 |
J Circ Biomark 2021; 10: 26-29 ISSN 1849-4544 | DOI: 10.33393/jcb.2021.2329 ORIGINAL RESEARCH ARTICLE |
 |
Pharmacokinetics of the disialoganglioside, GD2, a circulating tumor biomarker for neuroblastoma, in nonhuman primates
ABSTRACT
Background: The ganglioside GD2 is a potential circulating tumor biomarker for the childhood cancer neuroblastoma. Interpreting the levels of a circulating tumor biomarker depends in part on a knowledge of the biomarker’s clinical pharmacology.
Methods: We studied the plasma and cerebrospinal fluid (CSF) pharmacokinetics of the C18 lipoform of GD2 in two nonhuman primates with indwelling subcutaneous CSF lateral ventricular reservoir systems. GD2 was quantified with a validated high-performance liquid chromatography (HPLC)/tandem mass spectrometry assay. GD2 was administered as a short intravenous infusion and frequent plasma and CSF samples were drawn over 72 hours.
Results: GD2 plasma concentration declined monoexponentially with a half-life of 16 hours. Clearance was 0.0136 and 0.0131 L/h and volume of distribution (Vd) was 0.035 and 0.038 L/kg in the two animals. Vd was equivalent to plasma volume. Greater than 98% of GD2 in plasma is in a bound form consistent with its known association with lipoproteins and accounting for its limited volume of distribution. GD2 did not cross over from plasma into the CSF.
Conclusions: The pharmacokinetic profile of GD2 is favorable for a circulating tumor biomarker. This study demonstrates the value of characterizing the clinical pharmacology of circulating biomarkers to better understand their clinical behavior.
Keywords: Biomarker, Ganglioside, Neuroblastoma, Pharmacokinetics
Received: August 6, 2021
Accepted: November 22, 2021
Published online: December 3, 2021
Journal of Circulating Biomarkers - ISSN 1849-4544 - www.aboutscience.eu/jcb
© 2021 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
The ganglioside GD2 is a constituent of the plasma membrane of neuronal cells and is also expressed on the surface of the childhood cancer, neuroblastoma, and other cancers of neuroectodermal origin, such as melanoma. GD2 has a lipid domain (ceramide) that inserts into the plasma membrane and a 5-membered glycan domain that contributes to the glycocalyx on the cell surface. The glycan domain contains 2 sialic acid groups that are fully ionized in the physiological pH range.
GD2 circulates in low nanomolar concentrations in children, but its concentration is 30-fold elevated compared to controls in the serum of children with high-risk/high-stage neuroblastoma (1). We developed and validated a sensitive and specific high-performance liquid chromatography (HPLC)/tandem mass spectrometry assay to quantify the circulating lipoforms of GD2, and we are evaluating GD2 as a circulating tumor biomarker for neuroblastoma. The C18 lipoform (18-carbon fatty acid chain length in ceramide) is the predominant form of GD2 in the plasma of patients with neuroblastoma.
Interpreting the results of a circulating tumor biomarker depends in part on a knowledge of the biomarker’s clinical pharmacology. The steady-state circulating concentration of a tumor biomarker is determined by its rate of production by the tumor and by its clearance. Slow clearance results in accumulation of the biomarker in the plasma and enhances the sensitivity for detecting low tumor burden. However, a slowly cleared biomarker is not responsive to rapid changes in tumor burden (e.g., surgical resection). For example, an α-fetoprotein (AFP) concentration exceeding 100,000 ng/mL can take more than 2 months to fall into the reference range after complete resection of an AFP-producing tumor. Conversely, a rapidly eliminated tumor biomarker will not accumulate in the circulation, but its concentration may better reflect changes in tumor burden in real time.
We studied the pharmacokinetics and cerebrospinal fluid (CSF) penetration of GD2 after intravenous administration of the ganglioside to two nonhuman primates (NHPs) with indwelling subcutaneous CSF lateral ventricular reservoirs that allow for rapid, serial CSF sampling (2).
Methods
Chemicals
Purified human brain-derived GD2 was purchased from EMD Millipore Corp. (Billerica, MA) and contains two dominant lipoforms of GD2—D18: 1-18:0 (C18, molecular weight 1674.9 g/mol) and D18: 1-20:0 (C20). GD2 was dissolved in a small volume of dimethyl sulfoxide (DMSO), diluted in sterile normal saline, and filter sterilized through a 22-micron filter. A sample of the filtered drug solution was assayed for content of the C18 lipoform of GD2 to quantify the administered dose.
Animals
This study was approved by the National Cancer Institute Animal Care and Use Committee. Two adult male rhesus monkeys (Macaca mulatta), weighing 8.0 and 8.6 kg, respectively, were humanely utilized for this study and were cared for in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, 8th edition (3). Animals were socially housed when possible. Both subjects had previously undergone implantation of an indwelling lateral ventricular catheter, which was attached to a subcutaneously implanted CSF reservoir (2). The subjects also had subcutaneously implanted femoral venous access ports for sampling blood. Each subject had a veterinary physical and neurological examination and blood chemistries and complete blood counts performed prior to GD2 administration to ensure they were physiologically and neurologically within normal limits. After GD2 administration the subjects were observed for adverse events daily for 2 weeks and had biweekly clinical chemistries and complete blood counts.
GD2 was administered as a 10-minute intravenous infusion through a temporary catheter in the cephalic or saphenous vein. Blood was serially sampled from the femoral venous access port and CSF was sampled from the subcutaneous CSF reservoir.
Sample times and sample processing
Blood (3 mL) and CSF (0.3 mL) were collected prior to the GD2 infusion, at the end of the 10-minute infusion, and 0.25, 1, 2, 4, 6, 8, 24, 48, and 72 hours postinfusion. Blood was collected in heparinized tubes and placed on ice. Plasma was separated by centrifugation at 4°C. Plasma and CSF were stored frozen at −80°C until assayed.
Sample analysis
The concentration of the C18 lipoform of GD2 in plasma and CSF samples was quantified using a previously reported, validated HPLC/tandem mass spectrometry method with a lower limit of quantification of 3 nM (4). Human brain-derived GD2, which is made up of approximately 60% C20 and 40% C18 lipoforms, was used to construct the standard curves for the assay.
Pharmacokinetic analysis
A one-compartment pharmacokinetic model with first-order elimination was individually fit to the plasma concentration-time data from the 2 subjects using Phoenix NLME v.8.3 (Certara, Princeton, NJ). Model parameters are clearance (CL) and volume of distribution (Vd). The elimination rate constant (kel) was derived from CL/Vd, the half-life from 0.693/kel, the area under the concentration-time curve (AUC) from dose/CL, and the mean residence time (MRT) from 1/kel.
Protein binding
Human plasma was spiked with human brain-derived GD2 to achieve a 200 nM concentration of the C18 lipoform. Spiked plasma was centrifuged through a Vivaspin 500 concentrator with a 300,000 molecular weight cutoff (MWCO) polyethersulfone (PES) membrane (Sartorius, Goettingen, Germany). GD2 concentration was measured in the concentrate that remained above the PES membrane and the effluent that passed through the PES membrane.
Results
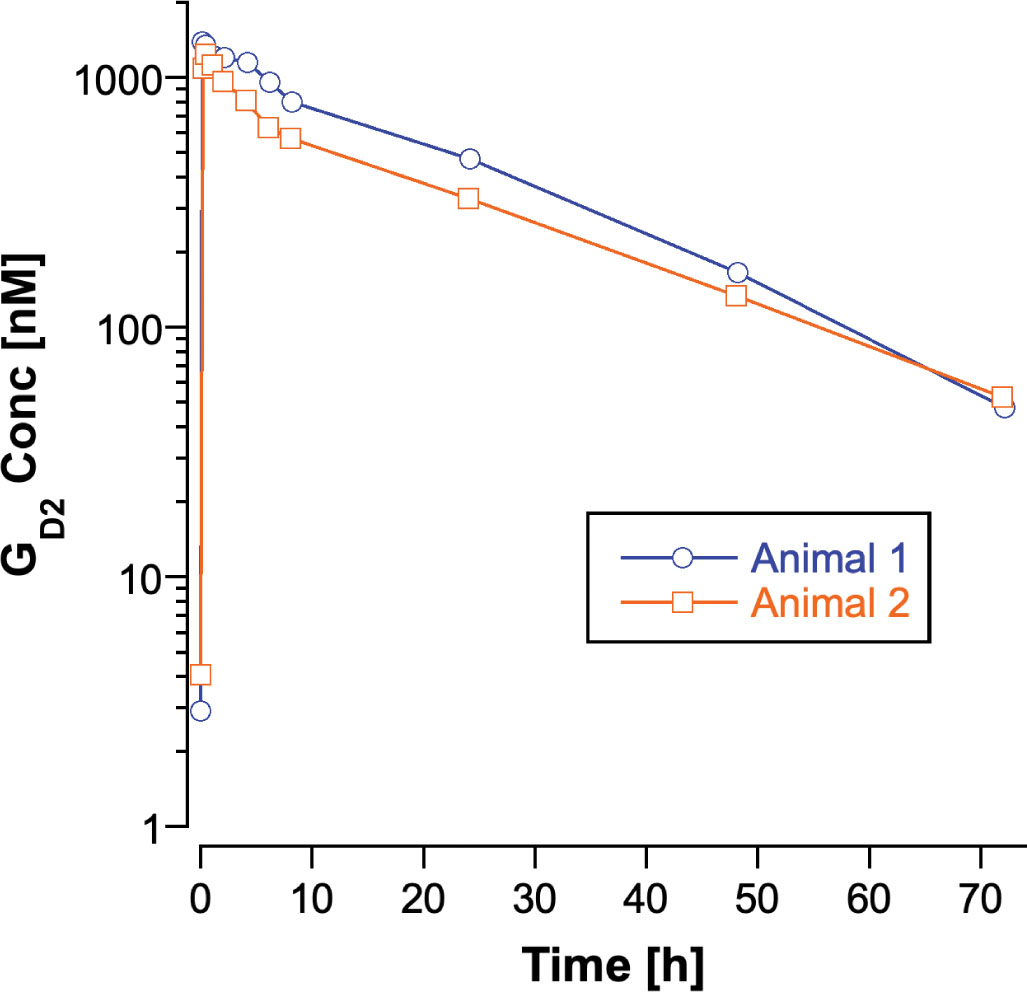
The predose concentrations of C18 GD2 in the 2 subjects were 5.0 and 3.7 nM in plasma and 4.0 and 8.9 nM in CSF. The predose plasma concentrations in the subjects are similar to GD2 concentrations in control human plasma (1). End-of-infusion GD2 plasma concentrations were 1,390 and 1,090 nM, and concentrations declined in the plasma in a mono-exponential fashion (Fig. 1). Pharmacokinetic parameters for C18 GD2 for the 2 subjects are listed in Table I. The administered dose of GD2 was estimated from the volume of drug solution administered and the concentration of C18 GD2 in the postfiltration drug solution. Intersubject variability in the pharmacokinetic parameters was minimal. The volume of distribution of GD2 approximated plasma volume (blood volume in adult rhesus macaques is 0.062 L/kg (5)). Clearance of GD2 from the circulation was slow with a half-life of 16 hours.
| Animal | Weight (kg) | Dose (nmol) | Volume of Distribution (L/kg) | Clearance (L/h) | kel (h−1) | AUC (nM × h) | Half-life (h) | MRT (h) |
|---|---|---|---|---|---|---|---|---|
| 1 (ZB39) | 8.6 | 402 | 0.0354 | 0.0136 | 0.0448 | 29,500 | 15.5 | 22.3 |
| 2 (ZJ57) | 8.0 | 313 | 0.0384 | 0.0131 | 0.0427 | 23,800 | 16.2 | 23.4 |
kel = first-order elimination rate constant; AUC = area under the concentration-time curve; MRT = mean residence time.
C18 GD2 concentrations remained at or near baseline levels in CSF throughout the 72-hour sampling period. The peak CSF concentration in animal 1 was 9.0 nM at the end of the infusion (baseline concentration was 4.0 nM), and CSF concentrations in animal 2 did not exceed the baseline concentration of 8.9 nM.
More than 98% of C18 GD2 in human plasma (200 nM) was retained in the plasma concentrate (above the membrane) after centrifugation through the Vivaspin 500 concentrator with a 300,000 MWCO PES membrane. The effluent GD2 concentration was 4.5 nM after 15 minutes of centrifugation.
Conclusions
The disialoganglioside GD2 is expressed in the cell membrane of neuroblastoma tumor cells and is shed into the circulation in patients with high-risk/high-stage disease (1,6). Prospective studies are ongoing to assess its potential utility as a circulating tumor biomarker for high-risk neuroblastoma. We characterized the pharmacokinetics of GD2 in a NHP CSF access model that has proven to be predictive of human plasma and CSF disposition for a wide variety of drugs (7) in order to better understand the clinical behavior of circulating GD2. The pharmacokinetic parameters from this study should prove useful for interpreting serial GD2 concentrations monitored over the course of a patient’s disease.
The pharmacokinetic characteristics of GD2 are favorable for a circulating tumor biomarker. The 16 hours half-life in plasma indicates that GD2 should be rapidly responsive to changes in tumor burden. After treatment, a new steady-state concentration should be reached within 3 to 4 days (5 half-lives), suggesting that plasma GD2 concentration could be useful for assessing treatment effect in near real time. The limited volume of distribution, which is equivalent to plasma volume in NHPs, enhances sensitivity because the GD2 is concentrated in (limited to) the compartment from which it is being measured. If GD2 were more widely distributed throughout the body, the concentration in plasma would be proportionally lower.
Ultrafiltration of plasma spiked with GD2 showed that it circulates in a bound form with a large molecular weight. This is consistent with the previously reported association of GD2 and other gangliosides with lipoproteins, which have molecular weights in excess of 3,000 kDa (8). GD2 is not detectable in lipoprotein-depleted plasma and is primarily associated with low-density lipoproteins (LDLs) (8). Binding of GD2 to LDL and, to a lesser extent, other lipoproteins accounts for the volume of distribution being limited to plasma volume, and likely also accounts for the lack of GD2 penetration into the CSF in the NHP CSF access model. Detecting GD2 in the CSF of patients with neuroblastoma could be an indicator of brain or meningeal tumor spread even in the presence of high plasma GD2 concentrations.
The anti-GD2 monoclonal antibody, dinutuximab, is a component of the frontline treatment of neuroblastoma. Circulating GD2 could theoretically bind to dinutuximab and block the binding of the antibody to GD2 on the surface of tumor cells. Dinutuximab binds to the glycan portion of GD2 that resides on the cell surface. The configuration of GD2 in LDL is likely to be similar to that in the cell membrane with the polar glycan portion on the surface of the lipoprotein complex and the ceramide portion extending into the nonpolar core. Therefore, even though GD2 is essentially all bound to lipoproteins in the circulation, the antigenic glycan portion may still be exposed on the surface for binding to dinutuximab.
Dinutuximab immunotherapy is currently administered in the final phase of neuroblastoma therapy, when plasma GD2 concentrations are likely to be low, but pilot studies are ongoing to investigate the use of dinutuximab in the initial phase of therapy when circulating GD2 concentrations are likely to be higher in some patients. Binding of the antibody to lipoprotein-associated GD2 could lower the efficacy of dinutuximab by limiting the amount of antibody available to bind to tumor cells.
The pharmacokinetic profile of GD2 is favorable for a circulating tumor biomarker. With its relatively short half-life, plasma GD2 concentrations should reflect changes in tumor burden with a minimal lag time, and the limited volume of distribution translates into higher concentrations in plasma, improving its sensitivity. This study demonstrates the value of characterizing the clinical pharmacology of circulating biomarkers to better understand their clinical behavior. The use of the NHP model that is predictive of human pharmacokinetics provided the opportunity to study the pharmacokinetics of GD2 after administration of a known dose and without interference from endogenous production. We plan to confirm the results using a more limited sampling approach in children with neuroblastoma after definitive treatment.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This project was funded by Alex’s Lemonade Stand Center of Excellence in Drug Development and Clinical Pharmacology Award and a grant from Cure Childhood Cancer.
References
- 1. Balis FM, Busch CM, Desai AV, et al. The ganglioside GD2 as a circulating tumor biomarker for neuroblastoma. Pediatr Blood Cancer. 2020;67(1):e28031. CrossRef PubMed
- 2. Lester McCully CM, Bacher J, MacAllister RP, et al. Development of a cerebrospinal fluid lateral reservoir model in rhesus monkeys (Macaca mulatta). Comp Med. 2015;65(1):77-82. PubMed
- 3. Guide for care and use of laboratory animals. 8th ed. Washington, DC: The National Academies Press 2011. Online
- 4. Busch CM, Desai AV, Moorthy GS, Fox E, Balis FM. A validated HPLC-MS/MS method for estimating the concentration of the ganglioside, GD2, in human plasma or serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1102-1103:60-65. CrossRef PubMed
- 5. Hobbs TR, Blue SW, Park BS, Greisel JJ, Conn PM, Pau FK. Measurement of blood volume in adult rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2015;54(6):687-693. PubMed
- 6. Ladisch S, Wu ZL, Feig S, et al. Shedding of GD2 ganglioside by human neuroblastoma. Int J Cancer. 1987;39(1):73-76. CrossRef PubMed
- 7. Baratz E, McCully C, Shih J, Warren K. Comparison of pharmacokinetic parameters between non-human primates and human patients. Neuro-oncol. 2018;20(suppl 2):i157. CrossRef
- 8. Valentino LA, Ladisch S. Localization of shed human tumor gangliosides: association with serum lipoproteins. Cancer Res. 1992;52(4):810-814. PubMed