 |
J Circ Biomark 2022; 11: 57-63 ISSN 1849-4544 | DOI: 10.33393/jcb.2022.2446 ORIGINAL RESEARCH ARTICLE |
 |
Diagnostic impact of CEA and CA 15-3 on chemotherapy monitoring of breast cancer patients
ABSTRACT
Introduction: Serum tumor markers have emerged as an effective tool to determine prognosis and treatment efficiency in different cancer types. This study aimed to explore the chemotherapy monitoring efficiency and prognostic sensitivity of tumor-associated cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in early (II) and late (IV) clinical stage breast cancer.
Methods: CA 15-3 and CEA serum levels were assessed in 56 breast cancer patients at early (n = 26) and late (n = 30) clinical stages with these primary inclusion criteria: those who received adjuvant chemotherapy AC (adriamycin and cyclophosphamide) or AC-T (adriamycin and cyclophosphamide followed by taxane) regimens and possessed tumors negative for human epidermal growth factor receptor 2 (HER2) based on a particle-enhanced turbidimetric assay.
Results: CA 15-3 had a higher elevation than CEA in the pretreatment group of breast cancer patients when compared to healthy controls. Late-stage patients showed higher positive serum levels than early-stage patients for both markers, with a preference for CA 15-3 over CEA. AC-T chemotherapy regimen treatment in both clinical stages revealed a significantly higher level of both markers as compared to the AC regime, with a preference for CA 15-3 over CEA in late stage. Both markers were significantly higher in the late-stage group as compared to early-stage groups for both chemotherapy regimens.
Conclusions: CA 15-3 is more efficient as a prognostic monitoring marker than CEA and reveals a positive connection between chemotherapy regimen system and staging, with increased observability in late-stage patients.
Keywords: Breast cancer, CA 15-3, CEA, Chemotherapy, Prognosis
Received: June 29, 2022
Accepted: October 3, 2022
Published online: November 7, 2022
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Breast cancer is the most common cancer among Jordanian females, accounting for 22.4% of cancer cases (1). After being diagnosed with breast cancer, the patient treatment plan includes a combination of surgery, radiation, hormone therapy, and chemotherapy. Disease progress is evaluated according to consistent measures (2) based on alterations in the size of the quantifiable lesions.
During chemotherapy treatment, metastatic breast cancer is generally examined using imaging techniques such as positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI). These techniques are costly, and their efficiency and accuracy in treatment evaluation may strongly affect patient outcomes (3).
Measuring differences in serum tumor markers has been established as a tool for evaluation of therapy effectiveness of different cancers (4,5). Cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) are the most frequent serum tumor markers used for breast cancer, even though their functionality remains controversial (4,6). Breast cancer markers are applied for therapy response speculations, after initial therapy observation, and as prognostic indicators.
CEA is expressed in the majority of human lung, pancreatic, and gastric cancers, in addition to breast carcinoma (7). Measurements of CEA in breast cancer are suggestive of lymph node involvement and tumor size. Consequently, CEA concentrations above 7.5 μg/L are linked to a higher possibility of subclinical metastases (8). The normal range of CEA levels was connected to a significantly better prognosis of patients at the time of diagnosis compared to those with elevated levels (9). Studies propose CEA as a useful marker for monitoring treatment response including chemotherapeutic ones (10-12).
CA 15-3 (MUC1) is a cell surface glycoprotein derived from the MUC1 gene. It’s expressed on the surface of various epithelial cell types and overexpressed in 90% of breast cancer cases (13). The elevated level of CA 15-3 is used to determine the relapse potential of breast cancer patients, and as a tool for therapeutic response evaluation at late stages (14). CA 15‐3 preoperative concentrations of early breast cancer patients have a notable relation to predict their outcomes (15).
Using serum tumor markers (CA 15-3 and CEA) permits the early identification of up to 60-80% of breast cancer patient metastasis (16). The serum levels of CA 15-3 and CEA were shown to be beneficial in the management of breast cancer patients and could aid as prognostic indicators and for observing disease development (17).
This study investigated the clinical importance of serum tumor markers CA 15-3 and CEA for monitoring Jordanian breast cancer patient responses to different chemotherapy regimens and their correlation with different clinical stages in addition to their prognostic value sensitivity.
Materials and methods
Patient cohort
Fifty-six female breast cancer patients were involved in this study using these main inclusion criteria: (1) human epidermal growth factor receptor 2 (HER2)-negative and (2) received adjuvant chemotherapy regimen AC (adriamycin and cyclophosphamide) or AC-T (adriamycin, cyclophosphamide, taxane). Table I shows the patient characteristics. Patients were categorized as follows: 26 patients (46.4%) were graded as stage II and 30 patients (53.5%) were graded as stage IV, patients who did not meet the criteria in Table 1 were excluded. Stage II or less was considered as early stage and stage II and above was considered as late stage as stated by the American Joint Committee on Cancer (AJCC) staging system (18). The median age between the two groups did not show any significant difference (p = 0.232).
| Patient parameter | n (%) |
|---|---|
| Age (years) | Median 49 |
| Range 43-55 | |
| Gender | Female |
| Clinical stage | |
| Stage II | 26 (46.4%) |
| Stage IV | 30 (53.5%) |
| Chemotherapy regime | |
| AC × 4 | 29 (51.7%) |
| AC × 4 followed by T × 4 | 27 (48.2%) |
| Histological type | IDC |
| HER2 receptor | Negative |
A = adriamycin; C = cyclophosphamide; HER = human epidermal growth factor receptor; T = taxane; IDC = Invasive ductal carcinoma.
Primary chemotherapy
The first group of AC regimen consisting of 14 stage II and 15 stage IV patients was treated with 4 cycles of adriamycin 50 mg/m2 and cyclophosphamide 1000 mg/m2 on day 1, which was repeated every 21 days. The second group of AC-T regimen consisting of 12 stage II and 15 stage IV patients received the previous chemotherapy regimen AC, followed by 4 cycles of taxane 80 mg/m2 every 21 days. The flowchart of patients is presented in Figure 1.
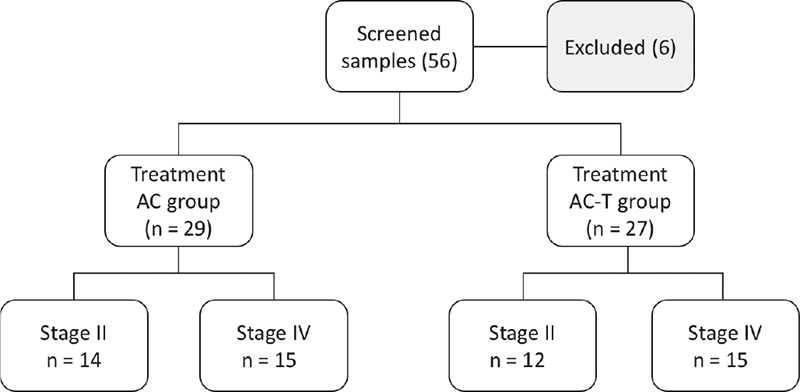
Fig. 1 - Flowchart of patients’ selection. AC (Adriamycin & Cyclophosphamide) or AC-T (Adriamycin & Cyclophosphamide followed by Taxane) regimes treatments. (Based on the oncology decision according to treatment guidelines).
Sample collection
Ethical approval for this study was acquired from Al Bashir Hospital, Amman, Jordan (#3345), and written informed consent was collected from all patients. CEA and CA 15-3 blood samples were gathered from patients for diagnosis after the third month of treatment protocol (fourth cycle).
CEA and CA 15-3 measurements
The serum was isolated by centrifugation (2,500 rpm for 10 minutes) of patients’ blood samples. Serum CEA and CA 15-3 levels were determined using an electrochemiluminescence immunoassay system (MODULAR ANALYTICS E170, Cobas e601; Roche, Germany): a particle-enhanced turbidimetric assay for CEA and immunoturbidimetric assay for CA 15-3. Marker assays were done using the commercial kits for CEA (Elecsys CEA, Cobas, Roche, Germany) and CA 15-3 (Elecsys CA 15-3, Cobas, Roche, Germany). A cut-off point of <5.0 μg/L (CEA) and <25.0 U/mL (CA 15-3) was used as indicated by the Roche Diagnostic Kit brochure. The CEA and CA 15-3 readings of 20 healthy females (con −) with inclusion criteria–does not have any type of cancer or chronic diseases, age ≥18 years, not on any type of medication, and 16 pre-chemotherapy breast cancer female patients (con +)—were obtained from Bio-lab laboratories.
Statistical analysis
Statistical analysis was performed using SPSS software. A t-test and Fishers test were performed to find out possible marker-level variations between target groups. To see if the differences in proportions were statistically significant, the chi-square test was utilized. When possible, the odds ratio was utilized to assess the relationship.
Results
This study was planned to determine the correlation between CEA and CA 15-3 levels’ elevation significance on monitoring response to Jordanian breast cancer female patients’ treatment with different chemotherapy regimens at early and late clinical stages.
CEA and CA 15-3 levels in breast cancer
The CEA and CA 15-3 serum levels were measured in all samples using ELISA. The serum levels of CEA (1.7 μg/L) and CA 15-3 (18.7 U/mL) were significantly increased (Fig. 2; p = 0.0005 and p = 0.0001), respectively, in the pre-chemotherapy group (con +) compared to the healthy group (con −): CEA (1.09 μg/L) and CA 15-3 (8.7 U/mL). The presented data revealed differentiation between CEA and CA 15-3 serum-level elevation of studied groups (Fig. 2), as we observed a stronger increase of CA 15-3 level (Fig. 2B) compared to CEA level (Fig. 2A). These results imply that CEA and CA 15-3 levels can be used efficiently to anticipate breast cancer tendency and provide a convenient detection method for breast cancer with a preference for CA 15-3 over CEA in sensitivity.
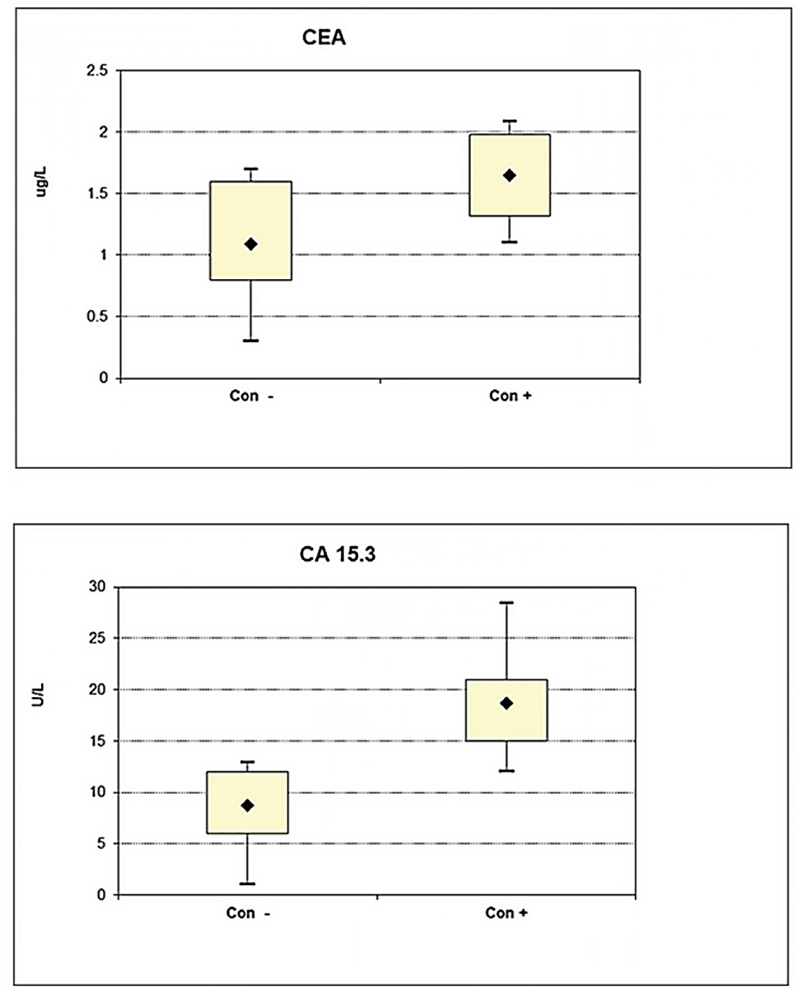
Fig. 2 - Difference in serum level between healthy non-cancer group (control –) and breast cancer patients groups before chemotherapy (control +) of (A) CEA (B) CA 15-3 markers. (*Significant increase differences for the serum levels of CEA and CA 15-3 (****P = 0.0005 and ****P = 0.0001) respectively in pre-chemotherapy group (con +) compared to healthy ones (con –)).
Positive serum levels of CEA and CA 15-3
In all-inclusive patient samples, elevated positive serum levels found in early and late stages were identified as follows: for CEA (II AC 4/14 [29%]), (II AC-T 4/11 [36%]), (IV AC 7/14 [50%]), and (IV AC-T 9/14 [64%]) of the breast cancer cases used the cut-off point <5.0 μg/L. As for CA 15-3 (II AC 5/14 [35.7%]), (II AC-T 7/11 [63.6%]), (IV AC 10/14 [71%]), and (IV AC-T 9/14 [64%]) of the breast cancer cases used the cut-off point <25 U/mL (Fig. 3). In total 8/25 (32%) of stage II and 16/28 (57%) of stage IV patients had higher levels of CEA than the cut-off point, while for CA 15-3 12/25 (48%) of stage II and 19/28 (68%) of stage IV patients had higher levels of CA 15-3 than the cut-off point (Fig. 3). In our study, a combined chemotherapy regimen demonstrated higher positive serum-level percentages for both markers as compared to a single chemotherapy regimen in the early-stage patient group; this elevation was notably stronger for CA 15-3 in comparison to CEA. The same result was obtained for CEA in the late-stage patient group; however, CA 15-3 behaved differently as positive serum levels were higher in a single chemotherapy regimen compared to a combined regimen. Overall, the late-stage patient group showed higher positive serum-level percentages compared to the early-stage group for both markers with a preference for CA 15-3 over CEA. These findings suggest that the serum levels of CA 15-3 might be more beneficial for observing chemotherapy response in advanced tumors than early diagnosis as compared with CEA.
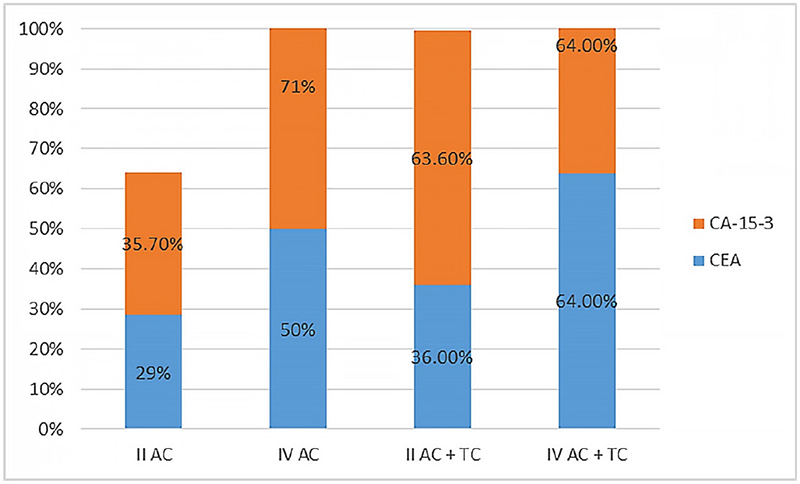
Fig. 3 - Positive serum levels of carcinoembryonic antigen (CEA) and cancer antigen (CA) 15-3 markers for both chemotherapy regimens (AC and AC-T) in early (II) and late (IV) clinical stages. AC = adriamycin and cyclophosphamide; AC-T = adriamycin and cyclophosphamide followed by taxane.
CEA and CA 15-3 levels based on chemotherapy type
Furthermore, the marker serum-level response and its association with the type of chemotherapeutic treatments in both early and late stages were investigated. Comparing the CEA levels between both treatment regimens AC (mean rank: 2.41) and AC-T (mean rank: 2.65) in early stage, the AC (mean rank: 8.16) and AC-T (mean rank: 17.45) in late stage showed no significant changes (p = 0.71 and p = 0.41), respectively (Fig. 4A). Conversely, the CA 15-3 levels behaved differently where it had shown a significant change (p = 0.056) comparing the AC-T (mean rank: 144.39) to AC (mean rank: 25.55) at late stage but not at early stage (p = 0.089) AC (mean rank: 25.00) and AC-T (mean rank: 43.27) (Fig. 4B). This elevation in response to a combined chemotherapy regimen was observed to be stronger in the case of CA 15-3 in comparison to CEA and more specifically in the late-stage patient group.
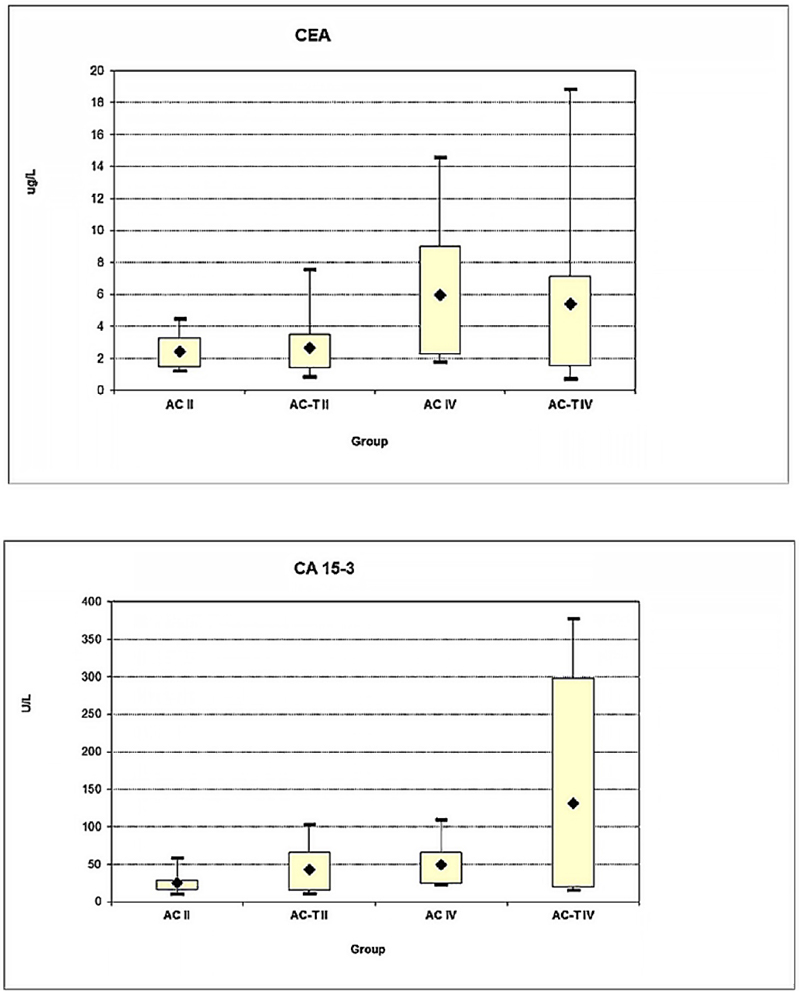
Fig. 4 - Effect of chemotherapy regime type AC or AC-T in both early (II) and late (IV) clinical stages on serum level response of (A) CEA and (B) CA 15-3 markers. (*Significant increase for the CA 15-3 serum level (*P = 0.05) comparing the AC-T to AC at late-stage IV).
CEA and CA 15-3 levels based on clinical stage
Analysis was performed to check if the CEA and CA 15-3 elevation response was associated with breast cancer progression in terms of different clinical stages (early and late) in each of the studied chemotherapeutic regimens. Comparing CEA levels between II (mean rank: 2.41) and IV (mean rank: 5.26) stages of AC treatment, II (mean rank: 2.65) and IV (mean rank: 5.83) stages of AC-T treatment, there was a significant change (p = 0.022) in stage IV AC but not in stage IV AC-T (p = 0.081) (Fig. 5A). The CA 15-3 level comparison ([II (mean rank: 25.00) and IV (mean rank: 49.35) stages of AC treatment], [II (mean rank: 34.27) and IV (mean rank: 130.96) stages of AC-T treatment]) showed a significant change in stage IV compared to stage II in both AC and AC-T treatments (p = 0.005 and p = 0.044), respectively (Fig. 5B).
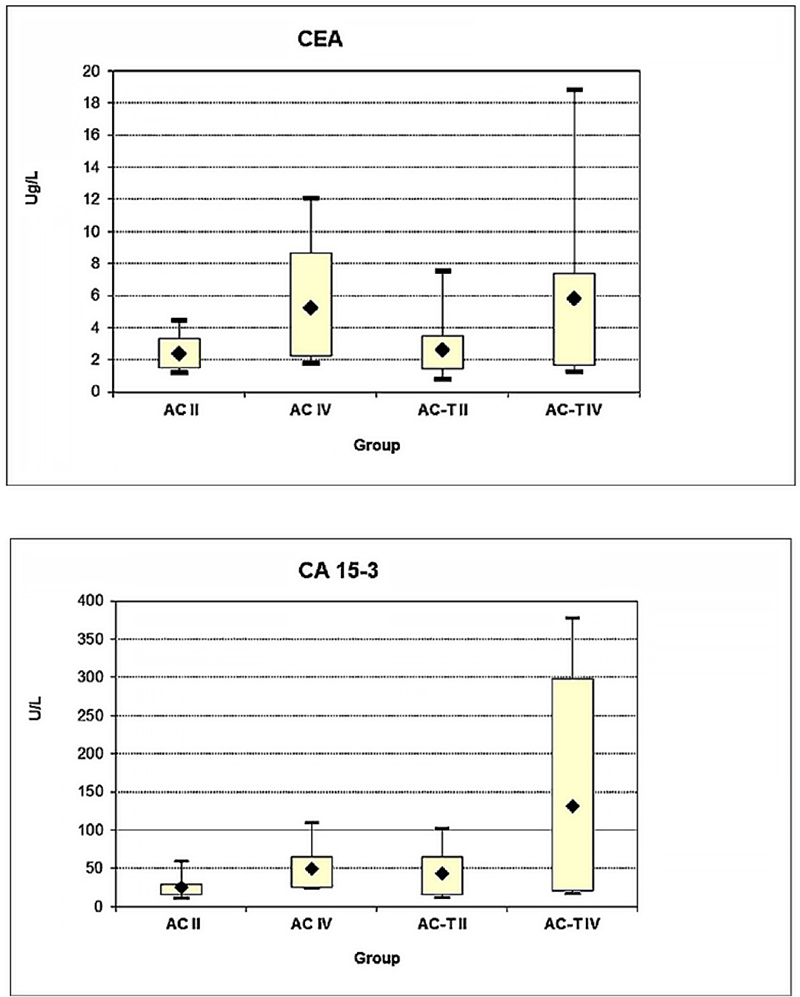
Fig. 5 - Effect of both clinical stages early (II) and late (IV) in association with chemotherapy regime type AC or AC-T on serum level response of (A) CEA and (B) CA 15-3 markers. (*Significant increase for the CEA serum level (*P = 0.02) for AC treatment at late-stage IV), (*Significant increase for the CA 15-3 serum level in late stage IV in both AC and AC-T treatments (*P = 0.005 and* P = 0.044) respectively.
The results revealed a significant connection between a change in marker level and clinical staging, as both CEA and CA 15-3 were significantly elevated in the late-stage patient group; more so than in the early-stage group in both chemotherapeutic regimens (Fig. 5). Collectively, these data suggest that CEA and CA 15-3 are predictive of chemotherapy response among different regimens throughout treatment and show differences between both early and late stages.
Discussion
Breast cancer is the most frequent cancer among Jordanian women. This study evaluated the significance of using CA 15-3 and CEA for monitoring different chemotherapy regimens since assessing prognosis using diagnostic markers is believed to help in patients’ therapeutic response anticipation, which is vital for evaluating the course of therapy and to avoid the side effects of worthless and inefficient treatments.
In the present study, CA 15-3 had shown a higher elevation as compared to CEA as both markers were significantly elevated in breast cancer patients at the time of diagnosis in comparison to healthy controls. These results imply that both CA 15-3 and CEA markers can efficiently predict breast cancer susceptibility and deliver benefit for breast cancer prognosis detection. The combination of both tumor markers (CA 15-3 and CEA) is important in breast cancer (19). CA 15-3 has better prognostic significance in relation to CEA (20). However, some studies have indicated that the prognostic value of CA 15-3 is lower than that of CEA (21), which demonstrates marker contradictory association in breast carcinogenesis (22).
Some studies have described the changes of CA-15-3 and CEA levels to be independent regardless of the breast cancer stage (23); however, our results showed that elevated serum levels of CEA and CA 15-3 above the cut-off point were identified in 8 (32%) and 12 (48%) of early-stage patients, and 16 (57%) and 19 (68%) of late-stage patients. More notable serum levels were elevated in the late stage than in the early stage with a preference for CA 15-3 over CEA. CA 15-3 and CEA elevation levels have been described as connected with clinic pathological parameters including advanced tumor, node, metastasis (TNM) stage (24).
The author analyzed the clinical impact of CA 15-3 and CEA to breast cancer patients with different chemotherapy regimens and in terms of different tumor clinical stages. This study found that CA 15-3 and CEA levels were shown to be higher in late stage and combined regimen as compared to early stage and single regimen, mainly with a preference for CA 15-3 at late stage over CEA. These results suggest that CA 15-3 and CEA serum levels can be an indicator for stage and chemotherapy regimen systems. Additionally, they suggest the clinical importance of CA 15-3 for follow-up as a prognostic variable during chemotherapy treatment of breast cancer patients.
CA 15-3 serum levels showed variations among breast cancer stages (9). CA 15-3 levels increase in all types of tumors; however, in breast cancer, it continues to increase as the tumor develops (25). Studies have reported that alterations of tumor marker levels are associated with a patient’s therapeutic response, in addition to imaging method evaluation (26). CA 15-3 flaring (125% over the baseline) has been noticed in breast cancer patients after chemotherapy and has been linked with higher chances of disease development (27). Increased CA 15-3 levels were found for locally progressed breast cancer patients who received primary chemotherapy (AC or AC-T regimen), which is an indicator of a poor response to treatment (28). The analysis of CA 15-3 levels upregulating during the first 4-6 weeks of a new therapy should be considered because false initial rises can happen (29-33). The chemotherapy influence on the temporary elevation of CA 15-3 followed by its decline could be a consequence of unsuitable early cancelation or change of chemotherapy regimen (30,34,35).
Previous studies indicated no connection between a breast cancer patient’s prior treatment and CEA levels (36). Based on our data, the CEA levels changed during the course of treatment and stages. CEA elevation for colorectal cancer patients has been noticed in the first 4-6 weeks after beginning chemotherapy (37,38). In addition, a chemotherapy regimen based on both irinotecan and oxaliplatin was found to induce a CEA flare and was correlated with good prognosis for colorectal cancer patients (39). The mechanisms by which chemotherapy induces CEA during cancer treatment remain to be elucidated. Several factors were described to have an influence and connection including hypothyroidism and inflammatory diseases (40,41). In some protocols CEA combined with CA 15-3 is used to observe the chemotherapy response for breast cancer patients, as it flares in the first 4-8 weeks of therapy as previously noted (42).
The current study promotes the option of monitoring CA 15-3 and CEA during adjuvant chemotherapy for breast cancer patients in Jordan as its results could contribute to treatment evaluation and be beneficial for customizing chemotherapeutic regimens in the future.
Conclusions
In conclusion, monitoring serum CA 15-3 and CEA levels for Jordanian breast cancer patients undergoing chemotherapy treatment provides prognostic indication and clinical information for evaluating tumor response as both markers had elevated levels with a preference for CA 15-3 over CEA in sensitivity.
Acknowledgments
The author acknowledges the biomedical scientists at Biolab Diagnostic Laboratories, represented by Dr. Amid Abdelnour, for their support of this work.
Disclosures
Conflict of interest: The authors declare that they have no conflict of interest.
Financial support: The author acknowledges the financial support from the deanship of scientific research at Al-Balqa Applied University Salt, Jordan (grant 118001).
Ethical approval: Ethical approval for this study was obtained from the ethical approval committee of Al Bashir Hospital Amman, Jordan (#3345).
References
- 1. Abdel-Razeq H, Mansour A, Jaddan D. Breast cancer care in Jordan. JCO Glob Oncol. 2020;6(6):260-268. CrossRef PubMed
- 2. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-216. CrossRef PubMed
- 3. Alunni-Fabbroni M, Müller V, Fehm T, Janni W, Rack B. Monitoring in metastatic breast cancer: is imaging outdated in the era of circulating tumor cells? Breast Care (Basel). 2014;9(1):16-21. CrossRef PubMed
- 4. Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 2012;14(1):R29. CrossRef PubMed
- 5. Liu L, Xu HX, Wang WQ, et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget. 2016;7(5):5943-5956. CrossRef PubMed
- 6. Falzarano R, Viggiani V, Michienzi S, et al. Evaluation of a CLEIA automated assay system for the detection of a panel of tumor markers. Tumour Biol. 2013;34(5):3093-3100. CrossRef PubMed
- 7. Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5(5):344-366. CrossRef PubMed
- 8. Molina R, Auge JM, Farrus B, et al. Prospective evaluation of carcinoembryonic antigen (CEA) and carbohydrate antigen 15.3 (CA 15.3) in patients with primary locoregional breast cancer. Clin Chem. 2010;56(7):1148-1157. CrossRef PubMed
- 9. Uehara M, Kinoshita T, Hojo T, Akashi-Tanaka S, Iwamoto E, Fukutomi T. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 2008;13(5):447-451. CrossRef PubMed
- 10. Dnistrian AM, Schwartz MK, Greenberg EJ, Smith CA, Schwartz DC. CA 15-3 and carcinoembryonic antigen in the clinical evaluation of breast cancer. Clin Chim Acta. 1991;200(2-3):81-93. CrossRef PubMed
- 11. Jezersek B, Cervek J, Rudolf Z, Novaković S. Clinical evaluation of potential usefulness of CEA, CA 15-3, and MCA in follow-up of breast cancer patients. Cancer Lett. 1996;110(1-2):137-144. CrossRef PubMed
- 12. Robertson JF, Jaeger W, Syzmendera JJ, et al; European Group for Serum Tumour Markers in Breast Cancer. The objective measurement of remission and progression in metastatic breast cancer by use of serum tumour markers. Eur J Cancer. 1999;35(1):47-53. CrossRef PubMed
- 13. Duffy MJ, Shering S, Sherry F, McDermott E, O’Higgins N. CA 15-3: a prognostic marker in breast cancer. Int J Biol Markers. 2000;15(4):330-333. CrossRef PubMed
- 14. Kurebayashi J. [Biomarkers in breast cancer]. Gan To Kagaku Ryoho. 2004;31(7):1021-1026. PubMed
- 15. Shering SG, Sherry F, McDermott EW, O’Higgins NJ, Duffy MJ. Preoperative CA 15-3 concentrations predict outcome of patients with breast carcinoma. Cancer. 1998;83(12):2521-2527. CrossRef PubMed
- 16. Jäger W, Eibner K, Löffler B, Gleixner S, Krämer S. Serial CEA and CA 15-3 measurements during follow-up of breast cancer patients. Anticancer Res. 2000;20(6D):5179-5182. PubMed
- 17. Zhao S, Mei Y, Wang J, Zhang K, Ma R. Different levels of CEA, CA153 and CA125 in milk and benign and malignant nipple discharge. PLoS One. 2016;11(6):e0157639. CrossRef PubMed
- 18. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-4. CrossRef PubMed
- 19. Vizcarra E, Lluch A, Cibrián R, et al. Value of CA 15.3 in breast cancer and comparison with CEA and TPA: a study of specificity in disease-free follow-up patients and sensitivity in patients at diagnosis of the first metastasis. Breast Cancer Res Treat. 1996;37(3):209-216. CrossRef PubMed
- 20. Geraghty JG, Coveney EC, Sherry F, O’Higgins NJ, Duffy MJ. CA 15-3 in patients with locoregional and metastatic breast carcinoma. Cancer. 1992;70(12):2831-2834. CrossRef PubMed
- 21. Ebeling FG, Stieber P, Untch M, et al. Serum CEA and CA 15-3 as prognostic factors in primary breast cancer. Br J Cancer. 2002;86(8):1217-1222. CrossRef PubMed
- 22. Banin Hirata BK, Oda JM, Losi Guembarovski R, Ariza CB, de Oliveira CE, Watanabe MA. Molecular markers for breast cancer: prediction on tumor behavior. Dis Markers. 2014;2014:513158. CrossRef PubMed
- 23. Moazzezy N, Farahany TZ, Oloomi M, Bouzari S. Relationship between preoperative serum CA 15-3 and CEA levels and clinicopathological parameters in breast cancer. Asian Pac J Cancer Prev. 2014;15(4):1685-1688. CrossRef PubMed
- 24. Agrawal AK, Jelen M, Rudnicki J, et al. The importance of preoperative elevated serum levels of CEA and CA15-3 in patients with breast cancer in predicting its histological type. Folia Histochem Cytobiol. 2010;48(1):26-29. CrossRef PubMed
- 25. Fu Y, Li H. Assessing clinical significance of serum CA15-3 and carcinoembryonic antigen (CEA) Levels in breast cancer patients: a meta-analysis. Med Sci Monit. 2016;22:3154-3162. CrossRef PubMed
- 26. Yang Y, Zhang H, Zhang M, Meng Q, Cai L, Zhang Q. Elevation of serum CEA and CA15-3 levels during antitumor therapy predicts poor therapeutic response in advanced breast cancer patients. Oncol Lett. 2017;14(6):7549-7556. CrossRef PubMed
- 27. Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52(3):345-351. CrossRef PubMed
- 28. Al-azawi D, Kelly G, Myers E, et al. CA 15-3 is predictive of response and disease recurrence following treatment in locally advanced breast cancer. BMC Cancer. 2006;6(1):220. CrossRef PubMed
- 29. Nisman B, Maimon O, Allweis T, et al. The prognostic significance of LIAISON(R) CA15-3 assay in primary breast cancer. Anticancer Res. 2013;33(1):293-299. PubMed
- 30. Kim HS, Park YH, Park MJ, et al. Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2009;118(1):89-97. CrossRef PubMed
- 31. Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi A. Correlation of serum and salivary CA15-3 levels in patients with breast cancer. Med Oral Patol Oral Cir Bucal. 2009;14(10):e521-e524. CrossRef PubMed
- 32. Ali HQ, Mahdi NK, Al-Jowher MH. The value of CA15-3 in diagnosis, prognosis and treatment response in women with breast cancer. J Pak Med Assoc. 2013;63(9):1138-1141. PubMed
- 33. Kobayashi S, Iwase H, Karamatsu S, Matsuo K, Masaoka A, Miyagawa T. The clinical value of serum CA15-3 assay postoperatively in breast cancer patients. Jpn J Surg. 1989;19(3):278-282. CrossRef PubMed
- 34. Di Gioia D, Heinemann V, Nagel D, et al. Kinetics of CEA and CA15-3 correlate with treatment response in patients undergoing chemotherapy for metastatic breast cancer (MBC). Tumour Biol. 2011;32(4):777-785. CrossRef PubMed
- 35. Devine PL, Duroux MA, Quin RJ, et al. CA15-3, CASA, MSA, and TPS as diagnostic serum markers in breast cancer. Breast Cancer Res Treat. 1995;34(3):245-251. CrossRef PubMed
- 36. Thriveni K, Krishnamoorthy L, Ramaswamy G. Correlation study of carcino embryonic antigen & cancer antigen 15.3 in pretreated female breast cancer patients. Indian J Clin Biochem. 2007;22(1):57-60. CrossRef PubMed
- 37. Sørbye H, Dahl O. Transient CEA increase at start of oxaliplatin combination therapy for metastatic colorectal cancer. Acta Oncol. 2004;43(5):495-498. CrossRef PubMed
- 38. Ailawadhi S, Sunga A, Rajput A, Yang GY, Smith J, Fakih M. Chemotherapy-induced carcinoembryonic antigen surge in patients with metastatic colorectal cancer. Oncology. 2006;70(1):49-53. CrossRef PubMed
- 39. Strimpakos AS, Cunningham D, Mikropoulos C, Petkar I, Barbachano Y, Chau I. The impact of carcinoembryonic antigen flare in patients with advanced colorectal cancer receiving first-line chemotherapy. Ann Oncol. 2010;21(5):1013-1019. CrossRef PubMed
- 40. Takahashi N, Shimada T, Ishibashi Y, Oyake N, Murakami Y. Transient elevation of serum tumor markers in a patient with hypothyroidism. Am J Med Sci. 2007;333(6):387-389. CrossRef PubMed
- 41. Klapdor R, Bahlo M, Babinski A. Atypical courses of serum tumor markers—4 case reports. Anticancer Res. 2003;23(2A):845-850. PubMed
- 42. Harris L, Fritsche H, Mennel R, et al; American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287-5312. CrossRef PubMed