 |
J Circ Biomark 2022; 11: 28-35 ISSN 1849-4544 | DOI: 10.33393/jcb.2022.2386 ORIGINAL RESEARCH ARTICLE |
 |
Soluble IL-33 receptor predicts survival in acute kidney injury
ABSTRACT
Introduction: The prediction of acute kidney injury (AKI)-related outcomes remains challenging. Herein we prospectively quantified soluble ST2 (sST2), the circulating isoform of the IL-33 receptor, in hospitalized patients with AKI.
Methods: In-hospital subjects with AKI of various etiology were identified through the in-hospital AKI alert system of the Brandenburg University hospital. sST2 was measured within a maximum of 48 hours from the time of diagnosis of AKI. The following endpoints were defined: in-hospital death, dialysis, recovery of kidney function until demission.
Results: In total, 151 individuals were included in the study. The in-hospital mortality was 16.6%, dialysis therapy became mandatory in 39.7%, no recovery of kidney function occurred in 27.8%. sST2 was significantly higher in nonsurvivors (p = 0.024) but did not differ in the two other endpoints. The level of sST2 increased significantly with the severity of AKI. Further differences were detected in subjects with heart insufficiency (lower sST2), and in patients that required ICU treatment, or ventilatory therapy, or vasopressors (all higher).
Conclusions: The current study suggests sST2 as biomarker of “acute distress”: it predicts post-AKI survival and substantially increases in subjects with a higher degree of cumulative morbidity under acute circumstances (e.g., ICU therapy, vasopressor administration).
Keywords: Acute kidney injury, IL-33, Soluble ST2, Mortality, Biomarker
Received: February 17, 2022
Accepted: May 16, 2022
Published online: June 6, 2022
Journal of Circulating Biomarkers - ISSN 1849-4544 - www.aboutscience.eu/jcb
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Acute kidney injury (AKI) occurs with increasing frequencies at hospitals in central Europe and the United States. It is being estimated that up to 18% of all hospitalized subjects develop AKI during the treatment course (1). The in-hospital mortality of hospital-acquired AKI has been reported to vary from 10% to 20% (1-3), with exceptionally low survival rates under intensive care conditions (4).
Interleukin-33 (IL-33) belongs to the Interleukin-1 family of cytokines (5). The cytokine was initially found within endothelial cell nuclei of so-called human high endothelial venules (HEV). Pichery and colleagues (6) detected the protein within the nuclei of murine cells in various tissues, such as epithelial cells, lymphoid organs, brain, and embryonic tissue. Within nuclei, IL-33 binds to chromatin (7); in the extracellular space however, it interacts with ST2. The latter exists as membrane-bound and soluble isoform (sST2), respectively (8). IL-33 has been shown to modulate the activity of several immunocompetent cells such as mast cells, group 2 innate lymphoid cells (ILC2s), T helper 2 cells, eosinophils, basophils, dendritic cells, macrophages, and others (9).
Early AKI recognition remains difficult, although new biomarkers have been identified in recent years (10). Future diagnostic criteria will most likely include markers of structural kidney damage (11). Until then, AKI is being diagnosed according to the 2012 published “KDIGO clinical practice guidelines for acute kidney injury” (12).
Experimental data suggest a critical role for IL-33 in the pathogenesis of AKI (13,14). Also, several studies evaluated IL-33 and sST2 as biomarkers in inflammatory and noninflammatory diseases. The literature on IL-33 reveals heterogenous findings, including protein elevation or suppression, or constant serum IL-33, depending on etiology and course of the disease (15-17). Also, IL-33 quantification has been associated with substantial difficulties. Lately we summarized the literature on the topic (18). For instance, Ketelaar et al. (19) used 4 different ELISA kits (Quantikine and DuoSet - R&D systems, respectively; ADI-900-201 - Enzo Life Sciences; SKR038 - GenWay Biotech Inc San Diego USA) for analyzing serum samples from asthma patients. The percentages of samples above the lower detection limit (LLD) were 0 (zero) in two kits (ADI-900-201 and SKR038). Also, the Quantikine kit showed only 2% of all samples above the LLD, the DuoSet kit in contrast was successful in at least 76%. Similar observations were made by Asaka et al. (20), who also employed the Quantikine kit. Finally, Riviere and colleagues (18) reported difficulties in IL-33 quantification as well. Regarding IL-33 and sST2 in conjunction, two studies were performed in AKI subjects so far. The first study revealed sST2 as an early predictor of acute kidney injury in patients with myocardial infarction (21). The second investigation showed sST2 to be AKI predictive in subjects undergoing cardiac surgery (22).
Herein, we prospectively analyzed serum sST2 levels in patients with newly onset AKI of various etiology. Three endpoints were defined: in-hospital death, the need for dialysis, and recovery of kidney function until demission.
Methods
Setting
This prospective observational study was conducted at the Brandenburg University Hospital in Brandenburg an der Havel, Germany. The hospital is part of the Brandenburg Medical School.
Study population and design
The study was approved by the local ethics committee of the Brandenburg Medical School Theodor Fontane in October 2019 (file no. E-01-20190820). All recruited participants were hospitalized patients of the University Hospital Brandenburg. Patients of multiple medical departments with newly onset AKI were included from May 2020 to June 2021. AKI was defined according to criteria 1 or 2 of 2012 revised KDIGO classification (23). The third criterion (urine output of below 0.5 mL/kg/h for at least 6 hours) was not applied since information on urine production was not available in all subjects. Serum IL-33 and sST2 levels were determined once at the time of initial diagnosis of AKI. All patients were over 18 years of age, were not previously receiving renal replacement therapy at the time of blood collection, and signed written informed consent. Preexisting chronic renal failure requiring dialysis, terminal disease with a strictly palliative treatment regimen, suspected or active COVID-19 disease, and age less than 18 years resulted in exclusion from the study. The AKI etiology was identified according to respective criteria for sepsis (24), cardiorenal syndrome types 1 or 3 (25), and hepatorenal syndrome (26). The diagnosis of obstruction was made by ultrasound analysis, the diagnoses of drug-induced, contrast-associated, and postsurgery AKI were made according to the history. Volume depletion or prerenal AKI was diagnosed if other causes were unlikely and if the patient presented clinical symptoms of volume depletion (e.g., dry skin in conjunction with low blood pressure and tachycardia).
Blood sampling and preanalytics
An automated AKI alert system has been implemented at the Brandenburg University Hospital in 2018. Elevated serum creatinine levels (according to the KDIGO criteria 1 or 2) that are measured during daily laboratory checks are registered by an electronic algorithm and transmitted to the nephrologist in charge. The messages exclusively contain a patient-related number and do not allow to identify individuals without the in-hospital database. After written informed consent was obtained, a standardized venous blood sample was collected in two 3.5 mL serum tubes (BD Vacutainer® SST™ II Advance). Blood was collected in the supine position with as little venous congestion as possible to avoid hemolysis. In patients with central venous line, blood was collected from that catheter. The filled blood tubes were stored upright for 30 minutes to maintain the clotting time specified by the manufacturer. This was followed by centrifugation at 1,400 g for 10 minutes at room temperature. Samples were stored in plastic tubes at constant −22°C until analysis.
Quantification of serum sST2
The quantification of sST2 was performed by using a commercially available kit: Human ST2/IL-33R Quantikine ELISA Kit (DST 200, R&D). The assay detects free and IL-33-complexed ST2. Analyses were performed in duplicates according to the manufacturer’s instructions. Sample predilution was adjusted individually to the concentrations. The range of assay sensitivity was 2.45-13.5 pg/mL. Reference blood samples from healthy adults were used for comparison.
Endpoints
Three primary endpoints were defined: in-hospital death, the need for dialysis, and recovery of kidney function until demission. The second criterion (need for dialysis) was fulfilled if one or more dialysis treatment sessions became mandatory. Dialysis was performed as hemodialysis, or hemodiafiltration, or slow extended daily dialysis (SLEDD), or as continuous veno-venous hemodiafiltration (CVVHD(F)). The respective procedure was chosen by the nephrologist in charge. Renal recovery was defined according to the criteria published by Fiorentino et al (27). It was diagnosed, if the last serum creatinine concentration did not differ from the initial value by more than 50%.
Statistics
Initially, results of sST2 quantification were tested for normality with the Kolmogorov-Smirnov test. Since data were not distributed normally, the Mann-Whitney test was applied for comparisons between two groups. Comparisons between three or more groups were performed with the Kruskal-Wallis test. The results are given as median + the interquartile range (IQR). Correlations were analyzed by calculating the Pearson correlation coefficient. A p-value of below 0.05 was considered as statistically significant. The Youden index (specificity + sensitivity −1) was employed for the identification of cut-off values, sensitivities and specificities were extracted from ROC (receiver operating characteristic) curves. Statistical analyses were performed with the following applications: WIZARD for MacOS (Version: 2.0.9, developer: Evan Miller, 2021) or Graphpad Prism® (Version 9.3.1).
Results
Baseline characteristics and outcomes
In total, 151 subjects were included in the study (females 62, males 89). The mean age of all individuals was 74.9 ± 13.4 years. The mean in-hospital treatment time was 16.2 ± 10.9 days. In-hospital mortality was 16.6%. Dialysis therapy became mandatory in 39.7%. Renal recovery occurred in 72.2%. All patient characteristics are summarized in Table I.
Etiology and severity of AKI
The most frequent AKI etiology was sepsis with 23.6%. Other etiologies were volume depletion; cardiorenal; contrast-induced (or associated); hepatorenal; drug-induced; obstruction; combined. More than 60% were diagnosed with AKIN (Acute Kidney Injury Network (28)) stage III (Tab. I).
Soluble ST2
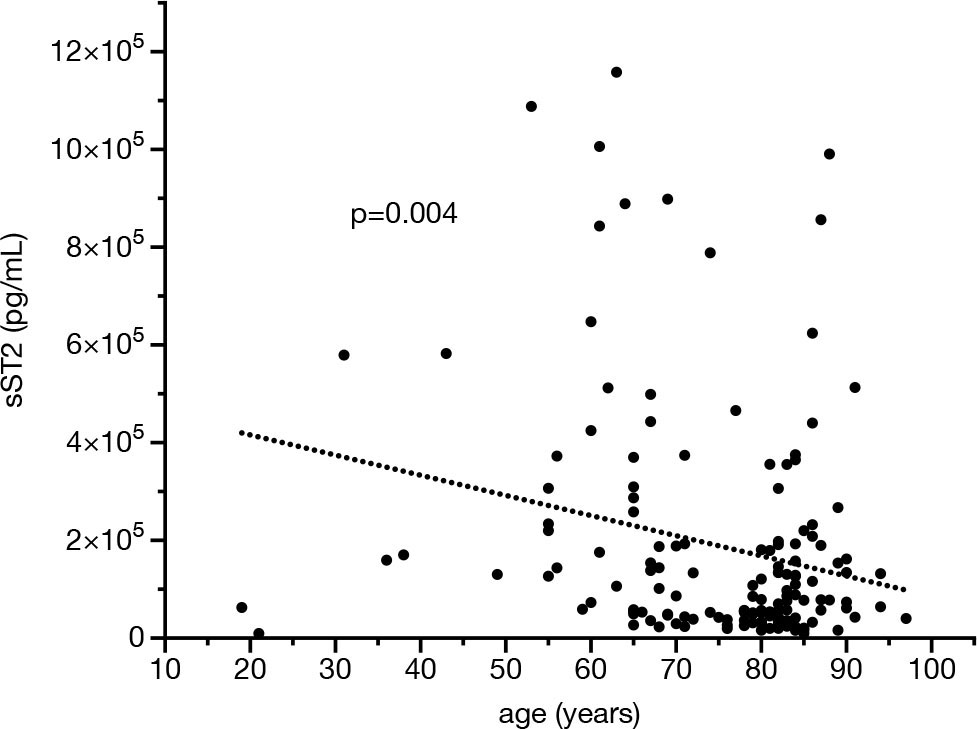
sST2 was quantified once, at the time of AKI diagnosis plus a maximum of 48 hours in some individuals. Serum ST2 levels correlated negatively with age (p = 0.004; r = −0.233) but not with the duration of in-hospital treatment (p = 0.228) (Fig. 1). Females did not significantly differ from males (p = 0.407). Only five patients were younger than 40 years. Out of these, only one subject showed sST2 levels of higher than 2 × 105 pg/mL as opposed to 25% of the individuals with age 60 or higher.
Endpoints
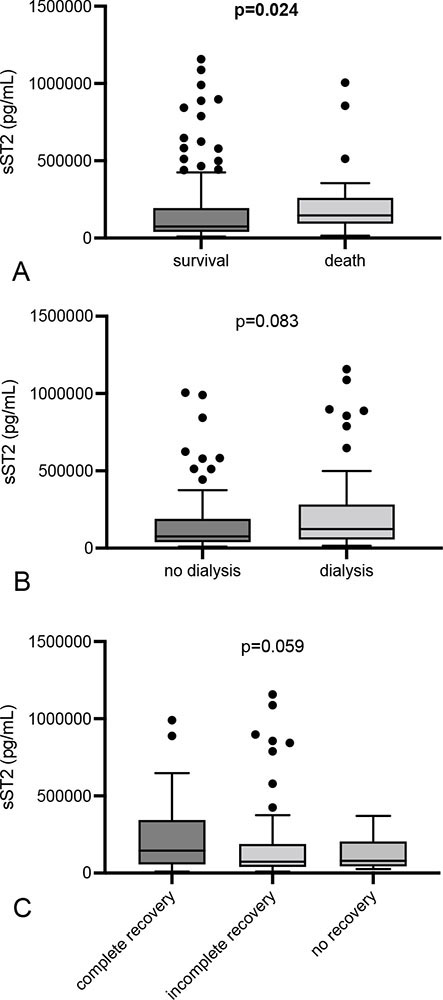
AKI patients with in-hospital death showed significantly higher serum sST2 at the time of diagnosis as compared to surviving subjects (146,100 [IQR 97,420-233,700] vs. 74,325 [IQR 40,030-192,900] pg/mL; p = 0.024) (Fig. 2). Additional analysis revealed a sST2 concentration of >86,110 pg/mL as cut-off (sensitivity 84%; specificity 55.56%). The risk of in-hospital death was 15.4% in subjects that reached the cut-off (prediction intervals 10.8%-21.4%). Patients requiring dialysis did not differ from those without the need for renal replacement therapy (123,550 [IQR 57,050-287,000] vs. 75,890 [IQR 38,560-189,600] pg/mL; p = 0.083) (Fig. 2). Subjects with renal recovery did not differ from patients without recovery (78,410 [IQR 43,520-192,900] vs. 132,900 [IQR 42,650-258,200] pg/mL; p = 0.48) (Fig. 2).
| Variable | Result |
|---|---|
| Age (years ± SD) | 74.9 ± 13.4 |
| Gender (females/males) | 62/89 |
| In-hospital treatment (days ±SD) | 16.2 ± 10.9 |
| AKI etiology (%) | |
| Sepsis | 23.6 |
| Volume depletion | 23.6 |
| Cardiorenal | 20.1 |
| Contrast-induced | 12.5 |
| Hepatorenal | 2.8 |
| Drug-induced | 1.4 |
| Postsurgery | 1.4 |
| Obstruction | 0.7 |
| Combined | 18.5 |
| Morbidities | |
| Preexisting CKD (%) | 73.5 |
| Arterial hypertension (%) | 88.4 |
| Diabetes mellitus (%) | 48 |
| Coronary artery disease (%) | 42.6 |
| Preexisting heart insufficiency (%) | 55.6 |
| Pulmonary disease (%) | 24.7 |
| Obesity (%) | 49 |
| History of neoplasia (%) | 27.8 |
| Dialysis initiated (%) | 39.7 |
| In-hospital death (%) | 16.6 |
| Recovery of kidney function (no/yes in %) | 27.8/72.2 |
| ICU treatment (%) | 32.5 |
| Ventilatory therapy (%) | 17.2 |
| Vasopressor therapy (%) | 16.6 |
Etiology and AKI stage
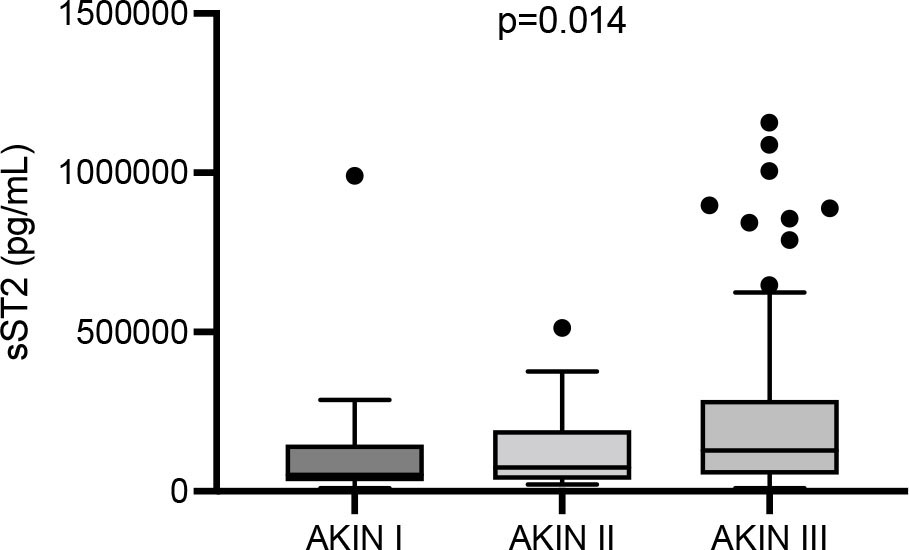
sST2 did not differ between all AKI types of a certain etiology (p = 0.2). The three most frequent entities (septic AKI; AKI due to volume depletion; cardiorenal AKI) were compared with all other entities combined. However, sST2 did not differ in any of the three analyses (septic AKI, p = 0.4; AKI due to volume depletion, p = 0.6; cardiorenal AKI, p = 0.39). sST2 significantly differed between the AKIN stages (28), the marker gradually increased from stage I to III (I: 51,830 [IQR 32,310-146,100] vs. II: 73,620 [IQR 35,650-191,200] vs. III: 128,500 [IQR 53,060-267,000] pg/mL; p = 0.014). The significance levels between AKIN stages I, II, and III were: I vs. II p = 0.99; II vs. III p = 0.35; I vs. III p = 0.01 (Fig. 3).
Morbidities
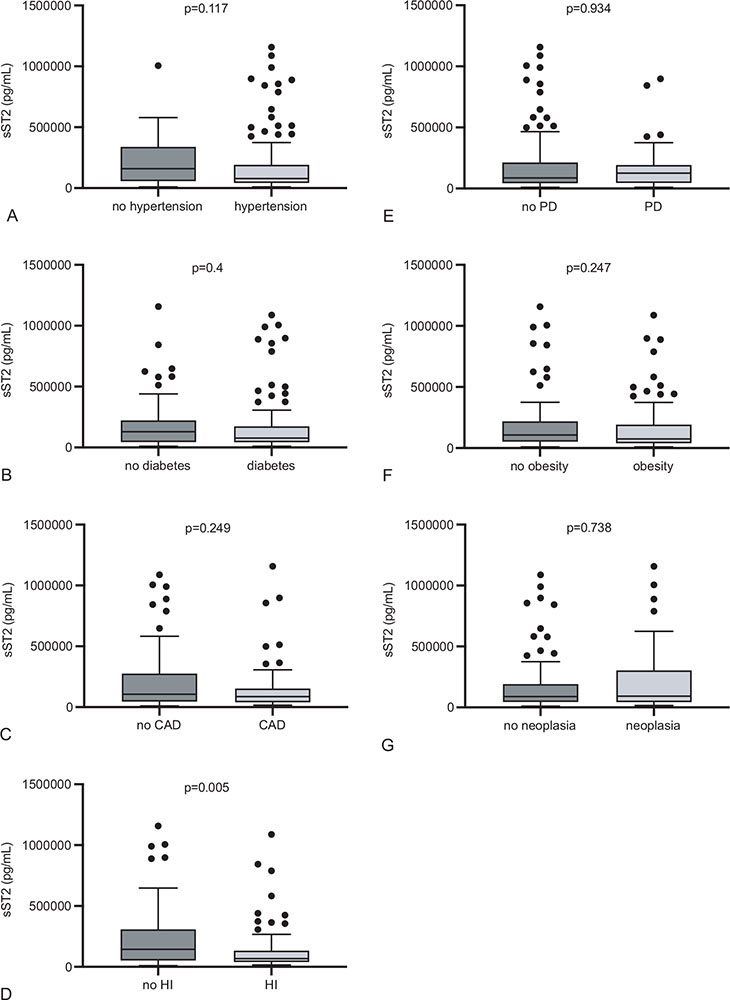
sST2 did not differ between patients with hypertension or diabetes mellitus as compared to subjects without the respective morbidity (p = 0.117 and p = 0.4). The same applied for pulmonary disease (one or more of the following diagnoses: chronic obstructive pulmonary disease, asthma, other), obesity, history of neoplasia, and coronary artery disease (p = 0.934, p = 0.247, p = 0.738, and p = 0.249). Patients with preexisting heart insufficiency, however, displayed lower serum sST2 than those without heart insufficiency (68,365 [IQR 39,280-134,000] vs. 144,850 [IQR 52,510-309,400] pg/mL; p = 0.005) (Fig. 4).
Treatment course
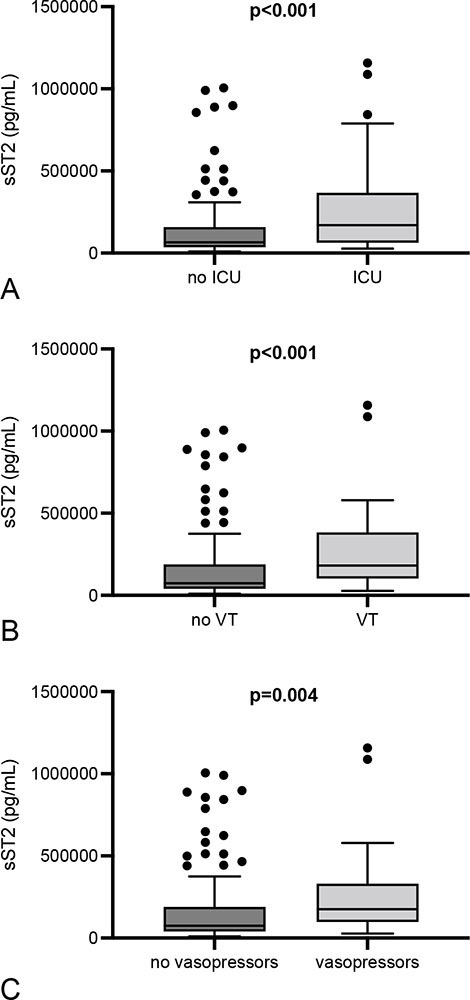
Patients that required treatment at the intensive care unit showed higher sST2 than subjects without ICU therapy (170,200 [IQR 63,970-364,800] vs. 65,745 [IQR 35,620-157,700] pg/mL; p < 0.001) (Fig. 3). Subjects that required ventilatory or vasopressor therapy displayed higher sST2 also (181,950 [IQR 107,700-369,800] vs. 73,620 [IQR 40,030-187,200] pg/mL; p < 0.001 and 175,500 [IQR 107,700-306,500] vs. 74,755 [IQR 40,030-188,400] pg/mL; p = 0.004) (Fig. 5).
Discussion
In the current study, we identified sST2 as novel predictor of survival in subjects with hospital-acquired AKI. To establish new biomarkers in AKI remains a fundamental goal in clinical nephrology. The majority of biomarker studies aimed (and still aim) to find parameters that allow AKI recognition as early as possible. Among the most widely studied molecules are Neutrophil Gelatinase-Associated Lipocalin (NGAL), Kidney Injury Molecule-1 (KIM-1), Liver-Fatty Acid Binding Protein (L-FABP), and the product of urinary Tissue Inhibitor of MetalloProtease-2 (TIMP-2) and Insulin-like Growth Factor-Binding Protein 7 (IGFBP7) (Schrezenmeier and colleagues provided an excellent summary (10).). Several studies additionally evaluated the prognostic value of certain marker molecules, particularly regarding the prediction of in-hospital survival. Hall and colleagues (29) found the urinary concentrations of NGAL, KIM-1, and IL-18 as predictive for the composite endpoint of AKI progression and in-hospital death. All markers were measured instantly if the AKI criteria were fulfilled. A 2011 published study evaluated both the diagnostic and prognostic potency of urinary NGAL (30). Subjects that reached the primary (composite) endpoint (AKI progression, dialysis, and death) showed higher NGAL levels at the time of inclusion. Survival prediction through both urinary NGAL and KIM-1 was also shown by Nickolas et al (31). Our findings did not only reveal higher sST2 in nonsurvivors but also gradually increased serum levels from AKI stages I to III according to KDIGO (12). Serum ST2 was also higher in subjects that either required vasopressors or ventilatory therapy or ICU treatment in general. Thus, elevated protein levels are apparently associated with a higher degree of cumulative morbidity under acute circumstances. Regarding permanent or preexisting diseases, the only difference occurred between subjects with versus without chronic heart insufficiency. Firouzabadi et al (16) failed to show higher or lower sST2 levels in heart insufficiency as opposed to healthy subjects. The individuals in this particular study did however not suffer from AKI. Regarding cardiac disease, soluble IL-33 receptor (sST2) has been shown to correlate with myocardial inflammation and fibrosis in rats with acute myocardial infarction (32).
In our study, the predictive value of sST2 was limited to the category survival. Tung and colleagues in contrast identified sST2 to be AKI predictive in patients with ST-segment elevation myocardial infarction (33). In our study cohort, subjects with versus without recovery of kidney function or dialysis did not differ in sST2. The recovery process was only assessed through serum creatinine, a marker that exclusively reflects the amount of glomerular filtration and by no means any adaptive or maladaptive responses within the renal tissue. Several studies included outcome analyses of post-AKI kidney function. Koyner and colleagues (34) identified IL-18, urinary albumin to creatinine ratio, and plasma NGAL to be associated with a higher risk of AKI progression. Comparable to our study, measurements were performed in close timely relation to AKI onset (at the day of AKI diagnosis, at least AKIN stage I). However, subjects exclusively received cardiac surgery. Caironi and colleagues (35) measured plasma proenkephalin A 119-159 (PenKid) in >900 septic subjects in order to identify associations with AKI onset and recovery of kidney (Albumin Italian Outcome Sepsis – ALBIOS – trial). Plasma PenKid was shown as useful not only in AKI but also in post-AKI recovery prediction. The most intriguing difference to our study was the inclusion of subjects with sepsis only. The same applies for the 2018 published Kid-SSS study (Kidney in Sepsis and Septic Shock study), which evaluated the same marker, measured within the first 24 hours after ICU admission (36). More than 580 were included. PenKid levels were associated with major adverse kidney events (MAKEs); low levels were suggestive for rapid recovery of kidney function. As opposed to sST2, PenKid reflects the amount of glomerular filtration with high sensitivity. In an observational cohort study, Schunk et al (37) measured the urinary Dickkopf-3 (DKK-3)/creatinine ratio in patients that received cardiac surgery. Some patients participated in the so-called “RenalRIP multicenter trial”. In this particular cohort, a urinary Dickkopf-3 (DKK-3)/creatinine ratio of >471 pg/mg was associated with higher risks for AKI and persistent renal dysfunction. DKK-3 is particularly secreted by stressed tubular epithelial cells (38). In the 2020 published RUBY study finally (39), urinary elevation of the C-C motif chemokine ligand 14 (CCL14) was shown to be predictive for persistent stage III AKI. In the same year, members of the “Acute Disease Quality Initiative Consensus Conference” published “Recommendations on Acute Kidney Injury Biomarkers” (40). Consensus statement number 9 suggests, “… novel biomarkers can be used for prediction of duration and recovery of AKI.” The recommendation received grade C (weak grade). Subsequently, the authors particularly discussed the PenKid and DKK-3 data.
Whether sST2 will presumably serve as marker of recovery prediction in AKI or not still needs to be elucidated more in detail. Herein, a heterogeneous group of AKI subjects was included, suffering from acute kidney dysfunction of various etiology. The data presented in the current study anyhow suggest a role of sST2 as biomarker of “acute distress”: it predicts post-AKI survival and substantially increases in subjects with a higher degree of cumulative morbidity under acute circumstances (e.g., ICU therapy, vasopressor administration). In this respect, two studies already evaluated the prognostic role of sST2 in sepsis (41,42).
The limitations of the current study shall be mentioned. Prehospital creatinine values were missing in many subjects. The AKI definition according to KDIGO (12) did not consider urine volumes since respective information was missing in too many individuals. Also, follow-up data after hospital demission were not available. Finally, it needs to be evaluated whether or not impaired kidney excretory function potentially modulates circulating sST2 per se. A recently initiated study in sepsis/septic shock will hopefully clarify this particular aspect.
In summary, sST2 may become clinically useful for risk stratification in AKI patients in the future. A respective study should therefore exclusively focus on AKI subjects treated under intensive care conditions. In any case, sST2 has for sure been identified as new candidate for risk prediction in AKI.
Acknowledgment
The authors thank Jana Friedrich for technical assistance.
Disclosures
Conflict of interest: The authors declare that they have no conflict(s) of interest.
Financial support: The study was supported by the Jackstädt-Stiftung.
Ethics statement: The study was formally approved by the ethics committee of the Medical School of Brandenburg (No.: E-01-20190820).
Author contributions: SE collected all samples and all patient-related clinical data. He also performed all measurements of sST2. He also assisted in writing. MH provided substantial knowledge and experimental expertise regarding quantification of sST2. SO provided substantial knowledge regarding quantification of sST2. KA helped to identify patients and collected patient-related clinical data. SP prepared figures and collected references. OR assisted in data analysis and manuscript writing. DP designed the study, provided funding, analyzed data, and wrote the manuscript. All authors approved the final version of the article.
References
- 1. Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607-625. CrossRef PubMed
- 2. Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol. 2012;7(4):533-540. CrossRef PubMed
- 3. Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25(6):1833-1839. CrossRef PubMed
- 4. Melo FAF, Macedo E, Fonseca Bezerra AC, et al. A systematic review and meta-analysis of acute kidney injury in the intensive care units of developed and developing countries. PLoS One. 2020;15(1):e0226325. CrossRef PubMed
- 5. Fields JK, Günther S, Sundberg EJ. Structural basis of IL-1 family cytokine signaling. Front Immunol. 2019;10:1412. CrossRef PubMed
- 6. Pichery M, Mirey E, Mercier P, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488-3495. CrossRef PubMed
- 7. Roussel L, Erard M, Cayrol C, Girard J-P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9(10):1006-1012. CrossRef PubMed
- 8. Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 axis in organ fibrosis. Front Immunol. 2018;9:2432. CrossRef PubMed
- 9. Cayrol C, Girard J-P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281(1):154-168. CrossRef PubMed
- 10. Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury – pathophysiological basis and clinical performance. Acta Physiol (Oxf). 2017;219(3):554-572. CrossRef PubMed
- 11. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. CrossRef PubMed
- 12. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672. CrossRef PubMed
- 13. Akcay A, Nguyen Q, He Z, et al. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol. 2011;22(11):2057-2067. CrossRef PubMed
- 14. Ferhat M, Robin A, Giraud S, et al. Endogenous IL-33 contributes to kidney ischemia-reperfusion injury as an alarmin. J Am Soc Nephrol. 2018;29(4):1272-1288. CrossRef PubMed
- 15. Chen Z, Hu Q, Huo Y, Zhang R, Fu Q, Qin X. Serum interleukin-33 is a novel predictive biomarker of hemorrhage transformation and outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2021;30(2):105506. CrossRef PubMed
- 16. Firouzabadi N, Dashti M, Dehshahri A, Bahramali E. Biomarkers of IL-33 and sST2 and lack of association with carvedilol therapy in heart failure. Clin Pharmacol. 2020;12:53-58. CrossRef PubMed
- 17. Behairy OG, Elsadek AE, Behiry EG, Elhenawy IA, Shalan NH, Sayied KR. Clinical value of serum interleukin-33 biomarker in infants with neonatal cholestasis. J Pediatr Gastroenterol Nutr. 2020;70(3):344-349. CrossRef PubMed
- 18. Erfurt S, Hoffmeister M, Oess S, et al. Serum IL-33 as a biomarker in different diseases: useful parameter or much need for clarification? J Circ Biomark. 2021;10:20-25. CrossRef PubMed
- 19. Ketelaar ME, Nawijn MC, Shaw DE, Koppelman GH, Sayers I. The challenge of measuring IL-33 in serum using commercial ELISA: lessons from asthma. Clin Exp Allergy. 2016;46(6):884-887. CrossRef PubMed
- 20. Asaka D, Yoshikawa M, Nakayama T, Yoshimura T, Moriyama H, Otori N. Elevated levels of interleukin-33 in the nasal secretions of patients with allergic rhinitis. Int Arch Allergy Immunol. 2012;158(s1)(suppl 1):47-50. CrossRef PubMed
- 21. Vyshnevska I, Kopytsya M, Hilоva Y, Protsenko E, Petyunina O. Biomarker SST2 as an early predictor of acute renal injury in patients with ST-segment elevation acute myocardial infarction. Georgian Med News. 2020;(302):53-58. PubMed
- 22. Lobdell KW, Parker DM, Likosky DS, et al. Preoperative serum ST2 level predicts acute kidney injury after adult cardiac surgery. J Thorac Cardiovasc Surg. 2018;156(3):1114-1123.e2. CrossRef PubMed
- 23. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Karger Publishers; 2012. CrossRef
- 24. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-810. CrossRef PubMed
- 25. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527-1539. CrossRef PubMed
- 26. Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164-176. CrossRef PubMed
- 27. Fiorentino M, Tohme FA, Wang S, Murugan R, Angus DC, Kellum JA. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One. 2018;13(6):e0198269. CrossRef PubMed
- 28. Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committe. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(5):1569-1574. CrossRef PubMed
- 29. Hall IE, Coca SG, Perazella MA, et al. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6(12):2740-2749. CrossRef PubMed
- 30. Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80(4):405-414. CrossRef PubMed
- 31. Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012 Jan 17;59(3):246-255. CrossRef PubMed
- 32. Sánchez-Más J, Lax A, Asensio-López MC, et al. Modulation of IL-33/ST2 system in postinfarction heart failure: correlation with cardiac remodelling markers. Eur J Clin Invest. 2014;44(7):643-651. CrossRef PubMed
- 33. Tung YC, Chang CH, Chen YC, Chu PH. Combined biomarker analysis for risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. PLoS One. 2015;10(4):e0125282. CrossRef PubMed
- 34. Koyner JL, Garg AX, Coca SG, et al; TRIBE-AKI Consortium. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905-914. CrossRef PubMed
- 35. Caironi P, Latini R, Struck J, et al; ALBIOS Study Investigators. Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem. 2018;64(9):1361-1369. CrossRef PubMed
- 36. Hollinger A, Wittebole X, François B, et al. Proenkephalin A 119-159 (Penkid) is an early biomarker of septic acute kidney injury: the Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018;3(6):1424-1433. CrossRef PubMed
- 37. Schunk SJ, Zarbock A, Meersch M, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394(10197):488-496. CrossRef PubMed
- 38. Fang X, Hu J, Chen Y, Shen W, Ke B. Dickkopf-3: current knowledge in kidney diseases. Front Physiol. 2020;11:533344. CrossRef PubMed
- 39. Hoste E, Bihorac A, Al-Khafaji A, et al; RUBY Investigators. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. 2020;46(5):943-953. CrossRef PubMed
- 40. Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3(10):e2019209. CrossRef PubMed
- 41. Hur M, Kim H, Kim HJ, et al; GREAT Network. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med. 2015;35(6):570-577. CrossRef PubMed
- 42. Hoogerwerf JJ, Tanck MWT, van Zoelen MAD, Wittebole X, Laterre P-F, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36(4):630-637. CrossRef PubMed