 |
Glob Reg Health Technol Assess 2025; 12: 9-15 ISSN 2283-5733 | DOI: 10.33393/grhta.2025.3199 ORIGINAL RESEARCH ARTICLE |
 |
Value contribution of leniolisib in the Treatment of Activated PI3Kδ syndrome (APDS) in Spain using Multi-Criteria Decision Analysis (MCDA)
ABSTRACT
Background: Activated phosphoinositide 3-kinase (PI3K) δ Syndrome (APDS) is an ultra-rare, potentially life-threatening disease that lacks approved treatments in Spain. This study aimed to apply Multi-Criteria Decision Analysis (MCDA) to assess the value of the first pharmacological treatment for APDS in Spain.
Methods: A multidisciplinary group of 8 experts evaluated the selective PI3Kδ inhibitor leniolisib against Standard of Care (SoC). An MCDA framework tailored for Orphan Drugs (ODs), consisting of 5 comparative and 2 quantitative non-comparative criteria, was used. Re-scoring followed a group discussion.
Results: Leniolisib scored higher than SoC in all criteria, including efficacy and safety. It was deemed highly valuable as the first disease-modifying treatment, with a positive therapeutic impact and potential to improve patients’ quality of life. Additionally, leniolisib may lead to cost savings. The supporting data was considered of high quality.
Conclusion: Based on MCDA methodology and stakeholder experience in APDS management, leniolisib is seen as a value-added treatment option compared to SoC in Spain.
Keywords: Activated phosphoinositide 3-kinase (PI3K) δ Syndrome (APDS), Decision-making, Multi-criteria Decision Analysis (MCDA), Ultra-rare disease, leniolisib
Received: July 9, 2024
Accepted: January 2, 2024
Published online: January 27, 2025
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2025 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Activated phosphoinositide 3-kinase delta syndrome (APDS) is a hereditary (autosomal dominant) ultra-rare disease, with an estimated prevalence of less than 1 per million worldwide (1-5). The disease was first described in 2013 with scarce published evidence available, as well as lack of guidelines to guide and harmonize clinical practice.
Multi-criteria decision analysis (MCDA) is an application of analytical methods capable of explicitly, objectively, systematically, and weighted consideration of various factors, using multiple criteria for decision-making. The aim is to obtain the overall value of the drug in an orderly, objective, pragmatic, and transparent manner (6,7).
Reflective MCDA methodology has been recently used to determine key value drivers in the treatment of APDS in Spain, providing a standardized MCDA framework to aid stakeholders in assessing the value contribution of any treatment directed to these patients (8).
The experts considered APDS to be a severe disease, linked to increased mortality risk, with high unmet needs, including scarcity of guidelines or protocols to guide disease management and the scarcity of disease-modifying treatments (9,10), limiting pharmacological therapeutic options currently available for symptomatic relief of immune deficiency and immune dysregulation-related symptoms treatments (9,11). Therapy is established on a patient-by-patient basis according to clinical symptomatology and includes antimicrobials, Immunoglobulin Replacement Therapy (IGRT) and off-label immunosuppressants and immunomodulators (i.e. corticosteroids, rituximab or sirolimus). To date, Hematopoietic Stem Cell Transplantation (HSCT) represents the only potentially curative treatment; however, in a recently published cohort by the ESID registry, only 17% of the patients underwent HSCT treatments (9). Clinical evidence (including long-term efficacy) is limited, and the most appropriate HSCT approach for APDS patients has not yet been fully defined. The study identified that current treatments present limited efficacy and relevant safety and tolerability issues that can lead to treatment discontinuation. The quality of evidence supporting current SoC was identified as another relevant gap, as available data are based on clinical experience and not on formal regulatory or published evidence. This was also reflected in the therapeutic impact criterion, which was considered moderate as resulting clinical outcomes were considered suboptimal.
APDS has been described as a high-cost disease, including pharmacological costs due to the use of combined therapies (including immunoglobulin replacement and hematopoietic stem-cell transplant), meaningful direct medical costs (high use of healthcare resources related to hospitalizations and management of complications) and indirect costs (burden assumed by patients and caregivers derived from recurrent hospital visits or disease complications, study and work absence, loss of productivity).
Current understanding of the etiopathogenesis of APDS supports the potential use of targeted therapy in the form of selective PI3Kδ inhibitors (8). Leniolisib (Pharming NV, Netherlands) is an oral, PI3Kδ selective inhibitor that specifically targets the causative factor resulting in the pathogenesis of the disease (12), addressing both immunodeficiency and immune dysregulation by restoring signaling homeostasis, normalizing immunophenotypes and reducing lymphoproliferation (12,13).
Leniolisib would represent the first approved therapy addressing the underlying cause of APDS and potentially modifying the long-term course of the disease (12-15). Leniolisib has been approved by the Food and Drug Administration (FDA) for the treatment of adult and pediatric (12 years of age and older) APDS patients (16), and it is currently undergoing evaluation by the European Medicines Agency (EMA) (17).
The aim of this study was to determine the relative value contribution of leniolisib in the treatment of APDS compared with SoC in Spain using a reflective MCDA-based methodology.
Methods
Study design and Evidence matrix development
The study was conducted between November 2023 and March 2024. It was designed following good MCDA methodological practices (18,19) and steps: 1. Defining the decision problem, 2. Selecting and structuring criteria, 3. Measuring performance, 4. Scoring alternatives, 5. Weighting criteria, 6. Calculating aggregate scores, 7. Dealing with uncertainty, 8. Reporting and examining of findings.
The OD-MCDA evidence matrix is a value determination framework incorporating criteria and attributes adapted to the particularities of the ODs and reflecting the decision-makers’ objectives and concerns, the selection of alternatives, and the obtention of evidence for the results of the alternatives for the selected criteria (Supplementary material) (20). The scoring is the assessment of the performance of treatment options against the identified criteria (20).
The process of assigning relative importance to each criterion in the MCDA matrix is called weighting. Experts’ appraisal requires the obtention of an indicator of added value from the combination of scores and weights. The criteria weighting step was excluded from the exercise and the well-established weighting by 98 evaluators and decision-makers from national and international organizations was used (21).
The OD MCDA framework used in this study is a specific evaluation framework developed from the EVIDEM framework (22) and validated by Spanish stakeholders involved in the evaluation of ODs and decision-making at the national, regional, and hospital levels (23). Since leniolisib had no price established in Spain at the time of the study, the pharmacological cost criterion was excluded from the framework. The non-comparative impact of the disease domain and the contextual criteria were also excluded since they had been previously scored in the previous APDS MCDA study (8).
The final MCDA framework used in this study consists of 4 domains, as shown in Table 1: Outcomes of intervention (3 criteria), type of benefit of intervention (1 criterion), economic consequences of intervention (2 criteria), and knowledge of intervention (1 criterion).
| Quantitative criteria |
|---|
|
Domain – Outcomes of intervention:
|
|
Domain - Type of benefit of intervention:
|
|
Domain – Economic consequences of intervention:
|
|
Domain – Knowledge of intervention:
|
The “Non-comparative criteria” scoring scale ranged from 0 to 5 (where 0 is the worst possible score and 5 is the best). Comparative criteria (efficacy/effectiveness, safety/tolerability, Patient Reported Outcomes (PROs), and economic) were scored on a scale ranging from −5 (leniolisib much worse compared to SoC) to +5 (leniolisib much better than SoC). Contextual criteria were scored using a three-point qualitative scale: positive, neutral, or negative. Reflections behind experts’ scores were collected in a qualitative manner.
Comparator selection
The relative value contribution of leniolisib was determined compared to the SoC. The therapeutic approach is individualized per patient and heterogeneous across centers, without an established, single reference SoC reported in the literature. Hence the SoC definition used in the clinical development program of leniolisib was used (24), including: immunoglobulins and immunomodulators (sirolimus, rituximab).
Literature review and matrix evidence development
A comprehensive literature review was performed in November 2023 according to a protocol with inclusion and exclusion criteria and according to the information needed to fill the MCDA matrix. Articles identified through the search were screened by title and abstract. Those articles that did not meet inclusion criteria were excluded. A full-text assessment was performed with those remaining (Fig. 1).
Published evidence was searched using the biomedical databases MEDLINE, Cochrane, and MEDES, and included languages were English and Spanish. It was complemented using grey literature sources such as Google Scholar, patient association websites, and available documents from official sources (e.g., EMA) Scientific Societies and Patient Associations web pages. No date restrictions were applied.
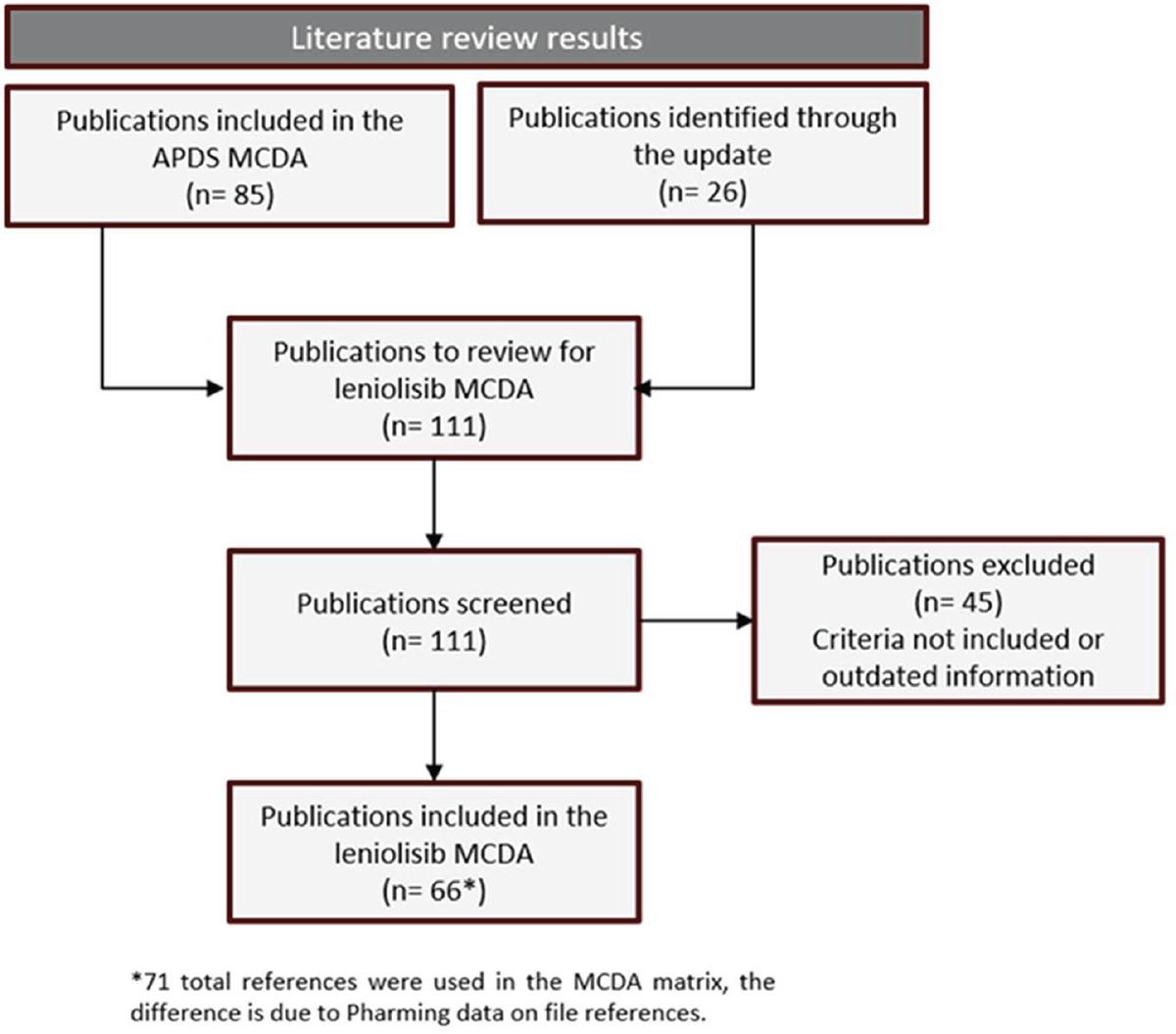
FIGURE 1 - Flowchart of the literature review.
The information extracted from the literature review was used to populate the MCDA matrix.
Expert panel design
The study was carried out through a multidisciplinary group of 8 experts (2 pediatric and 1 adult immunologist, 3 hospital pharmacists, 1 clinical pharmacology physician, and 1 ex-regional payer) from 6 different regions and 8 hospitals with the objective of collecting a wide range of perspectives. The experts are nationally recognized for their broad experience in the management of APDS and/or decision-making in Spain.
Experts received training on MCDA methodology being instructed to score each criterion individually, based on the information presented in the evidence matrix and their own experience and perspective.
Data analysis
Both initial and final value scores (before and after group discussion, respectively) and their relevant rationales were collected individually from each participant, transferred to a common database, and analyzed using Microsoft Excel software. For each quantitative criterion, the mean, Standard Deviation (SD), and the range of minimum and maximum scores were calculated. Comments and reflections on participants’ scores were analyzed and discussed in a qualitative manner. Results are shown as a percentage of experts who would consider that the drug would have a negative, neutral, or positive impact, according to each contextual criterion definition.
Results
Quantitative criteria scoring
Results of the quantitative criteria scoring are shown in Figure 2 (comparative criteria) and Figure 3 (non-comparative).
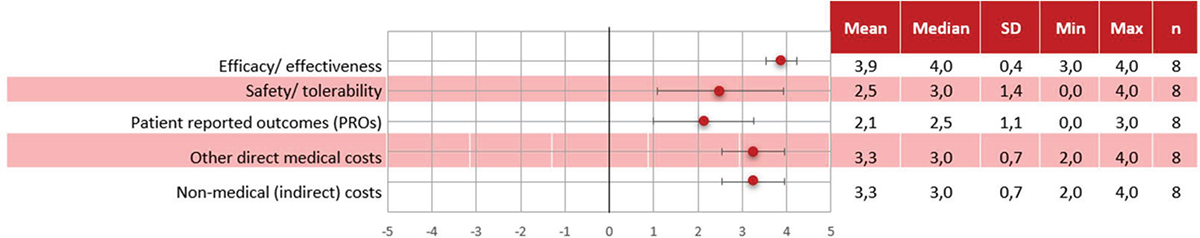
FIGURE 2 - Scoring results – Quantitative comparative criteria.
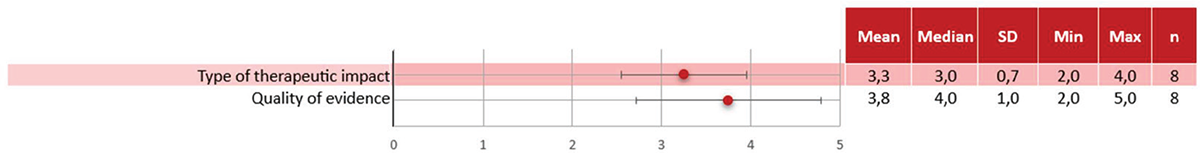
FIGURE 3 - Scoring results – Qualitative non-comparative criteria.
Leniolisib obtained positive and higher mean scores than SoC in all criteria. “Efficacy/effectiveness” achieved the highest score and with a high degree of concordance (3.9 ± 0.4). “Safety/tolerability” was considered positive although with low concordance (2.5 ± 1.4). “PROs” was also scored positively with a low level of concordance (2.1 ± 1.1). Leniolisib achieved a high score for “Other medical costs” with a moderate level of concordance among experts (3.3 ± 0.7) and “Non-medical (indirect) costs” also received a high score with a moderate level of concordance (3.3 ± 0.7).
Regarding the quantitative non-comparative criteria, “Type of therapeutic impact” was considered positive with a moderate level of concordance (3.3 ± 0.7) and “Quality of evidence” achieved a high score with a low level of concordance (3.8 ± 1.0).
Analyzing the results considering the different expert profiles, and although scoring was very similar across participants, some differences can be observed in the appreciation of the value contribution between clinicians and evaluators, as shown in Figure 4. The main differences in scoring were observed in the following criteria: “Safety/tolerability” received higher score from the clinicians (3.3) than from the evaluators (2.0); “PROs” received higher scoring from clinicians (3.0) than from evaluators (1.3); “Type of therapeutic impact” received a higher score from clinicians (4.0) than from evaluators (2.9); “Other medical costs” was scored higher by clinicians (3.7) than by evaluators (2.8).
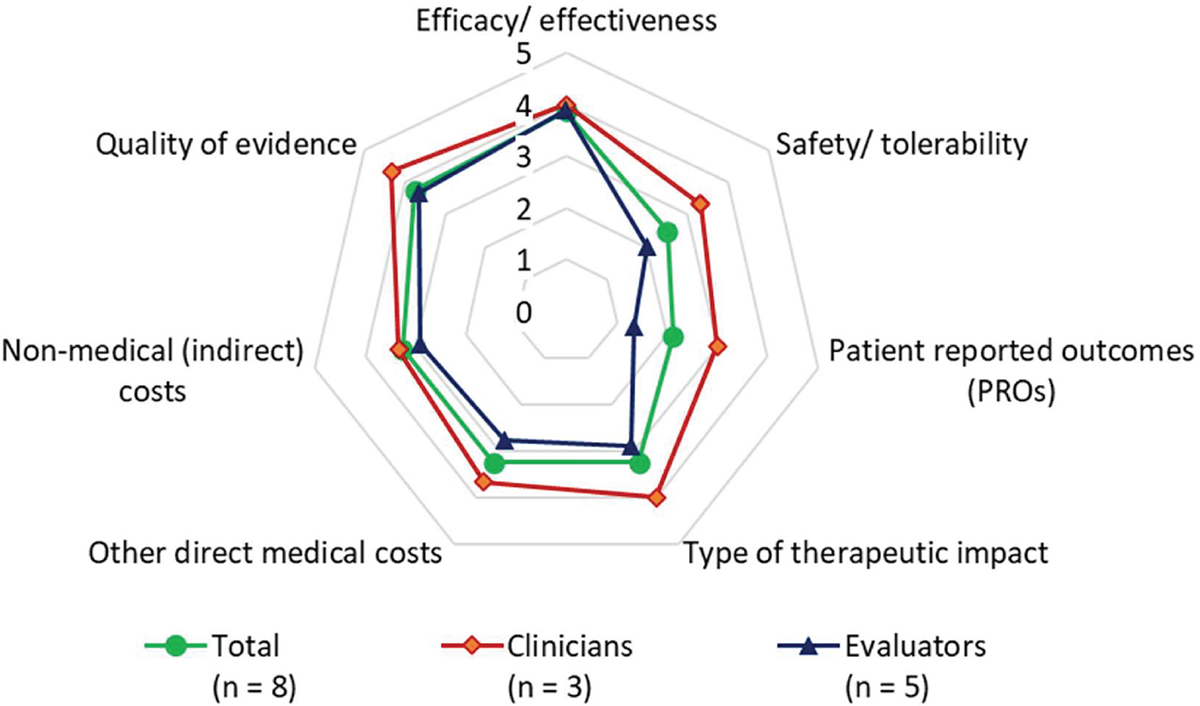
FIGURE 4 - Value contribution of leniolisib by expert profile.
Replicability and consistency (re-scoring)
The results of the analysis of the scores before and after the rescoring exercise showed no statistically significant differences between the initial scoring and the rescoring results. After rescoring, the average score of four criteria increased slightly: “Efficacy/effectiveness” (+0.3), “Other direct medical costs” (+0.2), “Non-medical indirect costs” (+0.2), and “PROs” (+0.1). Average score decreased (-0.1) slightly for the “Quality of evidence and grade of recommendation” criterion.
Discussion
To our knowledge, this is the first MCDA that estimates the value of a treatment for APDS in Spain based on an APDS MCDA value previously published.
The results suggest that leniolisib, the first treatment for APDS currently undergoing European regulatory review and already approved by the FDA is perceived by all stakeholders as a higher-value treatment when compared with SoC. Experts particularly valued the differential mechanism of action of leniolisib, inhibiting the production of phosphatidylinositol-3-4-5-trisphosphate (PIP3). PIP3 serves as an important cellular messenger specifically activating Akt (via PDK1) and regulates a multitude of cell functions such as proliferation, differentiation, cytokine production, cell survival, angiogenesis, and metabolism. Its mechanism of action confers leniolisib the potential to act as a disease-modifier, positively impacting the two key aspects of APDS: immune dysfunction and immune dysregulation.
Leniolisib scored higher than SoC in all criteria. The highest scoring criteria (score over 3) was “efficacy/effectiveness” due to the disease-modifying effect and the results observed during clinical development. The second highest scoring criteria was “Quality of evidence” given that the evidence available for leniolisib includes three clinical trials (one of which is triple-blinded, randomized, placebo-controlled) and an Open-Label Extension (OLE) study, which is rare in ODs, usually associated with lower levels of evidence. Both aspects were identified as important in the APDS MCDA study (8). The cost-related criteria (“Other direct medical costs” and “Non-medical indirect costs”) also received a high score, mainly because the clinical effect of leniolisib observed could reduce the use of resources extrapolated from the efficacy data. For example, since there would be a higher control of the disease, fewer hospitalizations and/or reduction of hospitalization days due to disease complications would be expected, including both hospital wards and intensive care units. Furthermore, a positive impact on sick leaves or travel expenses should also be expected from the patient/caregiver side. Some experts expressed that, despite reasonable extrapolation of the available efficacy data, this impact should be demonstrated in real clinical practice. Finally, the type of therapeutic impact was also highly scored by the experts, as leniolisib’s mechanism of action has the potential to modify the course of the disease, identified as an important unmet need in APDS.
“Safety/tolerability” received a lower score despite being scored in favour of leniolisib, mainly as the pivotal study duration was deemed limited by the experts to draw final conclusions. On the other hand, experts valued that there were no discontinuations related to leniolisib, which can be a relevant factor when treating a chronic disease. “Patient reported outcomes (PROs)” also received a lower score as experts considered that the evidence available was limited, but improvements were observed.
Although the study did not have the power to measure variations across different stakeholders’ profiles, differences in the scoring of some of the criteria might be explained by: “Safety/tolerability” reflecting that clinicians are more familiar with the management of adverse events derived from current SoC and felt more comfortable handling the uncertainty of leniolisib’s long term safety profile; “PROs” since physicians based their assessments of impact of therapies on quality of life as the direct observation and contact with the patient helps them to identify and understand the impact of treatments in quality of life beyond results from specific questionnaires; “Type of therapeutic impact” since clinicians valued the differential mechanism of action of leniolisib, suggesting a normalization effect on the immune system alterations observed while evaluators/pharmacists expected longer-term data and hard endpoints to prove this effect; and “Other medical costs” linked to the real-life experience from clinicians on impact on direct medical resources (material, human resources, time) involved in managing APDS patients and derived from an insufficient disease control with current SoC and the extrapolation of the available efficacy data of the anticipated impact of leniolisib, based on its clinical profile.
The comparative (pharmacological) cost of the intervention criterion was not included in the study because at the time of its performance, leniolisib had not yet achieved neither regulatory approval nor pricing and reimbursement approval in Spain.
The experts noted that the exercise was useful and interesting, and that the exchange of opinions and reflections shared during group discussion enriched analysis and individual assessments. Despite the multidisciplinary debate, some differences among stakeholders persisted due to personal perceptions and experiences and inherent aspects of each role. This highlights the importance of a multidisciplinary panel of experts in these types of studies to anticipate and understand how far or close they are in recognizing added value for each criterion, based on the same evidence and from their individual perspectives, as it occurs in real-life evaluation committees.
MCDA methodology is increasingly being used in the context of appraising ODs (25,26) in Spain. It is used at regional (22,23) and hospital level (27-29).
This study is not exempt from some limitations, for example, the size of the expert panel could be considered small. However, it is in line with other MCDA studies published (27,30-33) and, in some cases, it is bigger than some drug evaluation committees. Most of the experts participating in this study were also involved in the previous APDS-specific MCDA study conducted (8). This adds value to the coherence and consistency of the results obtained and granted that the experts had knowledge of the disease and the MCDA methodology. Another limitation of this study is that the OLE study is currently ongoing and final results are not available yet. The evaluation was based on the first OLE results data cut evaluation (34) and, although the experts considered that despite the limitation, the data trends observed in the pivotal trial were sufficient to perform an initial evaluation, further analysis could be interesting once the regulatory process is complete, and all the clinical and economic data become available. Establishing the relative value contribution of a new treatment for an ultra-rare disease and the decision to incorporate it in formularies what usually represents a challenge for healthcare systems. It must follow a holistic evaluation of the value provided, not limited to the traditional criteria of efficacy, safety, and cost, and reflecting the diverse perspectives of key stakeholders (18,19). The particularities of the clinical development of ODs and its epidemiologic, clinical and socioeconomic characteristics mean that there is greater uncertainty than in other, more prevalent therapeutic areas. International bodies and HTAs (35-37) have recognized it is advisable to establish a complementary evaluation system such as MCDA to complement existing ones and facilitate decision-making.
Defining first what represents value in a rare/ultra-rare disease such as APDS is critical before product appraisal is performed. This work complements and should be read in conjunction with previous work using MCDA methodology, determining first what represents value in APDS from the perspective of different key stakeholders in Spain (8), providing a disease-specific value framework against which current evaluation of the relative value contribution of leniolisib versus SoC could be determined. This study, by addressing multiple dimensions of value added by leniolisib versus SoC, can contribute to providing comprehensive, structured, and contextually relevant information to inform HTA and reimbursement decisions for the product, when available, in Spain.
Conclusion
To our knowledge, this is the first study to apply MCDA methodology to determine the value contribution of a treatment option for APDS in Spain. The findings of this study suggest that a robust, representative, and multidisciplinary sample of stakeholders in Spain perceived leniolisib as superior to SoC in all value criteria. The application of reflective MCDA methodology not only allows understanding the value perception of a new treatment in a holistic way taking into account a broad spectrum of value attributes and relative to available treatment alternatives, but also supports informed decision-making on the selection of the most appropriate therapy for these patients.
Disclosures
Conflict of interest: RA, CA, LIG, AH, ON, CP, CR-G, and JLT have received honoraria from Pharming for their participation in this study. KHH and RF are employees of Pharming. AG is an employee of Omakase Consulting, which received funding from Pharming for the development and performance of this study and the elaboration of the manuscript. None of the authors have received honoraria for the review of the manuscript.
Funding: This study was sponsored by Pharming Technologies B.V., Leiden, The Netherlands.
Authors contributions: AG participated in the conception and design of the study, evidence generation and data analyzes and interpretation and meetings moderation. AG has drafted the manuscript. RA, CA, LIG, AH, ON, CP, CR-G, and JLT performed the scores and evaluation of leniolisib. KHH and RF analyzed the data. All co-authors have reviewed and approved the manuscript.
References
- 1. Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. 2014;342(6160):866–71. PubMed
- 2. Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88-97. CrossRef PubMed
- 3. Lucas CL, Zhang Y, Venida A, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211(13):2537-2547. CrossRef PubMed
- 4. Deau MC, Heurtier L, Frange P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124(9):3923-3928. CrossRef PubMed
- 5. Orphanet: Síndrome de PI3K delta activado. Online (Accessed July 2024)
- 6. Goetghebeur MM, Wagner M, Khoury H, et al. Bridging health technology assessment (HTA) and efficient health care decision making with multicriteria decision analysis (MCDA): applying the EVIDEM framework to medicines appraisal. Med Decis Making. 2012;32(2):376-388. CrossRef PubMed
- 7. Frazão TDC, Camilo DGG, Cabral ELS, Souza RP. Multicriteria decision analysis (MCDA) in health care: A systematic review of the main characteristics and methodological steps. Vol 18. BMC Medical Informatics and Decision Making. BioMed Central Ltd; 2018. PubMed
- 8. Abad MR, Alerany C, Alsina L, et al. Determining value in the treatment of activated PI3Kδ syndrome in Spain: a multicriteria decision analysis from the perspective of key stakeholders. Glob Reg Health Technol Assess. 2024;11(1):124-130. CrossRef PubMed
- 9. Maccari ME, Wolkewitz M, Schwab C, et al; European Society for Immunodeficiencies Registry Working Party. Activated phosphoinositide 3-kinase δ syndrome: update from the ESID Registry and comparison with other autoimmune-lymphoproliferative inborn errors of immunity. J Allergy Clin Immunol. 2023;152(4):984-996.e10. CrossRef PubMed
- 10. Kumar BV, Connors TJ, Farber DL. Human T Cell Development, Localization, and Function throughout Life. Immunity. 2018;48(2):202-213. CrossRef PubMed
- 11. Elkaim E, Neven B, Bruneau J, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: A cohort study. J Allergy Clin Immunol. 2016;138(1):210-218.e9. CrossRef PubMed
- 12. Hoegenauer K, Soldermann N, Zécri F, et al. Discovery of CDZ173 (Leniolisib), Representing a Structurally Novel Class of PI3K Delta-Selective Inhibitors. ACS Med Chem Lett. 2017;8(9):975-980. CrossRef PubMed
- 13. Rao VK, Webster S, Dalm VASH, et al. Effective “activated PI3Kδ syndrome”-targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017;130(21):2307-2316. CrossRef PubMed
- 14. Fruman DA, Chiu H, Hopkins BD, et al. The PI3K Pathway in Human Disease. Cell. 2017;170(4):605-635. CrossRef PubMed
- 15. Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317-330. CrossRef PubMed
- 16. Food and Drug Administration. Leniolisib prescribing information. Online (Accessed July 2024)
- 17. European Medicines Agency (EMA). Online (Accessed July 2024)
- 18. Marsh K, IJzerman M, Thokala P, et al; ISPOR Task Force. Multiple Criteria Decision Analysis for Health Care Decision Making—Emerging Good Practices: Report 2 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19(2):125-137. CrossRef PubMed
- 19. Thokala P, Devlin N, Marsh K, et al. Multiple criteria decision analysis for health care decision making – An introduction: Report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health. 2016;19(1):1-13. CrossRef PubMed
- 20. Badia X, Chugani D, Abad MR, et al. Development and validation of an MCDA framework for evaluation and decision-making of orphan drugs in Spain. Vol. 7. Expert Opin Orphan Drugs. 2019;7(7-8):363-372. CrossRef
- 21. Badia X, Calleja M, Mirco A, et al. HT6 – DO SPAIN AND PORTUGAL EVALUATORS AND DECISION MAKERS GIVE THE SAME IMPORTANCE TO EVALUATION CRITERIA OF INNOVATIVE MEDICINES? Value Health. 2018;21:S9. CrossRef
- 22. Gilabert-Perramon A, Torrent-Farnell J, Catalan A, et al. DRUG EVALUATION AND DECISION MAKING IN CATALONIA: DEVELOPMENT AND VALIDATION OF A METHODOLOGICAL FRAMEWORK BASED ON MULTI-CRITERIA DECISION ANALYSIS (MCDA) FOR ORPHAN DRUGS. Int J Technol Assess Health Care. 2017;33(1):111-120. Accessed December 18, 2023. CrossRef PubMed
- 23. Gasol M, Paco N, Guarga L, et al. Early Access to Medicines: Use of Multicriteria Decision Analysis (MCDA) as a Decision Tool in Catalonia (Spain). J Clin Med. 2022;11(5):1353. Accessed December 18, 2023. CrossRef PubMed
- 24. Rao VK, Webster S, Šedivá A, et al. A randomized, placebo-controlled phase 3 trial of the PI3Kδ inhibitor leniolisib for activated PI3Kδ syndrome. Blood. 2023;141(9):971-983. CrossRef PubMed
- 25. Gil-Nagel A, Falip M, Sánchez-Carpintero R, et al. The contribution of fenfluramine to the treatment of Dravet syndrome in Spain through Multi-Criteria Decision Analysis. Epilepsy Behav. 2022;132:108711. CrossRef PubMed
- 26. Abad M, González-Meneses A, Gras E, et al. HTA130 Value Contribution of Olipudase Alfa Therapy for the Treatment of Non-Central Nervous System Manifestations of Acid Sphingomyelinase Deficiency (ASMD) by Multi-Criteria Decision Analysis (MCDA). Value Health. 2022;25(12):S321-S322. Accessed February 16, 2023. CrossRef
- 27. Roldán ÚB, Badia X, Marcos-Rodríguez JA, et al. MULTI-CRITERIA DECISION ANALYSIS AS A DECISION-SUPPORT TOOL FOR DRUG EVALUATION: A PILOT STUDY IN A PHARMACY AND THERAPEUTICS COMMITTEE SETTING. Int J Technol Assess Health Care. 2018;34(5):519-526. Accessed April 29, 2024. CrossRef PubMed
- 28. ORPHAR-SEFH. Manual para el desarrollo de un informe de evaluación de medicamentos huérfanos por parte del grupo ORPHAR-SEFH usando metodología de Análisis de Decisión Multicriterio. 2020. Online (Accessed July 2024)
- 29. Orpha-SEFH. Plan_Estratgico_OrPhar-SEFH_2024-2027. Online (Accessed July 2024)
- 30. Álvarez-Román MT, Cuervo-Arango I, Pérez-Santamarina R, et al. Determining the value contribution of emicizumab (Hemlibra®) for the prophylaxis of haemophilia A with inhibitors in Spain by multi-criteria decision analysis. Glob Reg Health Technol Assess. 2019;6(1):1-8. CrossRef
- 31. Domingo C, Fernandez M, Garin N, et al. Determining What Represents Value in the Treatment of Refractory or Unexplained Chronic Cough from the Perspective of Key Stakeholders in Spain Using Multi-Criteria Decision Analysis. Appl Health Econ Health Policy. 2023;21(1):119-130. CrossRef PubMed
- 32. Villanueva V, Carreño M, Gil-Nagel A, et al. Identifying key unmet needs and value drivers in the treatment of focal-onset seizures (FOS) in patients with drug-resistant epilepsy (DRE) in Spain through Multi-Criteria Decision Analysis (MCDA). Epilepsy Behav. 2021;122:108222. Accessed February 16, 2023. CrossRef PubMed
- 33. Taxonera C, Sala F, Martín I, et al. Determinación de la contribución de valor de filgotinib para el tratamiento de la colitis ulcerosa de moderada a grave mediante el análisis de decisión multicriterio (MCDA). Economía de la Salud. 2023;18(Jul):18. CrossRef
- 34. Rao VK, Kulm E, Šedivá A, et al. Interim analysis: open-label extension study of leniolisib for patients with APDS. J Allergy Clin Immunol. 2023;0(0). PubMed
- 35. Medicines Agency E. Revision 1 Human Medicines Development and Evaluation Benefit-risk methodology project Work package 4 report: Benefit-risk tools and processes Work package 4 report: Benefit-risk tools and processes Report by the EMA Benefit-Risk Methodology Project Team. 2012. Online (Accessed July 2024)
- 36. Health Organization Regional Office for Europe W. Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research. 2015. Online (Accessed July 2024)
- 37. Thokola P. 2011. Multiple criteria decision analysis (MCDA). REPORT BY THE DECISION SUPPORT UNIT. Online (Accessed July 2024)