 |
J Circ Biomark 2023; 12: 12-16 ISSN 1849-4544 | DOI: 10.33393/jcb.2023.2453 ORIGINAL RESEARCH ARTICLE |
 |
PIVKA-II or AFP has better diagnostic properties for hepatocellular carcinoma diagnosis in high-risk patients
ABSTRACT
Background: Hepatocellular carcinoma (HCC) is a lethal cancer. Two biomarkers were used for HCC diagnosis including alpha-fetoprotein (AFP) and protein induced by vitamin K absence-II or antagonist (PIVKA-II). However, data on biomarkers and HCC diagnosis are not consistent. This study aimed to evaluate if PIVKA-II, AFP, or a combination of both biomarkers had the best diagnostic properties for HCC.
Methods: This was a prospective study and enrolled patients 18 years or over with a high risk for HCC. AFP and PIVKA-II levels were calculated for HCC diagnosis. Diagnostic properties of both biomarkers were reported with sensitivity, specificity, and a receiver operating characteristic (ROC) curve.
Results: There were 260 patients with high risk for HCC in this cohort. Of those, 219 patients were diagnosed with HCC: confirmed by biopsy in 7 patients (2.69%) and by imaging in the others. Median values of AFP and PIVKA-II were 56 ng/mL and 348 mAU/mL, respectively. PIVKA-II level of 40 mAU/mL had sensitivity of 80.80%, while AFP of 10 ng/mL had sensitivity of 75.80%. A combination of PIVKA-II at 100 mAU/mL or over and AFP of 11 ng/mL gave sensitivity of 60.30%. The ROC curve of PIVKA-II plus AFP was significantly higher than the AFP alone (0.855 vs. 0.796; p = 0.027), but not significantly different from the PIVKA-II alone (0.855 vs. 0.832; p = 0.130).
Conclusion: PIVKA-II may have more diagnostic yield for HCC compared with AFP. It can be used alone without a combination with AFP.
Keywords: Hepatitis B virus, Hepatitis C virus, Sensitivity, Specificity
Received: July 8, 2022
Accepted: February 1, 2023
Published online: February 17, 2023
Journal of Circulating Biomarkers - ISSN 1849-4544 - www.aboutscience.eu/jcb
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Hepatocellular carcinoma (HCC) is a common cancer. It is the sixth most common cancer and the second highest in cancer-related death globally. In 2015, there were 854,000 new cases and 810,000 deaths in the world (1). There were three common causes of HCC: hepatitis B virus (HBV), alcohol, and hepatitis C virus (HCV). These causes related to HCC deaths in 33%, 30%, and 21%, respectively (1). High-risk patients for HCC were patients with cirrhosis, chronic HBV infection, or lesions identified on a surveillance ultrasound in patients with cirrhosis, chronic HBV infection, or prior HCC (2).
The main two markers for HCC are alpha-fetoprotein (AFP) and new biomarker: protein induced by vitamin K absence-II or antagonist (PIVKA-II). These markers play an important role in diagnosis, differential diagnosis, and prognosis. A nomogram of both AFP and PIVKA-II can be used to differentiate HCC and intrahepatic cholangiocarcinoma with an area under the receiver operating characteristic (ROC) curve of 95.1% (3). Both biomarkers can be used as a prognostic factor after several treatments such as radiofrequency, surgical resection, embolization, or radiotherapy (4-12). PIVKA-II level lower than 25 mAU/mL or equal after radiotherapy had long progression-free survival (p = 0.004) (4). Additionally, both biomarkers can indicate tumor size, tumor differentiation, vascular invasion, and those treated with hepatitis B/C antiviral agents (13-16).
As HCC is a lethal cancer with 5-year survival rate of under 15%, abdominal ultrasonography is used for HCC surveillance (17,18). However, its sensitivity was low at 63% (19). Biomarkers were studied and used as an alternative diagnostic method particularly for HCC including AFP and PIVKA-II (20-22). The advantage of AFP is widely available, while PIVKA-II is highly specific to HCC. Data on biomarkers and HCC diagnosis are not consistent. A report from the USA found that AFP was more sensitive than PIVKA-II (70% vs. 66%) in patients with HCC compared with patients with cirrhosis (23). On the other hand, a study from Nigeria found that PIVKA-II was more sensitive than AFP (96.8% vs. 62.9%) in patients with HCC vs. control with benign liver disease (24). A study from Korea recommends to use PIVKA-II combined with AFP to diagnose HCC in patients with HBV infection (25). These results showed that data on biomarkers and HCC diagnosis are not consistent and varied among countries, in which more data on this issue are required. This study aimed to evaluate if PIVKA-II, AFP, or a combination of both biomarkers had the best diagnostic properties for HCC.
Materials and methods
This was a prospective study conducted at University Hospital, Khon Kaen University, Thailand. Data were collected from the HCC project. The inclusion criteria were patients 18 years or over with a high risk for HCC. The high risk for HCC was defined by the American Association for the Study of Liver Diseases (AASLD) guidelines for HCC management (17): cirrhosis or presence of liver nodule(s) of 1 cm or over in size. Those with pregnancy, obstructive jaundice, vitamin K, or warfarin administration and presence of extrahepatic malignancy were excluded. The study protocol was approved by the ethics committee in human research, Khon Kaen University, Thailand (HE621134). This study was a part of HCC project of Khon Kaen University, Thailand.
Eligible patients provided a written informed consent prior to study participation. Data were collected as follows: baseline characteristics, laboratory results, and radiographic findings. Baseline characteristics included age, sex, etiology of cirrhosis, comorbid diseases, and the Child-Pugh score for cirrhosis. Laboratory tests in the study were platelet count, serum creatinine, prothrombin time, liver function test, AFP, and PIVKA-II. All samples were tested for AFP and PIVKA-II by using a test kit (µTASWako i30; FUJIFILM Wako Pure Chemical Corporation). Radiographic findings were numbers of liver mass, largest mass size (cm), and portal vein invasion. HCC was diagnosed by either confirmation by pathological findings or radiographic findings of arterial hypervascularity followed by venous and/or delayed phase or washout of contrast (17).
Statistical analyses
Descriptive statistics were used to calculate mean (1st-3rd interquartile ranges) or number (percentage) of the study population. AFP and PIVKA-II levels were calculated for HCC diagnosis by logistic regression analysis. Results were shown as various cutoff points with their diagnostic properties including sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, a ROC curve, and an area under the ROC curve with 95% confidence interval (CI). All statistical analyses were performed using STATA software version 10.1.
Results
There were 260 patients with a high risk for HCC in this cohort. Of those, 219 patients were diagnosed with HCC: confirmed by biopsy in 7 patients (2.69%) and by imaging in the others. The other 41 patients had a diagnosis of dysplastic nodule (25 patients; 60.98%), regenerative nodule (8 patients; 19.51%), hemangioma (5 patients; 12.20%), liver cyst (1 patient; 2.44%), fibronodular hyperplasia (1 patient; 2.44%), and hepatic adenoma (1 patient; 2.44%). Baseline characteristics and laboratory results are shown in Tables I and II. The median age was 58 years with male predominance (81.15%). The most common cause of HCC was HCV (43.85%) with a proportion of cirrhosis of 98.46%: mostly Child-Pugh score class A (81.15%). The median largest size of liver mass was 3.5 cm. The median values of AFP and PIVKA-II were 56 ng/mL and 348 mAU/mL, respectively.
The PIVKA-II level of 40 mAU/mL had sensitivity of 80.80% with 75.60% specificity, while AFP of 10 ng/mL had sensitivity of 75.80% with 65.90% specificity (Tabs. III and IV). A combination of PIVKA-II at 100 mAU/mL or over and AFP of 11 ng/mL gave sensitivity of 60.30% with 92.70% specificity. The ROC curve of PIVKA-II plus AFP was significantly higher than the AFP alone (0.855 vs. 0.796; p value = 0.027), but not significantly different from PIVKA-II alone (0.855 vs. 0.832; p value = 0.130), as shown in Figure 1.
| Factors | Median (1st-3rd quartile) or number (percentage) |
|---|---|
| Age, years | 58 (54-63) |
| Male sex, n (%) | 211 (81.15) |
| Etiology | |
| HBV | 98 (37.69) |
| HCV | 114 (43.85) |
| HBV plus HCV | 6 (2.31) |
| NAFLD | 14 (5.38) |
| ALD | 27 (10.38) |
| AIH | 1 (0.38) |
| Comorbid diseases | |
| None | 193 (74.23) |
| Diabetes | 38 (14.62) |
| Hypertension | 12 (4.62) |
| Cirrhosis | 256 (98.46) |
| Child-Pugh score A | 211 (81.15) |
| Child-Pugh score B | 36 (13.85) |
| Child-Pugh score C | 9 (3.46) |
AIH = autoimmune hepatitis; ALD = alcoholic liver disease; HBV = hepatitis B virus; HCV = hepatitis C virus; NAFLD = nonalcoholic fatty liver disease.
| Factors | Median (1st-3rd quartile) or number (percentage) |
|---|---|
| Platelet ´103/mm3 | 145 (102-220) |
| Creatinine (mg/dL) | 0.94 (0.80-1.15) |
| Prothrombin time (sec) | 12.3 (11.5-13.4) |
| Albumin (g/dL) | 3.9 (3.4-4.4) |
| Bilirubin (mg/dL) | 1.0 (0.6-1.6) |
| Alanine aminotransferase (U/L) | 51 (29-86) |
| Aspartate transaminase (U/L) | 74 (40-133) |
| Alkaline phosphatase (U/L) | 135 (88-196) |
| Alpha-fetoprotein (ng/mL) | 59 (6-1,598) |
| PIVKA-II (mA/mL) | 348 (31-10,442) |
| Radiographic findings | |
| Number of liver nodules, n (%) | |
| 1 | 156 (90.00) |
| 2 | 46 (17.69) |
| 3 | 12 (4.62) |
| ≥4 | 46 (17.69) |
| Largest size (cm) | 3.5 (1.8-7.5) |
| Portal vein invasion, n (%) | |
| No invasion | 181 (69.62) |
| Main portal vein invasion | 56 (21.54) |
| Non-main portal vein invasion | 23 (8.85) |
PIVKA-II = protein induced by vitamin K absence-II or antagonist.
| Cutoff | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|
| ≥40 | 80.80
(75.00-85.80) |
75.60
(59.70-87.60) |
3.31
(1.93-5.70) |
0.25 (0.18-0.35) |
| ≥60 | 78.50
(72.50-83.80) |
80.50
(65.10-91.20) |
4.03 (2.15-7.52) | 0.27 (0.20-0.36) |
| ≥80 | 75.30
(69.10-80.90) |
82.90
(67.90-92.80) |
4.41 (2.24-8.70) | 0.30 (0.23-0.39) |
| ≥100 | 72.10
(65.70-78.00) |
87.80 (73.80-95.90) | 5.92 (2.59-13.50) | 0.32 (0.25-0.40) |
| ≥120 | 69.40
(62.80-75.40) |
87.80
(73.80-95.90) |
5.69 (2.49-13.0) | 0.35 (0.28-0.44) |
HCC = hepatocellular carcinoma; LR+ = positive likelihood ratio; LR- = negative likelihood ratio; PIVKA-II = vitamin K absence-II or antagonist.
| Cutoff | Sensitivity | Specificity | LR+ | LR- |
|---|---|---|---|---|
| ≥10 | 75.80
(69.60-81.30) |
65.90
(49.40-79.90) |
2.22 (1.44-3.42) | 0.37 (0.27-0.51) |
| ≥12 | 73.10
(66.70-78.80) |
70.70
(54.50-83.90) |
2.50
(1.54-4.04) |
0.38 (0.28-0.51) |
| ≥14 | 69.40
(62.80-75.40) |
73.20
(57.10-85.80) |
2.59
(1.55-4.32) |
0.42 (0.32-0.55) |
| ≥20 | 65.30
(58.60-71.60) |
78.00
(62.40-89.40) |
2.97
(1.66-5.34) |
0.45 (0.35-0.57) |
| ≥200 | 44.70
(38.00-51.60) |
90.20
(76.90-97.30) |
4.59
(1.79-11.80) |
0.61 (0.52-0.72) |
AFP = alpha-fetoprotein; HCC = hepatocellular carcinoma; LR+ = positive likelihood ratio; LR- = negative likelihood ratio.
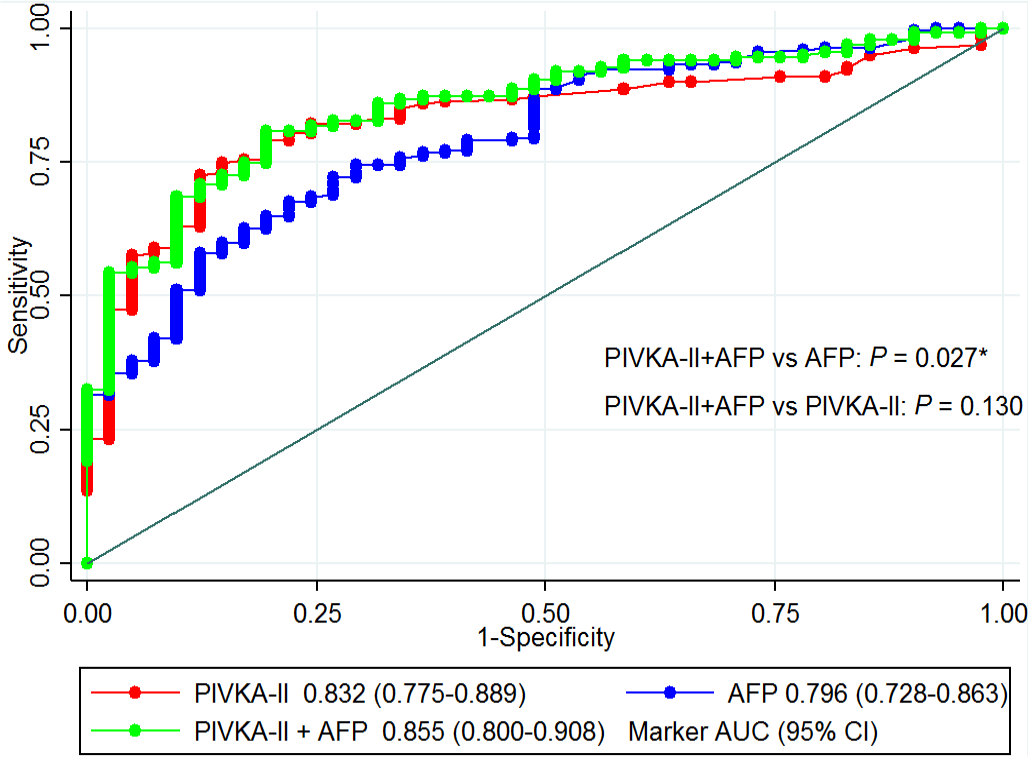
Fig. 1 - Receiver operating characteristic (ROC) curves and their area under the ROC curves (95% confidence interval) of protein induced by vitamin K absence-II or antagonist (PIVKA-II), alpha-fetoprotein (AFP), and the combination of both biomarkers to predict hepatocellular carcinoma (HCC) in patients with a high risk for HCC.
Discussion
This cohort found that PIVKA-II was more sensitive for HCC diagnosis than AFP and can be used alone without a combination with AFP.
The results of this study were comparable with two previous studies to detect HCC in cirrhosis patients with liver nodules of 1 cm or over (26-28). Note that this study had larger study population than the other two studies (260 vs. 128 vs. 90). With larger sample size in this study, PIVKA-II was more sensitive to detect HCC than AFP (80.80% vs. 75.80%) by the cutoff point of 40 mAU/L and 10 ng/mL, respectively. Compared with the study from France, the sensitivity of PIVKA-II was comparable (80.80% vs. 77%) as well as the cutoff point (40 vs. 42 mAU/L). However, this study had different results compared with the US study (23), in which AFP had better sensitivity than PIVKA-II in HCC with a sample size of 208 patients. AFP at 10.9 ng/mL had higher sensitivity at 66% compared with 56% sensitivity of PIVKA-II at 221.5 mAU/mL. These differences may be due to different control. In the previous study, patients with cirrhosis without liver mass served as controls, while non-HCC patients were cirrhotic patients with liver mass in this study. Another possible explanation is the property of PIVKA-II, which is an indicator of microvascular invasion (26). High PIVKA-II of over 90 mAU/mL had higher risk of microvascular invasion by 3.5 times (95% CI 1.08, 11.8; p value 0.043). In this study, 30.38% of patients had portal vein invasion, which may be an indicator of microvascular invasion (Tab. II).
In this study, we found that PIVKA-II can be used for HCC diagnosis without a need to combine with AFP. These results were different from the study from Korea (25). The areas under the ROC curve of these combinations were significantly different from PIVKA-II alone (0.912 vs. 0.870) or AFP alone (0.902 vs. 0.812) for those with liver cirrhosis in the previous study. Once again, these differences may be due to different study population. The previous study enrolled patients with HBV infection and categorized into three groups: non-cirrhotic HBV infection, cirrhosis without HCC, and HCC group (no data whether cirrhosis or not). Additionally, the cirrhosis group in the previous study may or may not have liver nodules like in this study. Note that HBV infection was accounted in only 37.69% in this study. Another explanation is different cutoff points for PIVKA-II and AFP. The cutoff points for these two markers in the previous study were 40 mAU/mL and 25 ng/mL, while the cutoff points in this study were 100 mAU/mL and 11 ng/mL. Note that a combination of these two biomarkers had lower sensitivity but higher specificity.
Even though both AFP and PIVKA-II are useful diagnostic markers for HCC, a previous report found that they may not be a good marker for small HCC nodules less than 2 cm as they have sensitivity of approximately 50% (29). However, they may be used for HCC detection particularly in hepatitis virus-related HCC (22,30). A study from China found similar findings as this study but different cutoff for both AFP and PIVKA-II (31). A combination of AFP and PIVKA-II model is better than AFP alone but comparable with PIVKA-II alone. Therefore, PIVKA-II may be used alone without a combination with AFP to diagnose HCC. Compared with benign liver disease, this study had PIVKA-II and AFP cutoff points at 40 mAU/mL and 10 ng/mL while the Chinese study had cutoff points of 43.47 mAU/mL and 21.47 ng/mL for PIVKA-II and AFP. The different cutoff points may be from different study population and sample size. This study had larger sample size and most patients (80%) had HBV or HCV as a cause, while the Chinese study did not show causes of HCC. Another study conducted with liver cirrhosis as a control group also found that similar findings of PIVKA-II alone were comparable with a combination of PIVKA-II and AFP for HCC diagnosis (32).
There are some limitations in this study. First, etiologies of cirrhosis in this study are varied; HCV was the most common cause (43.85%) followed by HBV infection (37.69%). The results of this study may not be applicable for other countries with different causes of cirrhosis or HCC. Second, we used different cutoff points for a combination of PIVKA-II and AFP as discussed earlier. Third, some associated factors with hepatitis virus or fatty liver such as sleep apnea were not studied (33-38). No predictor for HCC was studied as well as systematic review (39-42). Finally, note that control group in this study were those with high risk for HCC: presence of liver mass of 1 cm or more in size. As this study enrolled high-risk patients for HCC with various causes, various Child-Pugh score classification, various liver nodule sizes, these may lead to possible selection biases. Further studies are necessary before considering these biomarkers even for a general evaluation of the HCC diagnosis.
In conclusion, PIVKA-II may have more diagnostic yield for HCC compared with AFP. It can be used alone without a combination with AFP.
Acknowledgments
The authors would like to thank the Division of Research and Graduate Studies, and the Fundamental Fund of Khon Kaen University, Khon Kaen, Thailand.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contribution: All authors contributed equally to this manuscript.
References
- 1. Akinyemiju T, Abera S, Ahmed M, et al; Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683-1691. CrossRef PubMed
- 2. Lee S, Kim Y-Y, Shin J, et al. CT and MRI liver imaging reporting and data system version 2018 for hepatocellular carcinoma: a systematic review with meta-analysis. J Am Coll Radiol. 2020;17(10):1199-1206. CrossRef PubMed
- 3. Si Y-Q, Wang X-Q, Pan C-C, Wang Y, Lu ZM. An efficient nomogram for discriminating intrahepatic cholangiocarcinoma from hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:833999. CrossRef PubMed
- 4. Cho IJ, Jeong J-U, Nam T-K, et al. PIVKA-II as a surrogate marker for prognosis in patients with localized hepatocellular carcinoma receiving stereotactic body radiotherapy. Radiat Oncol J. 2022;40(1):20-28. CrossRef PubMed
- 5. Wang S-Y, Su T-H, Chen B-B, et al. Prothrombin induced by vitamin K absence or antagonist-II (PIVKA-II) predicts complete responses of transarterial chemoembolization for hepatocellular carcinoma. J Formos Med Assoc. 2022;121(6):1579-1587. CrossRef
- 6. Yanagaki M, Shirai Y, Hamura R, et al. Novel combined fibrosis-based index predicts the long-term outcomes of hepatocellular carcinoma after hepatic resection. Int J Clin Oncol. 2022;27(4):717-728. CrossRef PubMed
- 7. Yang Y, Li G, Lu Z, Liu Y, Kong J, Liu J. Progression of prothrombin induced by vitamin K absence-II in hepatocellular carcinoma. Front Oncol. 2021;11:726213. CrossRef PubMed
- 8. Sagar VM, Herring K, Curbishley S, et al. The potential of PIVKA-II as a treatment response biomarker in hepatocellular carcinoma: a prospective United Kingdom cohort study. Oncotarget. 2021;12(24):2338-2350. CrossRef PubMed
- 9. Hayashi M, Yamada S, Takano N, et al. Different characteristics of serum alfa fetoprotein and serum des-gamma-carboxy prothrombin in resected hepatocellular carcinoma. In Vivo. 2021;35(3):1749-1760. CrossRef PubMed
- 10. Mukund A, Vats P, Jindal A, Patidar Y, Sarin SK. Early hepatocellular carcinoma treated by radiofrequency ablation-mid- and long-term outcomes. J Clin Exp Hepatol. 2020;10(6):563-573. CrossRef PubMed
- 11. Lee Q, Yu X, Yu W. The value of PIVKA-II versus AFP for the diagnosis and detection of postoperative changes in hepatocellular carcinoma. J Interv Med. 2021;4(2):77-81. CrossRef PubMed
- 12. Kysela P, Kala Z, Zatloukal M, Raudenská M, Brančíková D. Hepatocellular carcinoma – prognostic criteria of individualized treatment. Klin Onkol. 2022;35(2):100-113. CrossRef PubMed
- 13. Si Y-Q, Wang X-Q, Fan G, et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect Agent Cancer. 2020;15(1):70. CrossRef PubMed
- 14. Basile U, Miele L, Napodano C, et al. The diagnostic performance of PIVKA-II in metabolic and viral hepatocellular carcinoma: a pilot study. Eur Rev Med Pharmacol Sci. 2020;24(24):12675-12685. PubMed
- 15. Degasperi E, Perbellini R, D’Ambrosio R, et al. Prothrombin induced by vitamin K absence or antagonist-II and alpha foetoprotein to predict development of hepatocellular carcinoma in Caucasian patients with hepatitis C-related cirrhosis treated with direct-acting antiviral agents. Aliment Pharmacol Ther. 2022;55(3):350-359. CrossRef PubMed
- 16. Su T-H, Peng C-Y, Chang S-H, et al. Serum PIVKA-II and alpha-fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J Formos Med Assoc. 2022;121(3):703-711. CrossRef PubMed
- 17. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022. CrossRef PubMed
- 18. de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(suppl 1):S75-S87. CrossRef PubMed
- 19. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47. CrossRef PubMed
- 20. Chi X, Jiang L, Yuan Y, et al. A comparison of clinical pathologic characteristics between alpha-fetoprotein negative and positive hepatocellular carcinoma patients from Eastern and Southern China. BMC Gastroenterol. 2022;22(1):202. CrossRef PubMed
- 21. Ji J, Liu L, Jiang F, et al. The clinical application of PIVKA-II in hepatocellular carcinoma and chronic liver diseases: a multi-center study in China. J Clin Lab Anal. 2021;35(11):e24013. CrossRef PubMed
- 22. Sun T, Li R, Qiu Y, Shen S, Wang W. New thresholds for AFP and des-γ-carboxy prothrombin in chronic liver disease depending on the use of nucleoside analogs and an integrated nomogram. Int J Gen Med. 2021;14:6149-6165. CrossRef PubMed
- 23. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. CrossRef PubMed
- 24. Ette AI, Ndububa DA, Adekanle O, Ekrikpo U. Utility of serum des-gamma-carboxyprothrombin in the diagnosis of hepatocellular carcinoma among Nigerians, a case-control study. BMC Gastroenterol. 2015;15(1):113. CrossRef PubMed
- 25. Seo SI, Kim HS, Kim WJ, et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3928-3935. CrossRef PubMed
- 26. Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848-854. CrossRef PubMed
- 27. Saitta C, Raffa G, Alibrandi A, et al. PIVKA-II is a useful tool for diagnostic characterization of ultrasound-detected liver nodules in cirrhotic patients. Medicine (Baltimore). 2017;96(26):e7266. CrossRef PubMed
- 28. Durazo FA, Blatt LM, Corey WG, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23(10):1541-1548. CrossRef PubMed
- 29. Tarao K, Nozaki A, Komatsu H, et al. Real impact of tumor marker AFP and PIVKA-II in detecting very small hepatocellular carcinoma (≤2 cm, Barcelona stage 0) – assessment with large number of cases. World J Hepatol. 2020;12(11):1046-1054. CrossRef PubMed
- 30. Chen Y, Yang Y, Li S, et al. Changes and clinical significance of PIVKA-II in hepatitis E patients. Front Public Health. 2022;9:784718. CrossRef PubMed
- 31. Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21(1):401. CrossRef PubMed
- 32. Xu F, Zhang L, He W, Song D, Ji X, Shao J. The diagnostic value of serum PIVKA-II alone or in combination with AFP in Chinese hepatocellular carcinoma patients. Dis Markers. 2021;2021:8868370. CrossRef PubMed
- 33. Khamsai S, Chootrakool A, Limpawattana P, et al. Hypertensive crisis in patients with obstructive sleep apnea-induced hypertension. BMC Cardiovasc Disord. 2021;21(1):310. CrossRef PubMed
- 34. Jeerasuwannakul B, Sawunyavisuth B, Khamsai S, et al. Prevalence and risk factors of proteinuria in patients with type 2 diabetes mellitus. Asia Pac J Sci Technol. 2021 [cited 2022 Jan 19];26(4):APST-26-04-02. Available from: Online
- 35. Soontornrungsun B, Khamsai S, Sawunyavisuth B, et al. Obstructive sleep apnea in patients with diabetes less than 40 years of age. Diabetes Metab Syndr. 2020;14(6):1859-1863. CrossRef PubMed
- 36. Sawunyavisuth B. What are predictors for a continuous positive airway pressure machine purchasing in obstructive sleep apnea patients? Asia Pac J Sci Technol. 2018:23(3):APST-23-03-10. CrossRef
- 37. Manasirisuk P, Chainirun N, Tiamkao S, et al. Efficacy of generic atorvastatin in a real-world setting. Clin Pharmacol. 2021;13:45-51. CrossRef PubMed
- 38. Tomar A, Bhardwaj A, Choudhary A, Bhattacharyya D. Association of obstructive sleep apnea with nocturnal hypoxemia in metabolic-associated fatty liver disease patients: a cross-sectional analysis of record-based data. J Family Med Prim Care. 2021;10(8):3105-3110. CrossRef PubMed
- 39. Tongdee S, Sawunyavisuth B, Sukeepaisarnjaroen W, Boonsawat W, Khamsai S, Sawanyawisuth K. Clinical factors predictive of appropriate treatment in COPD: a community hospital setting. Drug Target Insights. 2021;15:21-25. CrossRef PubMed
- 40. Charoentanyarak S, Sawunyavisuth B, Deepai S, Sawanyawisuth K. A point-of-care serum lactate level and mortality in adult sepsis patients: a community hospital setting. J Prim Care Community Health. 2021;12:21501327211000233. CrossRef PubMed
- 41. Boonwang T, Namwaing P, Srisaphonphusitti L, et al. Esports may improve cognitive skills in soccer players: a systematic review. Asia Pac J Sci Technol. 2022;27:APST-27-03-03.
- 42. Namwaing P, Ngamjarus C, Sakaew W, et al. Chest physical therapy and outcomes in primary spontaneous pneumothorax: a systematic review. J Med Assoc Thai. 2021;104(S4):S165-168. CrossRef