid (GCF) and serum in patients with chronic periodontitis. Hence, the aim of this study was to compare the levels of NT-proBNP in GCF and serum in patients with chronic generalized periodontitis.
Materials and methods: GCF and serum samples were collected in 19 patients with chronic periodontitis before and after NSPT after 6 weeks and the cumulative or reduction in values of NT-proBNP in GCF and serum was assessed. NT-proBNP levels in GCF and serum were determined by enzyme-linked immunosorbent assay.
Results: The concentrations of NT-proBNP were significantly reduced in GCF and serum after NSPT. Statistically significant difference of NT-proBNP concentration between pre- and postgroups in GCF was apparent (p < 0.0001), whereas statistically nonsignificant results in NT-proBNP serum levels when compared at baseline to postoperative state with mean of 61.77 (22.6 standard deviation [SD]) preoperatively and 72.67 (51.86 SD) postoperatively (p = 0.0007) was observed.
Conclusion: Significant reduction of NT-proBNP concentrations in GCF and serum in patients with chronic periodontitis subjected to NSPT was observed. This may account for a significant relation between periodontal disease, bacteremia, and CVD.
Keywords: Brain natriuretic peptide, Cardiovascular biomarker, Nonsurgical periodontal therapy, NT-proBNP, Periodontal disease
Received: July 8, 2022
Accepted: August 29, 2022
Published online: October 3, 2022
Journal of Circulating Biomarkers - ISSN 1849-4544 - www.aboutscience.eu/jcb
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Background
Periodontitis is a multifactorial inflammatory disease caused by interactions between periodontal microflora and host immune response (1). Perpetuating periodontal destruction is an important sequela of periodontitis that can lead to the subsequent loss of teeth. The expression of the disease results from the interaction of host defense mechanisms, microbial agents, and environmental and genetic factors.
Traditional clinical diagnosis of periodontal disease cannot reliably identify the sites of ongoing periodontal destruction and does not provide information about the patient’s susceptibility to disease, whether the disease is progressing or not, or the disease in remission, or the response to treatment will be positive or negative. Although specific microorganisms are implicated in periodontitis, many other aspects of tissue changes are known to negatively alter periodontal status. Based on this concept, serum, gingival tissue fluid, saliva, and tissue biopsy samples have now been investigated for periodontal adversities and their association with markers associated with systemic complications.
Gingival crevicular fluid (GCF) contains a variety of biochemical factors that may be used as biomarkers for the diagnosis or prognosis of periodontal tissue in health and disease. GCF, both stimulated and unstimulated, can be obtained noninvasively from various oral sites and has been shown to be a reliable predictor of disease activity. Due to the increased activity of the disease, many proteins and enzymes in GCF increase. Means obtained for each standard marker activity are observed at GCF, which is 20-fold higher than serum.
The prime objective of scaling and root planing (SRP) is to restore gingival health by completely removing the tooth surface elements that provoke the gingival inflammation, to provide a biologically acceptable root surface, and to facilitate oral hygiene.
Traditional methods for diagnosing periodontal diseases include clinical and radiographic examinations. These traditional measures provide information only about the existing disease and are incapable of predicting disease progression. As a result, advances in oral and periodontal disease diagnostic research are shifting toward methods for identifying and quantifying periodontal risk using objective measures such as biomarkers. One of these biomarkers that can be detected in several inflammatory conditions is N-terminal-pro-brain natriuretic peptide (NT-proBNP).
BNP is a 32 amino acid peptide that is released primarily from the ventricular myocardium in response to increased myocardial wall tension. BNP is produced as a prohormone that is cleaved into two peptides: the active hormone BNP; and a biologically inactive NT-proBNP. Because of its longer half-life compared to active BNP, it has been suggested that serum NT-proBNP may be a viable biomarker for cardiovascular disease (CVD) (1). This peptide is also associated with an increased risk of CV events, cerebral ischemia, and all causes that increase the mortality rate.
In a previous study, patients with periodontitis showed significantly higher NT-proBNP serum levels than subjects without periodontitis. Serum NT-proBNP levels increased as periodontal disease destruction progressed (2). Recent studies have shown an association between periodontitis and elevated serum NT-proBNP levels (1).
To the best of our knowledge, the relationship between GCF and serum levels of NT-proBNP in chronic periodontitis and the effect of SRP on their levels have not been explored. Hence, this study aims to compare the levels of NT-proBNP in serum and GCF in patients with chronic generalized periodontitis before and after SRP.
Materials and methods
The study was conducted at the Department of Periodontology, Faculty of Dental Sciences, M. S. Ramaiah University of Applied Sciences in collaboration with the Department of Microbiology, Ramaiah Medical College and Hospital, Bangalore. Study period was from January 2020 to November 2021.
This study was a nonrandomized comparative interventional study in which serum and GCF samples were collected from 19 patients with chronic periodontitis. The reason for using serum rather than saliva as an indicator for analysis is that serum is still the best body fluid for evaluating many biomarkers reflecting systemic processes, and substitution should be used with caution. Furthermore, while salivary cortisol levels may be reflective of systemic levels, other immune biomarkers in saliva, particularly cardiac, such as interleukin (IL)-6, soluble IL-6 receptor, and C-reactive protein (CRP) cytokines, have failed to show significant correlations to paired plasma samples. Around 45 days of SRP and cumulative or decreased NT-proBNP levels for both parameters were collected. GCF and serum were evaluated. Because the sensitivity and specificity of cardiac markers are influenced by several factors such as time of presentation, treatment, diagnostic thresholds, kinetic and half-life, the recall period was 45 days. Because NT-proBNP has a shorter half-life and full recovery and reattachment of periodontal apparatus can take up to 6 weeks, 45 days was chosen as the optimal recall interval. Subjects referred to the Department of Periodontology who met the selection criteria were evaluated and included in the study. A total of 19 patients who met the selection criteria were considered sample size for this study. GCF and serum samples were taken before and after SRP. All subjects received nonsurgical periodontal therapy (NSPT), which includes SRP and subgingival debridement.
Inclusion criteria were: Patients within the age 25-50 years, systemically healthy patients, subjects with ≥18 completely erupted teeth, subjects with presence of bleeding on probing, probing pocket depth ≥5 mm, and clinical attachment level ≥6 mm. Exclusion criteria were: atherosclerotic vascular disease (i.e., CVD, stroke, and peripheral artery disease), immunological disorders, arthritis/osteoporosis, history of periodontal intervention within the last 6 months, anti-inflammatory and nonsteroidal anti-inflammatory therapy within 3 months prior to periodontal assessment, and pregnancy or lactation.
The research was performed in accordance with the Declaration of Helsinki of the World Medical Association (2008) and was approved by the Ethics Committee for Human Trials of M. S. Ramaiah University of Applied Sciences with reference no: EC-2020/PG/13. Informed consent was obtained from each patient or their relatives after full explanation of the periodontal examination, GCF, and blood sample withdrawal.
The sample size has been estimated using GPower software v. 3.1.9.4.
Considering the effect size to be measured (dz) at 50%, power of the study at 80%, and the margin of the error at 10%, the total sample size needed was 19.
Collection of serum and GCF NT-proBNP before SRP
NT-proBNP in serum and GCF levels was assessed in patients with periodontitis before SRP. Complete periodontal examination was performed in all subjects (Fig. 1).
The GCF samples from deepest probing depth were collected for NT-proBNP assessment (Fig. 2). The GCF samples from all the patients were collected after 24 hours following baseline examination to avoid contamination of the sample with blood. GCF samples were obtained from the sites with deepest probing depth. Supragingival plaque of the intended tooth was removed with piezoelectric ultrasonic scaler before sampling. The tooth was dried prior to obtaining the sample. The GCF collection was done using micropipettes with proper isolation of the site with cotton rolls.

Fig. 1 - Preoperative scaling and root planing.
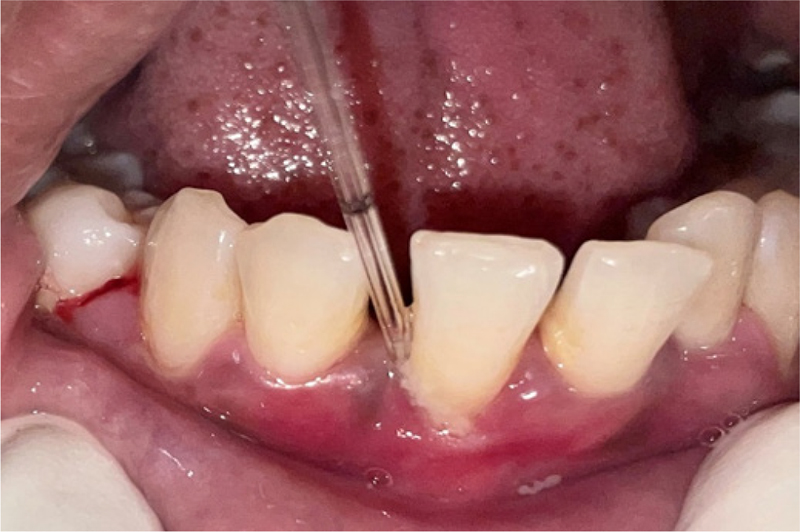
Fig. 2 - Collection of gingival crevicular fluid samples.
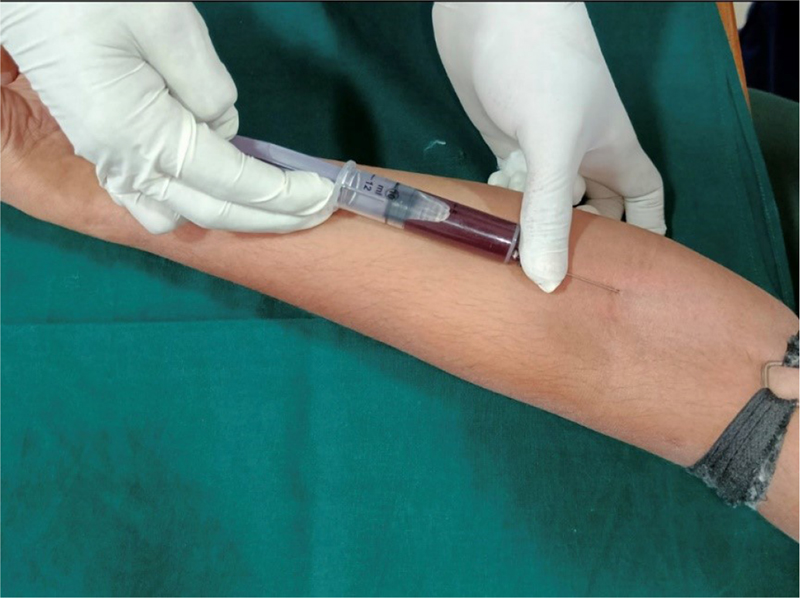
Fig. 3 - Collection of blood sample from antecubital vein.
Blood samples were obtained in the morning (Fig. 3). This is important because the human body is subjected to variations depending on the time of day; due to this variability of parameters during the day the values are observed to alter, which is reflected in the laboratory results (3), and both the Canadian and US hematology guidelines endorse this view. Although still in debate, fasting before drawing blood is not recommended because the body starts to use its own protein, especially with a small supply of fat. This can lead to glucose levels being too low and even to increased amounts of ketone compounds or a reduction in iron and hemoglobin levels. Subjects should be seated and relaxed and asked not to be anxious as it may cause the body to stimulate and release adrenaline. Therefore, a blood test preceded by physical effort or anxiousness will manifest itself in the form of altered blood serum levels (4). Briefly, 2 mL of venous blood was collected from the antecubital fossa by venipuncture using a 20-gauge needle with a 2 mL syringe. Blood samples were allowed to clot at room temperature and, after 1 hour, serum was separated from blood by centrifugation and 0.5 mL of extracted serum was immediately transferred to 1.5-mL aliquots. Each aliquot was stored at −80°C until required for analysis. Ultrasonic SRP procedure was performed.
Collection of serum and GCF NT-proBNP after SRP
NT-proBNP in serum and GCF levels was assessed in patients with chronic generalized periodontitis after SRP after 45 days.
Complete periodontal examination was performed in all subjects and all the clinical parameters were assessed. The procedure for assessing the serum and GCF levels was repeated, blood samples were obtained in the morning. Briefly, 2 mL of venous blood was collected from the antecubital fossa by venipuncture using a 20-gauge needle with a 2 mL syringe. Blood samples were allowed to clot at room temperature and, after 1 hour, serum is separated from blood by centrifugation (Fig. 4) and 0.5 mL of extracted serum was immediately transferred to 1.5-mL aliquots. Each aliquot was stored at −80°C until required for analysis.

Fig. 4 - Serum immediately after centrifugation.
The GCF samples from the deepest probing depth were collected for NT-proBNP assessment after reevaluating for any local factors postoperatively (Fig. 5). The GCF samples from all the patients were collected after 24 hours following baseline examination to avoid contamination of the sample with blood. GCF samples were obtained from the sites with deepest probing depth (Fig. 6). The tooth was dried prior to obtaining the sample. The GCF collection was done using micropipettes with proper isolation of the site with cotton rolls.

Fig. 5 - Postoperative scaling and root planing.
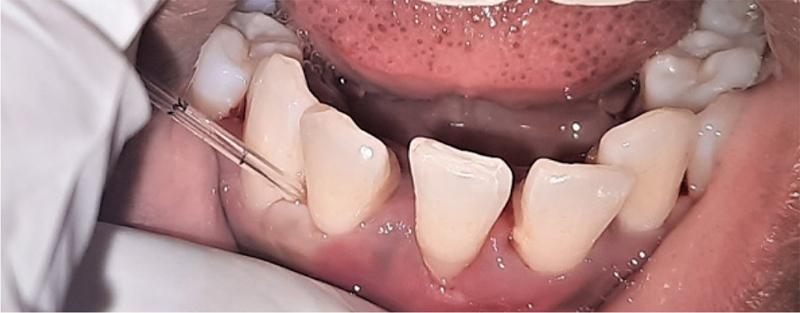
Fig. 6 - Postoperative gingival crevicular fluid collection.
Comparing the levels of NT-proBNP in serum and GCF
The levels of NT-proBNP in serum and GCF in patients before and after SRP were compared, and the results will determine whether NSPT has an effect on serum and GCF NT-proBNP levels.
Standard preparation
Reconstitution of original BNP standard with 1 mL of sample diluent was done. Standards were kept for 15 minutes with gentle agitation before making further dilutions. Additional standards were prepared by serially diluting the standard stock solutions as given in Table I.
| Standard Conc. (pg/mL) | Standard Vial | Dilutions |
|---|---|---|
| 2000 | Standard no. 8 | Reconstitute with 1 mL sample diluent |
| 1000 | Standard no. 7 | 300 µL Standard no. 8 + 300 µL sample diluent |
| 500 | Standard no. 6 | 300 µL Standard no. 7 + 300 µL sample diluent |
| 250 | Standard no. 5 | 300 µL Standard no. 6 + 300 µL sample diluent |
| 125 | Standard no. 4 | 300 µL Standard no. 5 + 300 µL sample diluent |
| 62.5 | Standard no. 3 | 300 µL Standard no. 4 + 300 µL sample diluent |
| 31.25 | Standard no. 2 | 300 µL Standard no. 3 + 300 µL sample diluent |
| 0 | Standard no. 1 | 300 µL sample diluent |
BNP = brain natriuretic peptide; GCF = gingival crevicular fluid.
Table I indicates the standard values or concentrates obtained from the serum and GCF samples of BNP, which procures a standard curve to the measure the values in the samples.
NT-proBNP enzyme-linked immunosorbent assay (ELISA) quantitative assay procedure:
- Bring all the reagents to room temperature before use (Fig. 7)
- Pipette standards 1-8 samples – about 100 µL (Fig. 8)
- Incubate for 90 minutes
- Wash 1× wash buffer; Decant, 4 × 300 µL
- Pipette biotinylated anti-BNP 100 µL
- Incubate 60 minutes (37°C)
- Wash 1× wash buffer; Decant, 4 × 300 µL
- Pipette streptavidin: Horseradish peroxidase (HRP) conjugate 100 µL
- Incubate 30 minutes
- Wash 1× wash buffer; Decant, 4 × 300 µL
- Pipette tetramethylbenzidine (TMB) substrate 90 µL
- Incubate in dark for 10 minutes.
- NT-proBNP level detection was performed by ELISA in an ELISA plate analyzer.
- Pipette stop solution 50 µL
- Measure 450 within 15 minutes
Anti-BNP antibody was precoated in 96-well plates and biotin-conjugated anti-BNP antibody was used as detection antibody. Then, the calibrator, test sample, and biotin-conjugated detection antibody were added to the well and washed with wash buffer. HRP was added and unbound conjugates were washed with wash buffer. TMB substrate will be used to visualize the HRP enzymatic reaction. The TMB will be catalyzed by HRP to give a blue-colored product, which will turn yellow after the addition of the acid solution stops (Fig. 9). The density of the yolk will be proportional to the amount of NT-proBNP sample collected in the plate. The optical density absorbance (O.D.) reading should be recorded at 450 nm in a microplate reader and then the NT-proBNP concentration should be calculated (Fig. 10). Demographic, clinical, and historical information for certain diseases will also be recorded.
Fig. 7 - N-terminal-pro-brain natriuretic peptide enzyme-linked immunosorbent assay kit.
Fig. 8 - (Left) 8 standards in vials. (Right) Adding serum and gingival crevicular fluid samples in the microtiter plate.
Fig. 9 - Pipetting stop solution – blue to yellow.
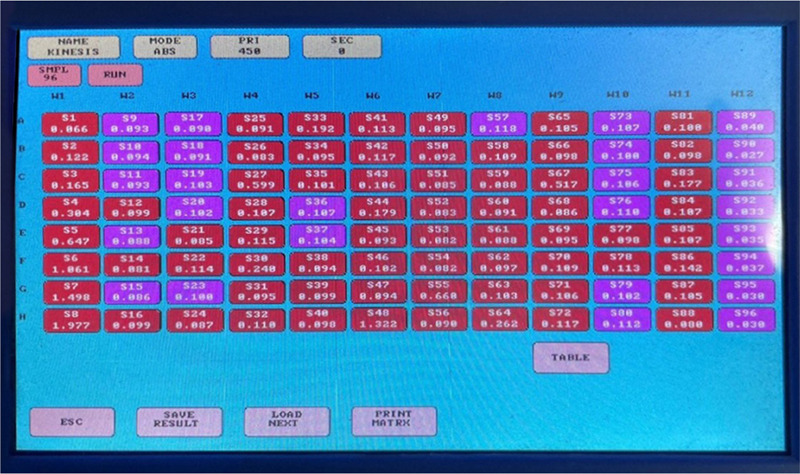
Fig. 10 - Screenshot of estimated values obtained.
Standard curve was generated using the obtained standard concentration to measure the concentrations of NT-proBNP in GCF and serum (Fig. 11).
Statistical analysis
Statistical Package for the Social Sciences (SPSS) for Windows (Version 22.0, Released 2013; Armonk, NY: IBM Corp.) was used for providing statistical analysis.
Descriptive statistics
Descriptive analysis of all the explanatory and outcome parameters was done using mean and standard deviation for quantitative variables, frequency and proportions for categorical variables.
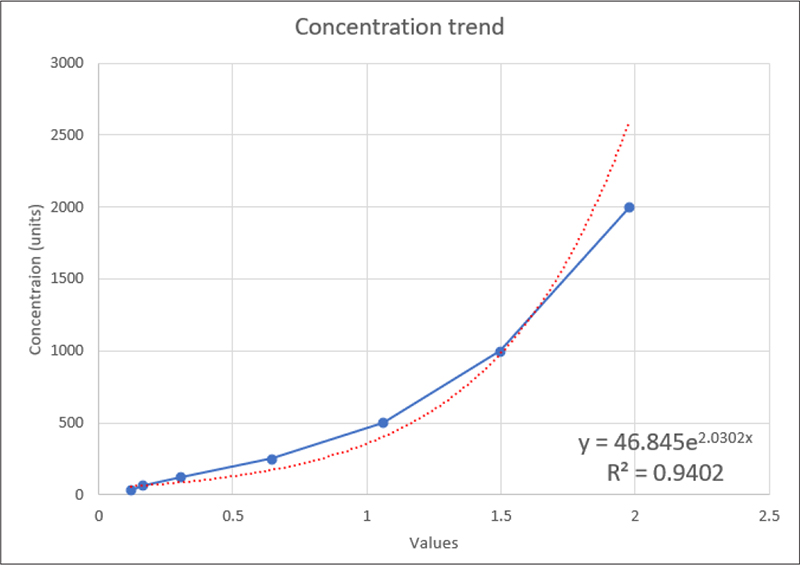
Fig. 11 - Standard curve for concentration trend.
Inferential statistics
Student’s paired t-test was used to compare the mean values of clinical parameters and NT-proBNP levels (GCF and serum) before and after SRP periods. Pearson correlation test was used to assess the relationship between clinical parameters and NT-proBNP levels (GCF and serum) before and after SRP periods. Stepwise multiple linear regression analysis was performed to predict the variation in the NT-proBNP levels (GCF and serum) in context to clinical parameters before and after SRP periods. The level of significance (p-value) was set at p < 0.05 and any other relevant test, if found appropriate during the time of data analysis, were dealt accordingly.
Processing the ELISA kits and estimating the contorted BNP values in GCF and serum were done in the Department of Microbiology, Ramaiah Medical College, Bangalore.
Results
In total, 19 subjects were recruited and treated, and their GCF and serum samples were collected prior to and post-SRP. The sample population comprised of 11 females and 9 males (Fig. 12) and were in the age range of 18 to 50 years (Fig. 13). There was no significant difference between the two groups regarding age and gender.
The purpose of this intervention study was to estimate GCF and serum NT-proBNP levels 45 days before and after the intervention. In this study, nonsurgical periodontal SRP was selected as the treatment modality and NT-proBNP levels were evaluated 45 days after NSPT.

Fig. 12 - Graphical presentation of subjects’ gender distribution taken from collection of gingival crevicular fluid and serum samples.
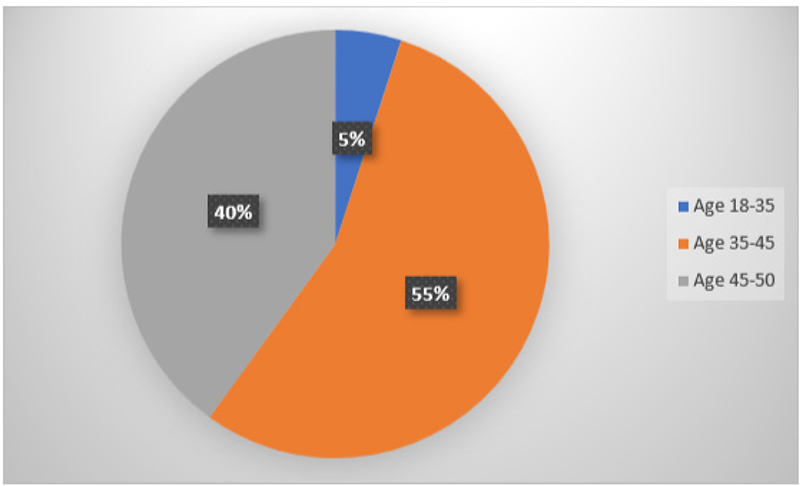
Fig. 13 - Graphical representation of age distribution of subjects for collection of gingival crevicular fluid and serum.
Table II signifies the pre- and postoperative serum values. Although few subjects showed reduction in concentrations of serum NT-proBNP, however, upon estimation it was found that, in 36.8% of subjects’ (n = 7) serum NT-proBNP exhibited higher values even after NSPT. Nevertheless, upon clinical evaluation of periodontal parameters, periodontal status of the subjects was improved. Hence, serum concentration values were not statistically significant (p > 0.0005).
Table III signifies the pre- and postoperative NT-proBNP GCF values. The table indicates that there is a significant reduction in GCF post-SRP. Subjects showed drastic reduction in GCF-BNP values, whereas 36.8% subjects (n = 7) showed slight increased concentrations in GCF levels. Clinical evaluation of periodontal parameters showed that periodontal status of the subjects was improved. Hence, GCF concentration values were statistically significant (p < 0.0001).
There is a significant increase in NT-proBNP serum levels when compared at baseline to postoperative state with the mean of 61.77 (22.6 standard deviation [SD]) preoperatively and 72.67 (51.86 SD) postoperatively (p = 0.0007) (Tab. IV).
Table V shows that there is a significant decrease in GCF levels of NT-proBNP when compared at baseline to postoperative state; this could indicate GCF as a promising indicator for assessing NT-proBNP levels in patients with periodontitis.
| BNP Serum Samples | ||
|---|---|---|
| Sample ID | Preoperative | Postoperative |
| D129 | 56.58 | 56.81 |
| D584 | 56.69 | 56.47 |
| D570 | 56.58 | 55.67 |
| D569 | 57.27 | 55.44 |
| D557 | 56.01 | 55.33 |
| D124 | 55.22 | 55.33 |
| D048 | 55.78 | 52.91 |
| D045 | 57.27 | 56.24 |
| D541 | 56.24 | 59.53 |
| D397 | 56.35 | 58.45 |
| D099 | 57.74 | 279.69 |
| D569 | 57.62 | 56.35 |
| D875 | 59.04 | 56.01 |
| D868 | 57.39 | 57.04 |
| D587 | 55.89 | 57.74 |
| D035 | 56.35 | 79.74 |
| D577 | 55.44 | 57.98 |
| D453 | 158.05 | 133.81 |
| D273 | 58.21 | 55.78 |
BNP = brain natriuretic peptide.
| BNP GCF Samples | ||
|---|---|---|
| Sample ID | Preoperative | Postoperative |
| D129 | 59.16 | 56.81 |
| D584 | 76.26 | 58.45 |
| D570 | 56.81 | 58.09 |
| D569 | 58.57 | 59.40 |
| D557 | 69.18 | 58.21 |
| D124 | 56.81 | 57.21 |
| D048 | 57.51 | 57.16 |
| D045 | 58.21 | 50.5 |
| D541 | 57.86 | 58.21 |
| D397 | 56.69 | 58.21 |
| D099 | 57.27 | 62.50 |
| D569 | 57.16 | 57.98 |
| D875 | 58.92 | 55.11 |
| D868 | 59.40 | 50.81 |
| D587 | 58.09 | 49.48 |
| D035 | 67.37 | 50.40 |
| D577 | 56.58 | 50.09 |
| D453 | 57.62 | 50.29 |
| D273 | 685.93 | 49.79 |
BNP = brain natriuretic peptide; GCF = gingival crevicular fluid.
| Serum | Mean (± SD) | p-Value |
|---|---|---|
| Preoperative | 61.77 (±22.6) | 0.0007 |
| Postoperative | 72.67 (±51.86) |
NT-proBNP = N-terminal-pro-brain natriuretic peptide; SD = standard deviation.
| Serum | Mean (± SD) | p-Value |
|---|---|---|
| Preoperative | 90.11 (±140.11) | <0.0001 |
| Postoperative | 54.93 (±4.23) |
GCF = gingival crevicular fluid; NT-proBNP = N-terminal-pro-brain natriuretic peptide; SD = standard deviation.
Analysis was done by Fischer’s analysis test because the significance of the deviation from p-value can be calculated exactly, rather than relying on an approximation that becomes exact in the limit as the sample size grows to infinity, as with many statistical tests. Significant difference of BNP concentration between pre- and postgroups in GCF was apparent and the mean concentration in the pre-SRP group was high when compared to post-SRP group, which had evidently reduced postintervention (p < 0.0001).
Statistically nonsignificant difference of BNP concentration between pre- and postgroups in serum was observed and similarly the mean concentration of BNP in serum was seen to be increased postintervention as compared to preintervention (p = 0.0007).
Discussion
Chronic periodontitis is a common condition, affecting 45.9% of the US adult population aged 30 years and older (5). Chronic periodontitis causes loss of connective tissue that supports teeth and alveolar bone, which, if left untreated, is a major cause of tooth loss in adults (6). According to the case definitions of the Centers for Disease Control and Prevention and the American Academy of Periodontology, the estimated prevalence of early/moderate and severe periodontitis is 37.1% and 8.9% in adults, respectively, in the United States (5).
In addition, since diabetes has a great impact on periodontal disease and treatment, we excluded patients with such systemic diseases (7). NT-proBNP also has an indirect effect on pancreatic insulin-producing β-cells through the accumulation of intracellular iron. Patients with CVD, neurodegenerative disease, chronic infections (e.g., tuberculosis and degenerative disease), cancer, and liver disease were also excluded as these diseases impair the onset, progression, and outcome of periodontal disease and may affect the NT-proBNP concentration levels (8).
This study shows that patients with periodontitis have higher serum and GCF NT-proBNP levels. Moreover, as periodontal disease progresses, serum and GCF NT-proBNP levels increase, which reduce adequately after SRP. BNP is a vital cardiovascular biomarker that is actively detected in periodontal diseases; SRP aids in the improvement in clinical parameters, which may be due to resolution of the inflammatory response and the cessation of periodontal destruction, which makes a direct impact on this biomarker and reduces its potential pathogenicity and leads to their lowered concentrations in GCF and serum (9). This could be explained by invasion of periodontal pathogen into vascular endothelial cells in order to evade the immune cells. Endothelial invasion by Porphyromonas gingivalis is facilitated by hemagglutinin A, hemagglutinin B, and fimbriae A. Fimbriae A increases expression of different adhesion molecules on the endothelial cells of blood vessels and expression of inflammatory mediators such as IL-6, IL-8, and cyclooxygenase-2. In fact, lipopolysaccharide (LPS) and fimbriae A could coordinate in the proinflammatory stimulation of arterial endothelium by P. gingivalis. Aggregatibacter actinomycetemcomitans also upregulates the formation of adhesion molecules, however, in lesser amount (10). This suggests that CVD and periodontal disease have a bidirectional relationship (11) and periodontal intervention has vital influence on periodontal and CVD parameters, which may perhaps decrease the risk of CVD.
In this study, during the estimation of NT-proBNP concentrations in GCF we found that there is substantial decrease in the levels post-SRP when compared at baseline. Significant difference of NT-proBNP concentration between pre- and poststate in GCF was apparent and the mean concentration in the pre-SRP group was high when compared to post-SRP group, which had evidently reduced postintervention (p < 0.0001). However, when concentration of NT-proBNP in serum was estimated, few subjects did not show significant reduction. Upon estimation it was found that the serum levels in NT-proBNP with subjects having periodontitis revealed increased values. However, upon clinical evaluation of periodontal parameters, periodontal status of the subjects was improved. This would be because of elimination of local factors and patient education along with maintenance. Hence, serum concentration values were not statistically significant (p = 0.0007).
In accordance with the present study, Mahendra Mohan et al assessed CRP levels after SRP evaluated at baseline, 1 month and 45 days in patients with diabetes mellitus and chronic periodontitis (DM-CP) and nondiabetic chronic periodontitis (NDM-CP) patients and showed similar results, suggesting that the CRP levels in both GCF and serum were higher in DM-CP patients than in NDM-CP patients (12). Although there was a significant improvement in both the groups, greater improvement was observed in both GCF and serum samples of DM-CP patients. Our results are consistent with the results of this study, showing a significant reduction in GCF and serum NT-proBNP levels after SRP compared to baseline and 45 days. Both showed a decrease, but a greater decrease in BNP concentration was observed with GCF compared to serum.
However, Paschalina Goutoudi et al concluded that periodontal therapy reduced IL-8 levels in GCF (13). This finding is consistent with our study where NT-proBNP levels decreased in GCF after SRP. However, there is a result contrary to our study that the concentration of NT-proBNP before and after periodontal intervention is not statistically significant. A close association was observed between periodontal destruction and NT-proBNP levels. Patients with periodontitis initially presented with higher periodontal inflammation with high NT-proBNP levels while when SRP was performed, these values decreased after 45 days; NT-proBNP levels decreased significantly in GCF and serum, because there is an inflammatory response and it stops the destruction of periodontal disease affecting NT-proBNP, thereby reducing their levels.
Samah H. Elmeadawy et al have shown that NSPT significantly reduces serum VCAM1 levels. The VCAM1 protein mediates the adhesion of lymphocytes, monocytes, eosinophils, and basophils to the vascular endothelium and may be involved in the development of atherosclerosis and rheumatoid arthritis (14). Another study by Cui D et al has shown reduced VCAM1 expression and macrophage/monocyte infiltration in the vascular wall of mice treated with NSPT (15). Both studies were consistent with our study that GCF NT-proBNP levels decreased 45 days after receiving SRP.
Deng et al however reported NSPT had no significant effect on CRP, total cholesterol, low-density lipoprotein cholesterol, and triglycerides (16). Also, Zeng et al concluded NSPT was associated with carotid atherosclerosis but statistical heterogeneity was substantial, hence the results were obsolete and trajected toward showing NSPT having no effects on CVD biomarkers (17). These studies showed contradictory results when compared to our study. Our study showed substantial homogeneity in results where NSPT has an effect on the NT-proBNP levels in GCF and serum in patients with periodontitis.
Gupta et al (2015) correlated the levels of sCD40 L and MCP-1 in serum and GCF of patients with chronic periodontitis before and after SRP. sCD40 L and MCP-1 are the acute precipitators of CVD in subjects with periodontitis, and Gupta et al concluded that a positive correlation observed between sCD40 L and MCP-1 levels and detected reduced levels of GCF and serum after SRP, which suggests this phenomenon as one of the pathways that may lead to the propagation of cardiovascular events in patients with periodontal disease (18). This is in accordance with our study as NT-proBNP levels are reduced post-SRP, which shows a positive correlation between CVD and periodontitis and may prevent the risk of CVD.
For this study, SRP was found to significantly reduce NT-proBNP levels in GCF in patients with chronic periodontitis compared to baseline values. This decrease in NT-proBNP levels at the end of the study can be explained by the effect of SRP on local factor removal. Therefore, it can reduce the inflammatory response and, as a result, the mediators that stimulate the production in the acute phase protein.
We were unable to compare the results of the present study on the effect of SRP on serum or GCF levels of NT-proBNP in patients with chronic periodontitis with other studies because to our knowledge this is the first study to evaluate NT-proBNP levels in GCF and serum in patients with periodontitis before and after SRP.
The significant increase in the concentration levels of NT-proBNP in serum may perhaps be due to the following reasons:
- Time Factor: NT-proBNP is a cardiac biomarker that is detected in patients with periodontitis. The reduction of the levels in serum requires supplemental time in the blood stream to get stabilized. Also, evidences regarding CVD biomarkers suggest the recall period as 3-6 months for reevaluation.
- Periodontal Invention: Evidence by Alka S. Waghmare et al concluded that bacteremia frequently occurs immediately after SRP (19). Similarly, in our study SRP may have led to bacteremia and therefore had aggravated the levels of serum NT-proBNP for a brief time.
This study had a number of shortcomings. The study recruited a small sample size. Further investigations with larger numbers of subjects and of a longer duration are needed to better understand the role of periodontal therapy on the improvement of the cardiac status. Blinding was done only at the statistician level. Thus, multilevel blinding can be incorporated in future studies.
Conclusion
Till date and to the best of our knowledge this is the first study that aims to compare the levels of NT-proBNP in serum and GCF in patients with chronic generalized periodontitis before and after SRP. Even with the limitations of this study, we can conclude that NSPT has a reducing effect on the serum and GCF NT-proBNP levels in chronic periodontitis patients. In addition, serum and GCF BNP levels represent a potential biomarker of chronic periodontitis and may indicate NSPT may avoid the risk of CVD events by reducing systemic inflammation caused by local factors. Hence, it can be concluded that NSPT reduces NT-proBNP concentrations in patients with chronic periodontitis and may evade the future risk of CVD.
Compliance with ethics guidelines
The study protocol is approved by the University Ethics Committee for Human Trials of M. S. Ramaiah University of Applied Sciences (Ref no. EC-2020/PG/13). Written informed consent has been obtained from the participants.
Availability of data and materials: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Authors’ contribution
IF: Clinical investigator; funding; data collection; manuscript preparation
BS: Designing of the study; clinical investigator; data analysis
UY: Literature search; manuscript review
SFK: Study concept; manuscript preparation, editing and review
MN: Manuscript preparation and review
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Leira Y, Blanco J. Brain natriuretic peptide serum levels in periodontitis. J Periodontal Res. 2018;53(4):575-581. CrossRef PubMed
- 2. Jensen J, Ma LP, Fu MLX, Svaninger D, Lundberg PA, Hammarsten O. Inflammation increases NT-proBNP and the NT-proBNP/BNP ratio. Clin Res Cardiol. 2010;99(7):445-452. CrossRef PubMed
- 3. Dale JC, Steindel SJ, Walsh M. Early morning blood collections: a College of American Pathologists Q-Probes study of 657 institutions. Arch Pathol Lab Med. 1998;122(10):865-870. PubMed
- 4. Sorita A, Patterson A, Landazuri P, et al. The feasibility and impact of midnight routine blood draws on laboratory orders and processing time. Am J Clin Pathol. 2014;141(6):805-810. CrossRef PubMed
- 5. Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611-622. CrossRef PubMed
- 6. Dikbas I, Tanalp J, Tomruk CO, Koksal T. Evaluation of reasons for extraction of crowned teeth: a prospective study at a university clinic. Acta Odontol Scand. 2013;71(3-4):848-856. CrossRef PubMed
- 7. Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000. 2014;64(1):139-153. CrossRef PubMed
- 8. Iacopino AM, Cutler CW. Pathophysiological relationships between periodontitis and systemic disease: recent concepts involving serum lipids. J Periodontol. 2000;71(8):1375-1384. CrossRef PubMed
- 9. Sophia K II, Suresh S, Sudhakar U Sr, et al. Comparative evaluation of serum and gingival crevicular fluid periostin levels in periodontal health and disease: a biochemical study. Cureus. 2020;12(3):e7218. CrossRef PubMed
- 10. Khlgatian M, Nassar H, Chou HH, Gibson FC III, Genco CA. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect Immun. 2002;70(1):257-267. CrossRef PubMed
- 11. Dhadse P, Gattani D, Mishra R. The link between periodontal disease and cardiovascular disease: how far we have come in last two decades? J Indian Soc Periodontol. 2010;14(3):148-154. CrossRef PubMed
- 12. Mohan M, Jhingran R, Bains VK, et al. Impact of scaling and root planing on C-reactive protein levels in gingival crevicular fluid and serum in chronic periodontitis patients with or without diabetes mellitus. J Periodontal Implant Sci. 2014;44(4):158-168. CrossRef PubMed
- 13. Goutoudi P, Diza E, Arvanitidou M. Effect of periodontal therapy on crevicular fluid interleukin-6 and interleukin-8 levels in chronic periodontitis. Int J Dent. 2012;2012:362905. CrossRef PubMed
- 14. Elmeadawy SH, El-Sharkawy HM, Elbayomy A. Effect of nonsurgical periodontal therapy on systemic pro-inflammatory and vascular endothelial biomarkers and serum lipid profile in chronic periodontitis patients. Egyptian Dent J. 2017;63(3):2421-2433. CrossRef
- 15. Cui D, Li H, Lei L, Chen C, Yan F. Nonsurgical periodontal treatment reduced aortic inflammation in ApoE(-/-) mice with periodontitis. Springerplus. 2016;5(1):940. CrossRef PubMed
- 16. Linkai D, Chunjie L, Qian L, Yukui Z, Hongwei Z. Periodontal treatment for cardiovascular risk factors: a systematic review. West China J Stomatol. 31(5):463-467. PubMed
- 17. Zeng XT, Leng WD, Lam YY, et al. Periodontal disease and carotid atherosclerosis: a meta-analysis of 17,330 participants. Int J Cardiol. 2016;203:1044-1051. CrossRef PubMed
- 18. Gupta M, Chaturvedi R, Jain A. Role of cardiovascular disease markers in periodontal infection: understanding the risk. Indian J Dent Res. 2015;26(3):231-236. CrossRef PubMed
- 19. Waghmare AS, Vhanmane PB, Savitha B, Chawla RL, Bagde HS. Bacteremia following scaling and root planing: a clinico-microbiological study. J Indian Soc Periodontol. 2013;17(6):725-730. CrossRef PubMed