 |
Glob Reg Health Technol Assess 2025; 12: 61-69 ISSN 2283-5733 | DOI: 10.33393/grhta.2025.3194 ORIGINAL RESEARCH ARTICLE |
 |
D.I.Ri.M.O. project: deprescription, inappropriateness evaluation and therapeutic reconciliation in hospital medicine
ABSTRACT
Background: In the Italian healthcare landscape, the management of chronic pathologies is a priority. Often, the elderly patient suffers from several pathologies at once and is commonly on polytherapy: this can easily bring potentially harmful errors in drug therapy. The D.I.Ri.M.O. project took place in an Internal Medicine department and aimed to reduce medication errors and improve the state of health through the Pharmacological Reconciliation procedure.
Methods: From June to October 2022, the team archived therapies for 70 hospitalized patients aged over 65 years and suffering from two or more chronic diseases. For each patient enrolled, the team developed a reconciliation board; afterward, the physician and the pharmacist proceeded to remodulate therapies, especially in those patients with serious interactions.
Results: The team collected 287 drug interactions and then classified them according to the Intercheck Web software classification: 36 class D (very serious), 49 class C (major), 174 class B (moderate), and 28 class A (minor). The modified therapies at discharge were 77.14%. This restriction brought about the removal of unnecessary drugs. After six months, the team observed an improvement in the health conditions of the patients enrolled.
Conclusions: By increasing the patient’s awareness and reducing the number of potentially inappropriate prescriptions, it is possible to improve the effectiveness of therapies. It is also possible to look at a saving policy to make the economic resources better allocated.
Keywords: Comorbidity, Deprescription, Elderly patient, Hospitalization, Pharmacological reconciliation, Polytherapy
Received: July 22, 2024
Accepted: February 12, 2025
Published online: March 3, 2025
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2025 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
The rising average age of the Italian population and the growing number of patients with multiple health conditions are challenges that the National Health Service (NHS) and its stakeholders face daily (1). The Italian state legitimizes the NHS to guarantee the population the pharmacological and home care it needs (2,3).
The improvement of socio-economic health conditions and the increase in survival led to a deep modification of the scenarios and treatment needs, resulting in a progressive growth in chronic diseases (4). That’s why elderly subjects are often in polytherapy, and the prevalence of pharmacological interactions is between 3.2% and 30% (5).
It is, therefore, essential to know the characteristics of chronic diseases to better understand how they differ from acute pathologies (6).
The presence of various clinical conditions necessitates interventions from multiple specialists. This can lead to complex medication regimens overlapping within the treatment plan. Multiple prescriptions are often associated with an inevitable reduction in compliance, therapeutic duplications, and an increase in inappropriate prescriptions (7).
In the last few years, health workers in Italy and in the rest of the world have started to understand the importance of deprescribing (8,9). Despite the guidelines highlighting the importance of deprescribing, there are several obstacles that this intervention has to face (10,11,12).
Scientific evidence suggests that in populations at high risk of adverse effects, deprescribing may be beneficial even if, to make the deprescription intervention part of the routine practice, a targeted approach is required (13,14). Some other studies have debated the discontinuation of drugs in particular conditions (15,16).
The aim of this work was to verify the actual implications that the activity of pharmacological reconciliation among chronic patients can have in the short- and long-term in real life. The team wanted to provide a photograph of the selected sample as a projection of what the effects could be on the entire population (Figure 1). In this context, the pharmacist has a fundamental role in supporting the doctor in the therapy review.
Potentially inappropriate prescriptions (PIPs), defined as those therapies where the risk of adverse events exceeds the expected benefit, have been recognized among the main factors that can contribute to determining the appearance of predictable adverse reactions (17).
A review of studies published in 2007 reported that, in geriatric settings, drug-drug interactions (DDIs) are responsible for 5% of hospital admissions (18).
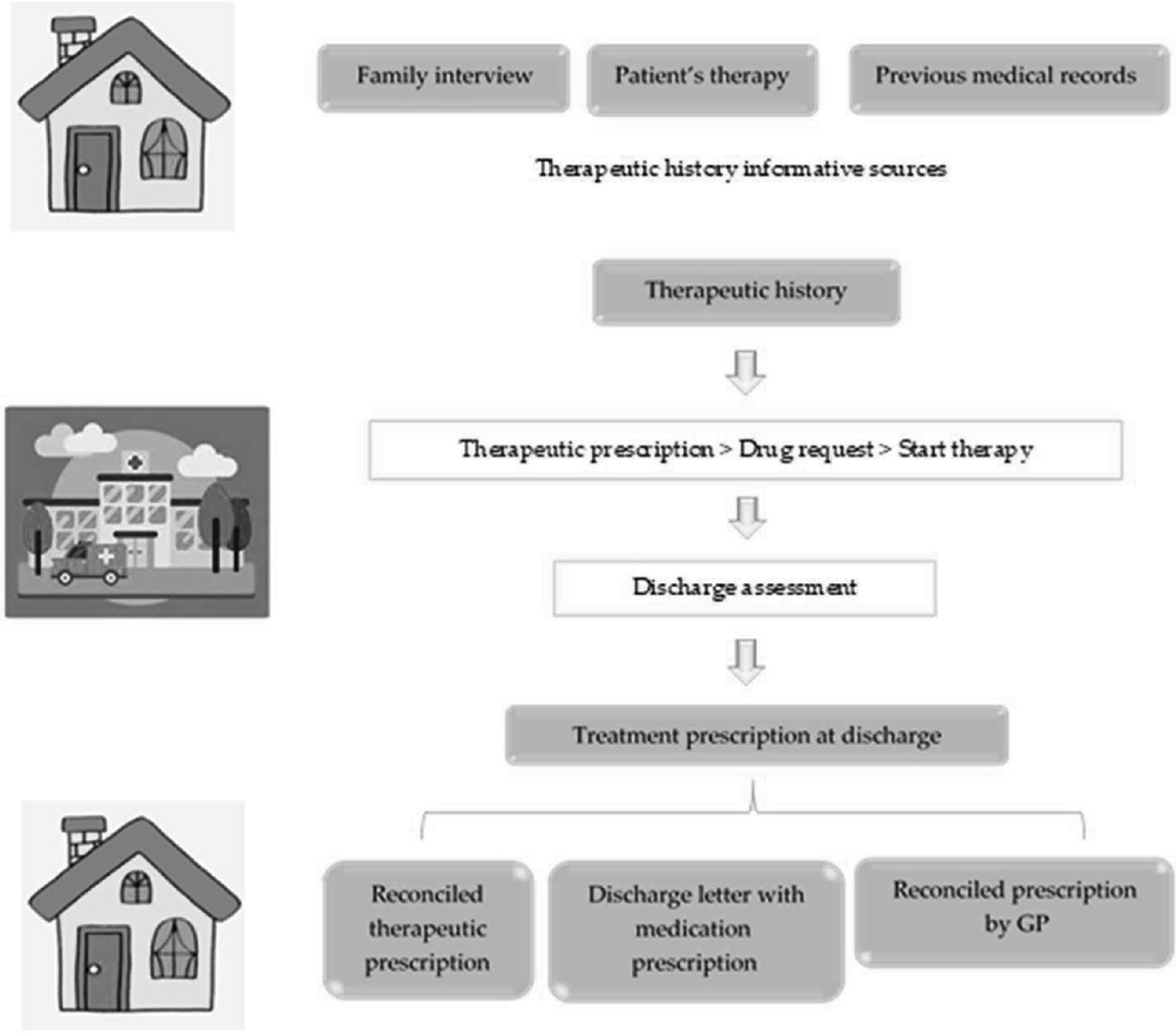
FIGURE 1 - Pharmacological reconciliation flow-chart.
The project was carried out pursuing two main objectives: the reduction of potentially inappropriate prescriptions and the improvement of the health status of chronic patients who access the Unit of Internal Medicine.
Materials and Methods
The concurrence between doctors and pharmacists has led to a project that aims to reduce duplications of therapy and to ensure an improvement in patient’s health status, the D.I.Ri.M.O. project: “Deprescription, inappropriateness evaluation and therapeutic reconciliation in hospital medicine.” The project was approved and deliberated by the General Directorate of the LHA BAT with resolution no. 742/2022, published in the relevant Praetorian Register.
Enrolment of patients
From June 2022 to October 2022, in an Internal Medicine ward (P.O. “Bonomo” Andria, Italy), 70 patients who met the following criteria were enrolled:
- age ≥ 65 years;
- simultaneous presence of at least 2 chronic diseases;
- ongoing therapy with drugs belonging to classes “A,” “C,” and “R” according to the Anatomical, Therapeutic, and Chemical (ATC) drug classification.
Patients suffering from neurological diseases, taking drugs belonging to the ATC “N” category, and patients unable to sign the informed consent were excluded from the project. A code associated with each patient ensured the anonymization of the data. At the time of enrollment, a data collection form reporting the patient’s habits and therapy was completed. Due to the absence of a unanimous standardization of the terminology to define the severity of a pharmacological interaction, there is a wide intervariability of the available databases (19). The choice of the Mario Negri Institute software called “Intercheck Web” was dictated by the need for speed of operation and the possibility of drawing up a PDF report for each patient used as a reconciliation form.
Patients were enrolled using the random sampling method. In the selected period, all patients were enrolled according to the aforementioned inclusion/exclusion criteria until the required number of 70 patients was reached. Patients who were enrolled and then died in the hospital were excluded from the project as it was not possible to carry out follow-up on them. This code was used both for completing the data collection form and for drafting the therapeutic reconciliation form.
The team obtained therapeutic reconciliation sheets for each patient enrolled. The drug interactions found were classified according to four classes of clinical relevance, going from A, minor to D, contraindicated, or very serious. Inappropriateness was also assessed using the Beers, START, and STOPP criteria.
The classification criteria for drug interactions in classes A-D are fundamental for identifying and managing interactions between medications. A clearer definition of these criteria can help healthcare professionals make more informed decisions regarding patients’ pharmacological therapy.
Class A: Interactions without clinical significance.
Class B: Potentially significant interactions, but with a low risk of adverse effects.
Class C: Interactions that require monitoring or adjustments to therapy.
Class D: Severe interactions that require immediate intervention.
The same tool permits the evaluation of the interaction using Beers, START, and STOPP criteria, which are essential for evaluating inappropriate pharmacological therapy in older adults. The Beers Criteria identify high-risk medications to avoid, while the START Criteria focus on treatments that may be omitted. The STOPP Criteria help pinpoint inappropriate prescriptions, providing lists of medications to consider discontinuing based on clinical evidence. Together, these criteria enhance medication safety and efficacy for elderly patients.
The team used three indices to evaluate the improvement in the patient’s health status: Charlson, Barthel, and Exton–Smith. The Charlson index attributes a score related to the severity of the disease, in particular, the probability of death within one year (20, 21). The Barthel index or scale aims to establish the patient’s degree of independence in carrying out common daily activities [Activities of Daily Living (ADL)]. The Exton–Smith Scale represents a sensitive tool for assessing the risk of developing a pressure sore by considering the patient’s general physical state, mental state, walking, motility, and continence. The patient’s probability of developing pressure sores or ulcers is an important indicator of his degree of independence.
General practitioners, informed of the patient’s discharge from the ward, were tasked with reviewing the patients’ chronic therapy and simplifying the treatment as per the deprescribing form. They also assessed the patient’s overall health status.
The project provides a 6-month follow-up evaluation of the three indexes.
After the conclusion of the project, evaluations in terms of cost-effectiveness were carried out. The healthcare costs relating to each patient included in the project in the years 2021 and 2022 were computed, considering the expenses for drugs, hospital admissions, and outpatient services (specialist visits, laboratory tests, and diagnostic procedures).
Statistical Analysis
A descriptive statistical analysis was conducted. Categorical variables are provided as numbers and percentages, while continuous variables are given as mean ± standard deviation (SD). The means of each of the three clinical indices (Charlson, Barthel, and Exton–Smith) were compiled at hospitalization and at follow-up, and then the percentage variations were calculated.
In cost analysis, outliers, namely those patients whose costs exceeded more than 3 times the standard deviation over the mean value, were excluded.
Healthcare costs were computed in the 6-month period before (range: December 2021 to May 2022) and after (range: November 2022 to May 2023) the time of enrollment (range: June 2022 to October 2022), and the changes were presented as percentage of delta increase (Δ%). Statistical analyses were performed using Stata 12.0 SE software.
Results
A total of 70 patients were enrolled, 38 (54.2%) male and 32 (45.8%) female.
The average age was 83 years: 23 patients were under 80 years old (32.9%), 37 were between 80 and 90 years old (52.8%), and 10 were over 90 years old (14.3%) (Table 1).
Among them, 53 (75.7%) of the patients took more than 5 drugs. Everyone was taking at least one drug belonging to the ATC categories “A,” “C,” and “R” previously described. The average number of drugs taken per patient was 6.53.
At the time of enrollment, the three indices (Charlson, Barthel, and Exton–Smith) were compiled for each patient. The maximum hospital stay was 18 days, and the minimum was 3 days. The average length of stay was 9 days.
The average values of the three indexes at the time of hospitalization of the enrolled patients were 3.06 for the Charlson Index, 13.88 for the Exton-Smith Index, and 47.42 for the Barthel Index.
| Overall patients (N = 70) | |
|---|---|
| Age (years), mean ± SD | 83 ± 5.75 |
| Age classes, n (%) | |
| • <80 years | 23 (32.9%) |
| • 80-90 years | 37 (52.8%) |
| • >90 years | 10 (14.3%) |
| Gender, n (%) | |
| • Female | 32 (45.8%) |
| • Male | 38 (54.2%) |
| Charlson Comorbidity Index, mean ± SD | 3.06 ± 2.08 |
| Barthel scale, mean ± SD | 47.42 ± 36.27 |
| Exton–Smith scale, mean ± SD | 13.88 ± 4.13 |
| Length of hospitalization (days), mean ± SD, min-max | 9 ± 4.34, (3-18) |
| Drugs, mean ± SD | 6.53 ± 2.94 |
| Drug classes, n (%) | |
| • > 5 drugs | 53 (75.7%) |
The value of the Charlson index shows that each patient had at least three different comorbidities, with the exception of pathologies such as a metastatic solid tumor, diabetes, or liver disease, which gave a score greater than 1. Regarding the Exton–Smith index, the maximum value was 20, which indicates a high level of autonomy and lucidity. The average value obtained indicates that most patients had an intermediate degree of autonomy, although they were not completely independent. Finally, for the Barthel index, the maximum value is 100 points. The average value obtained is just under 50 points. This is easily understandable given the advanced age of the patients enrolled and the increasingly worse conditions in which they arrive at the ward compared to those of normal daily life.
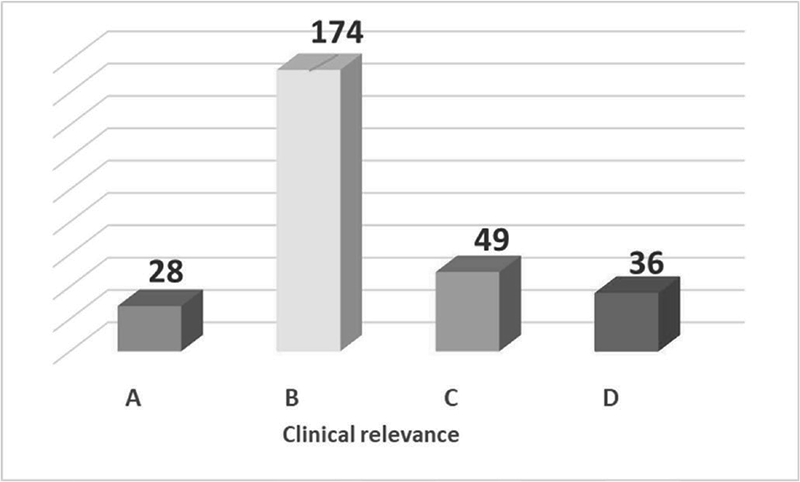
FIGURE 2 - Pharmacological interactions archived.
Then, 70 pharmacological recognition forms were processed. They were obtained by including the pharmacological therapy carried out at home before hospitalization and specifying some of the patient’s lifestyle habits (for example, smoking, alcohol, grapefruit juice, and food supplements). The files were then consulted by the doctor and the pharmacist at the time of discharge to remodulate, where possible, the therapy and carry out deprescription activities.
The processed cards returned a total of 287 interactions (Figure 2) distributed as follows:
- 28 class A (minor clinical relevance);
- 174 class B (moderate clinical relevance);
- 49 class C (major clinical relevance);
- 36 class D (contraindicated or very serious).
| INTERACTIONS | ||||||
|---|---|---|---|---|---|---|
| Patient Code | Age | Charlson comorbidity index at admission | A | B | C | D |
| 1 AND | 78 | 2 | 1 | |||
| 2 AND | 92 | 4 | 3 | 2 | 2 | |
| 3 AND | 71 | 1 | 2 | 14 | 3 | |
| 4 AND | 89 | 1 | ||||
| 5 AND | 81 | 4 | 1 | 7 | 3 | 1 |
| 6 AND | 80 | 6 | 1 | |||
| 7 AND | 84 | 1 | 3 | |||
| 8 AND | 84 | 3 | ||||
| 9 AND | 90 | 4 | 2 | 14 | 5 | 2 |
| 10 AND | 76 | 1 | ||||
| 11 AND | 91 | 3 | 4 | 1 | ||
| 12 AND | 81 | 7 | 1 | 4 | 2 | 3 |
| 13 AND | 89 | 5 | 1 | |||
| 14 AND | 84 | 5 | 2 | 7 | ||
| 15 AND | 82 | 3 | ||||
| 16 AND | 84 | 5 | 1 | 1 | ||
| 17 AND | 83 | 6 | 1 | 1 | ||
| 18 AND | 81 | 7 | 2 | 1 | ||
| 19 AND | 72 | 1 | ||||
| 20 AND | 77 | 0 | ||||
| 21 AND | 89 | 2 | 1 | 5 | ||
| 22 AND | 83 | 1 | 1 | |||
| 23 AND | 77 | 2 | 6 | |||
| 24 AND | 81 | 1 | 2 | 2 | 2 | |
| 25 AND | 77 | 2 | 1 | |||
| 26 AND | 76 | 0 | 2 | |||
| 27 AND | 72 | 0 | 2 | 3 | ||
| 28 AND | 80 | 3 | ||||
| 29 AND | 73 | 2 | 1 | 5 | ||
| 30 AND | 80 | 1 | 2 | 2 | ||
| 31 AND | 75 | 8 | 2 | |||
| 32 AND | 86 | 4 | 2 | |||
| 33 AND | 89 | 2 | 1 | 1 | 2 | |
| 34 AND | 81 | 4 | 6 | 2 | 1 | |
| 35 AND | 80 | 1 | 3 | 1 | ||
| 36 AND | 74 | 5 | 3 | 11 | 3 | 3 |
| 37 AND | 81 | 1 | 6 | |||
| 38 AND | 83 | 0 | ||||
| 39 AND | 86 | 5 | ||||
| 40 AND | 90 | 6 | ||||
| 41 AND | 84 | 3 | 1 | 1 | 2 | |
| 42 AND | 81 | 3 | 1 | 2 | 1 | |
| 43 AND | 76 | 6 | 1 | 3 | ||
| 44 AND | 91 | 5 | 1 | |||
| 45 AND | 88 | 1 | 2 | |||
| 46 AND | 87 | 1 | 1 | 4 | ||
| 47 AND | 85 | 1 | 2 | 1 | ||
| 48 AND | 89 | 3 | 2 | |||
| 49 AND | 86 | 2 | 6 | |||
| 50 AND | 81 | 3 | 1 | 1 | 1 | |
| 51 AND | 89 | 2 | 2 | 2 | ||
| 52 AND | 85 | 3 | 3 | 2 | 6 | |
| 53 AND | 85 | 7 | 2 | 1 | ||
| 54 AND | 85 | 3 | 5 | 1 | 1 | |
| 55 AND | 86 | 5 | 1 | |||
| 56 AND | 87 | 0 | 1 | |||
| 57 AND | 89 | 2 | ||||
| 58 AND | 73 | 0 | 1 | |||
| 59 AND | 90 | 1 | 2 | 1 | ||
| 60 AND | 78 | 4 | 1 | 6 | 1 | 1 |
| 61 AND | 73 | 1 | 1 | 1 | 1 | |
| 62 AND | 91 | 1 | 1 | |||
| 63 AND | 90 | 4 | 2 | 1 | ||
| 64 AND | 76 | 7 | 2 | 2 | ||
| 65 AND | 81 | 3 | 2 | |||
| 66 AND | 75 | 5 | 2 | 1 | ||
| 67 AND | 90 | 1 | ||||
| 68 AND | 76 | 3 | 4 | |||
| 69 AND | 89 | 2 | 2 | 6 | 2 | 1 |
| 70 AND | 79 | 1 | ||||
The most frequently found DDIs (in number) were those of moderate clinical relevance.
It was possible to deprescribe in 77.14% of cases. In five cases, it was not possible to carry out deprescribing activities: in particular, three patients were transferred to other wards; one patient died immediately before discharge; another was mistakenly enrolled even though he was not taking home medications.
There were 65 patients on whom it was possible to carry out a revaluation of therapy. In particular, 54 were discharged with simplification of therapy, and 11 continued the previous therapy without any change (Figure 3).
By analyzing each discharge case by case, it was possible to arrive at some considerations: a change of device and inhalation drug was carried out 3 times. This decision was certainly guided by reasons of prescriptive appropriateness (22).
In four cases, cholecalciferol-based drugs were deprescribed. This behavior may have two reasons: on the one hand, the achievement of optimal vitamin D values in the patient; on the other, the fact that its dispensing has now been limited to stricter conditions than before (23).
Drugs to control hypercholesterolemia were removed in six patients. Achieving normal blood cholesterol values is a very rare event, especially in elderly patients who have an increasing long-term risk of fatal and nonfatal cardiovascular outcomes that can get worse if they discontinue statins (24). However, the reason beyond the choice of deprescribing statins may be due to recent scientific evidence suggesting that for patients on primary prevention with no particular critical issues, the long-term of statins do not seem to provide substantial clinical benefits (25-27).
As regards proton pump inhibitors (PPIs), in three cases, these drugs were completely eliminated from the patient’s home therapy, 22 patients started therapy with the pump inhibitor, and in 13 cases, the molecule taken was modified (Table 4).
| Active ingredient | Number of times it has been deprescribed |
|---|---|
1. Acetylsalicylic Acid (ASA) - B01AC06 |
10 |
2. Furosemide - C03CA01 |
9 |
3. Allopurinol - M04AA01 |
7 |
4. Atorvastatin - C10AA05 |
6 |
5. Canrenone - C03DA01 |
5 |
6. Bisoprolol - C07AB07 |
4 |
7. Colecalciferolo (Vitamin D3) - A11CC05 |
4 |
8. Warfarin - B01AA03 |
4 |
9. Acido folico (Folic Acid) - B03BB01 |
3 |
10. Clopidogrel - B01AC04 |
3 |
11. Doxofillina - R03DA04 |
3 |
12. Esomeprazolo (Esomeprazole) - A02BC05 |
3 |
13. Ferroso solfato (Ferrous Sulfate) - B03AA07 |
3 |
14. Metformina (Metformin) - A10BA02 |
3 |
15. Pantoprazolo (Pantoprazole) - A02BC02 |
3 |
16. Ramipril - C09AA05 |
3 |
17. Rifaximina (Rifaximin) - A07AA09 |
3 |
| ATC | PPI | New prescriptions | Shift | Disposal |
|---|---|---|---|---|
| A02BC01 | Omeprazole | 1 | 1 | 1 |
| A02BC05 | Esomeprazole | 3 | 4 | 1 |
| A02BC02 | Pantoprazole | 17 | 6 | 1 |
| A02BC03 | Lansoprazole | 1 | 2 | / |
Pantoprazole is the most used molecule. The therapeutic choice is certainly motivated by reasons of prescriptive appropriateness, given that it is an effective and very rapid active ingredient. However, it is still useful to draw up an evaluation that also places emphasis on pharmaceutical expenditures.
Reasoning in terms of cost (net of co-payment and pay-back) per Defined Daily Dose (DDD), i.e. cost per average daily maintenance dose of each drug, the most convenient molecule appears to be omeprazole (DDD € 0.285) and the prescription of more expensive active molecules like lansoprazole, pantoprazole or esomeprazole should be reserved for those patients who are not candidates for treatment with omeprazole (DDD €0.395 lansoprazole, DDD €0.372 pantoprazole, DDD €0.333 esomeprazole).
Prescribing a molecule at the lowest cost for the NHS allows for a better allocation of resources.
In seven patients, the diabetes treatment regimen was adjusted. Specifically, patients who were initially prescribed oral therapies, such as metformin or repaglinide, transitioned to subcutaneous injectable insulin therapy. Guidelines are oriented towards early insulin therapy in diabetic patients because it guarantees a more rapid restoration of physiological postprandial insulinemic profiles, improving glucose tolerance and reducing episodes of hypoglycemia. Thus, the risk of weight gain is controlled by the benefits of improved glycemic control (28).
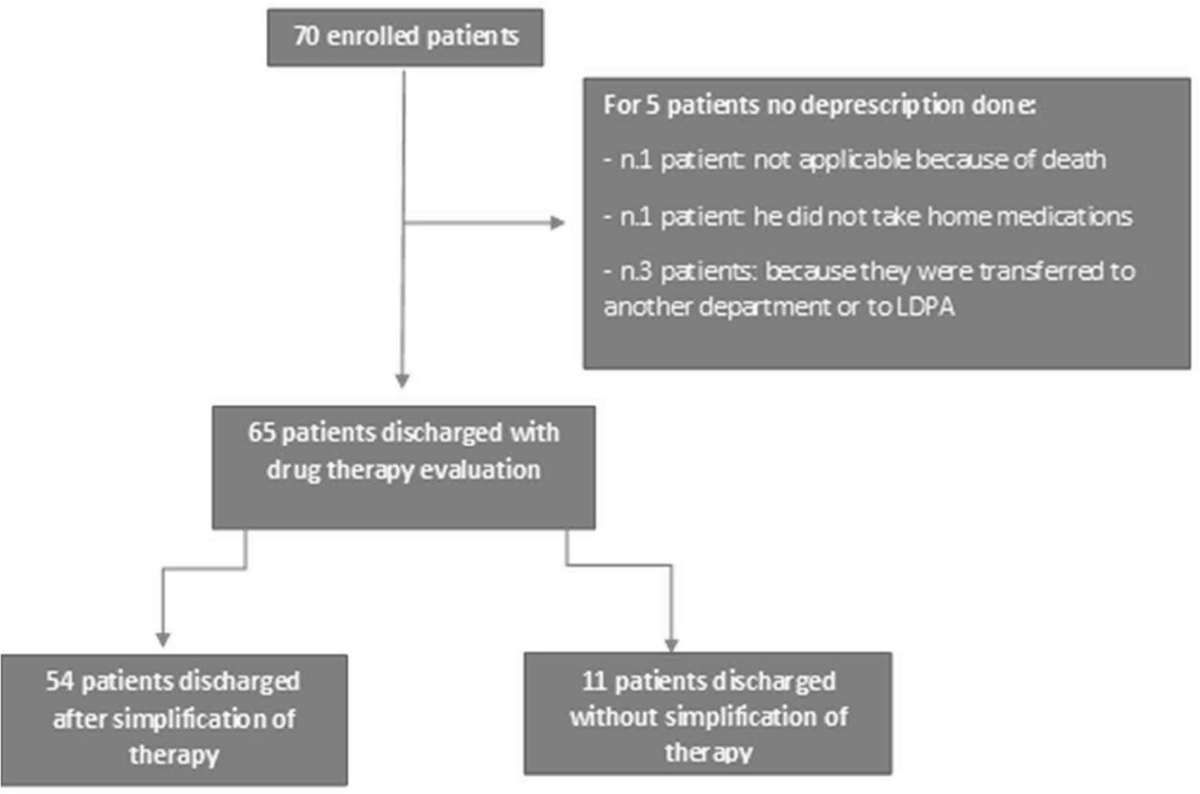
FIGURE 3 - Flow-chart reporting the deprescription activity.
It is important to point out that, for the purposes of quantifying the deprescribing interventions, any replacements of the anticoagulant drug taken orally [warfarin or new oral anticoagulants (NOAC)] with heparin were not considered, as these are due to normal clinical practice.
The current findings dispute the need to preoperatively withhold aspirin treatment in patients undergoing some particular interventions (29). Also, regarding therapies with warfarin or NOAC, the discussion is open on the timing of periprocedural interruption of treatment based on their pharmacodynamics and pharmacokinetics features (30).
Follow-up
Six months later, of the 70 patients, 25 (35.7%) died, 12 did not answer the phone call, and 33 interviews were possible. Using a computer database system, the team verified that the patients who did not answer the phone call were still alive.
As can be seen in Figure 4, the Charlson index is the only one that did not change over time: no other comorbidities were established in the patients enrolled in the period from discharge to follow-up.
The average Exton–Smith index increased by 11.53%, which is indicative of the fact that the level of autonomy of the patients interviewed improved. The Barthel index increased by 25.58%. Positive considerations can be made regarding the patient’s mental state of clarity, level of continence, and bed rest. An improvement in the Barthel index also translates into a reduction in the possibility of developing bedsores (Figure 4).
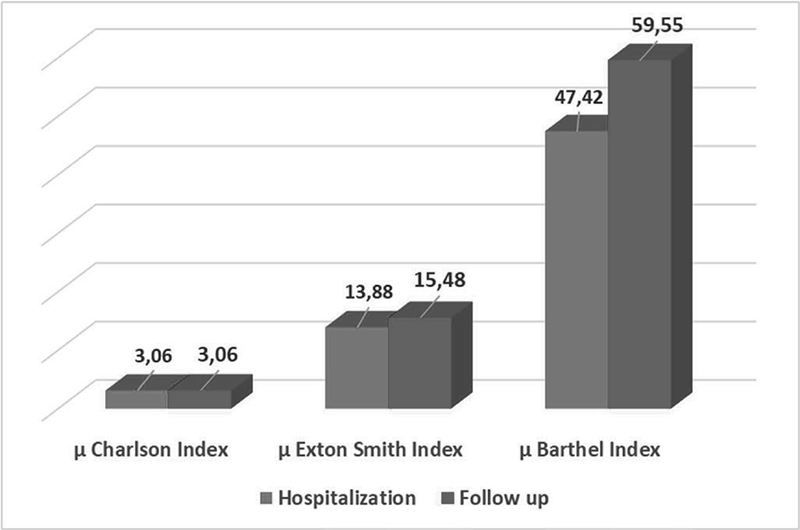
FIGURE 4 - Comparison between the mean values of the indices compiled at hospitalization and at follow-up.
Analyzing the performance of the individual indices, we noticed that the Barthel index increased or remained the same in 23 cases, while it decreased in 10 cases.
The Exton–Smith index increased in 24 patients and decreased in 9 patients.
It is noted that, with the exception of the Charlson index, the trend of the scores was more or less comparable. In both cases, an increase is significant in terms of a general improvement in the patient’s state of health.
The sum of the values collected is always 33, equal to the number of interviews carried out.
Cost-effectiveness
As regards the pharmaceutical expenditures incurred by the patients included in the project, considering the two semesters before and after the patient’s enrollment, pharmaceutical expenditures were observed in 58.6% of patients (41 out of 70). This percentage was raised when considering the patients on whom deprescription was performed (54 out of 70), resulting in higher pharmaceutical spending in 59.3% of cases. In terms of economic value, the cost increase for medications was 47.3%, specifically €77,229.26 in 6 months before inclusion and €113,550.83 in the 6 months after (Table 5). Of €113,550.83 for pharmaceutical expenditures, €46,304.45 (40.8%) were for new drugs prescribed after reconciliation.
Similarly, expenditures for hospitalization also rose by 34.97%, accounting for €157,853.55 in 6 months before inclusion and for €213,058.64 in the 6 months after (Table 5). It is important to underline that the costs relating to hospitalizations were calculated net of the hospitalization that allowed enrollment in the project. Different examinations can be made regarding outpatient services (i.e., specialist visits, laboratory tests, and diagnostic procedures. Among the 70 patients enrolled, there was a reduction of −37.28 in the expenses related to the provisions of outpatient services, which were respectively €35,547.17 and 22,294.28 in 6 months before and after inclusion (Table 5). Nevertheless, it is important to consider that hospitalization costs are significantly influenced by the advanced age and the severity of the clinical conditions of the observed patients. These factors represent independent variables that directly impact costs, making it difficult to establish a direct causal link between these expenses and the pharmacological reconciliation activities. Consequently, variations in hospitalization costs cannot be regarded as a significant indicator of the impact of reconciliation activities, as they are primarily related to the intrinsic characteristics of the patients rather than specific interventions.
An initial analysis suggests that the deprescribing intervention improves the patient’s health status but is accompanied by an increase in pharmaceutical spending in the following months and in hospitalizations.
Such results should be contextualized into broader considerations. The use of new drugs in the two-year period examined and their prescription by medical specialists has, in some cases, determined up to a doubling of pharmaceutical spending per patient (ATC L01FC01, B03XA01, L01FF05, L01FG01). The high average age of the patients enrolled led to a rapid and often unstoppable worsening of the patient’s general clinical conditions, especially when they were suffering from chronic and disabling pathologies. This inevitably leads to an increase in hospital admissions in elderly patients. Finally, it is also important to highlight how the year 2021, due to the COVID-19 pandemic, saw congestion in hospital facilities, especially in Southern Italy, which caused delays in all those hospitalizations and scheduled interventions for the following year.
| 6 months before enrollment | 6 months after enrollment | ||
|---|---|---|---|
| Time intervals (ranges) | December 2021 to May 2022 | November 2022 to May 2023 (€) | Δ% |
| Pharmaceutical expenditures (€) | 77,229.26 | 113,550.83* | 47.03 |
| Hospitalization (€) | 157,853.55 | 213,058.64 | 34.97 |
| Outpatient services*** (€) | 35,547.17 | 22,294.28 | -37.28 |
*€46,304.45 (40.8%) of €113,550.83 for new drugs only found post-reconciliation.
**Outpatient services included specialist visits, laboratory tests, and diagnostic procedures.
These results should be viewed in the light of some limitations. Although the reduction in drug prescriptions might provide several clinical and economic benefits, representing a hot topic of current research, it should be acknowledged that the limited sample size and the short follow-up prevent us from drawing firm conclusions on the generalizability of our findings or their transferability on a national scale. However, similar published studies seem to suggest that 6 months is a suitable timespan to highlight the positive rebounds of medication reconciliation on pharmaceutical expenditures [33]. Lastly, since the administrative databases are primarily conceived for reimbursement purposes, they do not allow the trace of some information, including disease severity, so it was not possible to introduce this variable into the stratification of the cost analysis. Lastly, the present analysis only provides descriptive statistics, with no comparative purposes at the moment. Further studies on a larger population are needed to highlight possible significant differences in the results.
Conclusions
The modification of the home therapy and the subsequent integration with the drugs prescribed at discharge avoided therapeutical duplication and informed the patient about the possible interactions, even non-serious ones, that could arise from the drugs usually taken.
There is still poor evidence of the actual savings resulting from the deprescribing interventions. A discussion is open about the concept that cost savings brought by deprescribing must be counterbalanced with the cost of the intervention itself and the healthcare personnel dedicated to it. However, reducing the drugs taken by a patient in polypharmacy feasibly brings economic savings, although further research still needs to be conducted (31).
The cost analysis suggests an increase in the pharmaceutical expenditure of the enrolled patients, but this rise is affected by the prescription of new and high-cost drugs. Furthermore, the general worsening of patients’ clinical conditions due to their age and pre-existing pathologies has led to an increase in hospitalization costs. It must also be considered that the activity of counseling at the time of deprescription may have resulted in a greater awareness of the patient about the therapies and, consequently, to better adherence, with an increase in pharmaceutical costs, but which, in the long run, will make possible a decrease in costs for exacerbations (32).
Therefore, an overall approach is necessary. The improvement in the patient’s health status, undeniably documented by the three evaluation indices, was accompanied by a reduction in access to outpatient services (i.e., specialist visits, laboratory tests, and diagnostic procedures) and a reduction in the costs deriving from them.
The D.I.Ri.M.O. project suggested that reducing the number of drugs taken by the patient is a viable option, and this might provide benefits in terms of reduction of side effects and improved compliance. Applying this procedure on a large scale might make it possible to optimize the management of chronic patients in both home and hospitalization settings.
A lower number of daily administrations inevitably translates into an increase in the effectiveness of the therapies. The collaboration of multiple professional figures is essential in a not-too-distant future in which the management of chronic conditions will be the main challenge for health services.
Disclosures
Conflicts of Interest: The authors declare no conflicts of interest.
Financial support: This research received no external funding.
Author contributions: Conceptualization, M.G.P., C.P. and D.A.; methodology, M.G.P., C.P. and D.A.; software, M.G.P. and C.P.; validation, C.P. and D.T.; formal analysis, M.G.P.; investigation, M.G.P., A.T., C.P., S.L., D.L.; resources, C.P., L.D.E., C.N., B.I., M.M.; writing—original draft preparation, M.G.P.; writing—review and editing, C.P., D.A., D.T.; supervision, C.P., S.L., D.A., D.T.; project administration, C.P., S.L., D.A. All authors have read and agreed to the published version of the manuscript.
Data availability statement: The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
References
- 1. AIFA – Italian Medicines Agency: “The use of medicines in Italy – OsMed 2021 Report” Update of 07.29.2022. Online (Accessed July 2024)
- 2. Prime Ministerial Decree 01.12.2017: “Definition and updating of the essential levels of assistance, referred to in article 1, paragraph 7, of the legislative decree of December 30 1992, n. 502” Official Gazette General Series, n. 65 of March 18 2017. Online (Accessed July 2024)
- 3. Ministerial Decree n. 77, 23.05.2022: “Regulation establishing the definition of models and standards for the development of territorial assistance in the National Health Service”. Online (Accessed July 2024)
- 4. Presidency of the Council of Ministers. “Italy tomorrow- National Recovery and Resilience Plan”. Online (Accessed July 2024)
- 5. Gnjidic D, Johnell K. Clinical implications from drug-drug and drug-disease interactions in older people. Clin Exp Pharmacol Physiol. 2013;40(5):320-325. CrossRef PubMed
- 6. Ministry of Health: “National Chronicity Plan”. Last update 15.09.2016. Online (Accessed July 2024)
- 7. Ministry of Health. “Ministerial Recommendation n°17- Failure to reconcile drug therapy can cause serious harm to patients” Last update: December 2014. Online (Accessed July 2024)
- 8. Crisafulli S, Poluzzi E, Luunghi C, et al. Deprescribing as a strategy for improving safety of medicines in older people: Clinical and regulatory perspective. Frontiers in Drug Safety and Regulation 2022; 2 CrossRef
- 9. Zazzara MB, Cangni A, Cas Da R, et al. Medication use and costs among older adults aged 90 years and older in Italy. Frontiers in Pharmacology 2022; 13 CrossRef
- 10. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200-1205. CrossRef PubMed
- 11. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137. CrossRef PubMed
- 12. Reeve E, To J, Hendrix I, et al. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793-807. CrossRef PubMed
- 13. Luymes CH, Poortvliet RKE, van Geloven N, et al. Deprescribing preventive cardiovascular medication in patients with predicted low cardiovascular disease risk in general practice – the ECSTATIC study: a cluster randomized non-inferiority trial. BMC Med. 2018;16(1):5. CrossRef PubMed
- 14. Mahlknecht A, Wiedermann CJ, Sandri M, et al. Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomized controlled trial. BMC Geriatr. 2021;21(1):659. CrossRef PubMed
- 15. Jowett S, Kodabuckus S, Ford GA, et al; OPTiMISE investigators. Cost-effectiveness of antihypertensive deprescribing in primary care: a markov modelling study using data from the OPTiMISE trial. Hypertension. 2022;79(5):1122-1131. CrossRef PubMed
- 16. Caldeira D. Deprescribing cardiovascular drugs in low-risk patients increases the risk of uncontrolled blood pressure and LDL-cholesterol. BMJ Evid Based Med. 2018;23(6):235-236. CrossRef PubMed
- 17. Sukumar S, Orkaby AR, Schwartz JB, et al. Polypharmacy in older heart failure patients: a multidisciplinary approach. Curr Heart Fail Rep. 2022;19(5):290-302. CrossRef PubMed
- 18. Barrons R. Evaluation of personal digital assistant software for drug interactions. Am J Health Syst Pharm. 2004;61(4):
380-385. CrossRef PubMed - 19. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. CrossRef PubMed
- 20. Becker ML, Kallewaard M, Caspers PW, et al. Hospitalizations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641-651. CrossRef PubMed
- 21. Radovanovic D, Seifert B, Urban P, et al. AMIS Plus Investigators. Validity of Charlson Comorbidity Index in patients hospitalized with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart. 2014;100(4):288-294. CrossRef PubMed
- 22. Nota 99 per la Prescrizione di farmaci ad uso inalatorio nei pazienti affetti da Broncopneumopatia Cronica Ostruttiva (BPCO); Agenzia Italiana del Farmaco: Rome, Italy, 2021. Online (Accessed July 2024)
- 23. Nota 96 per la Prescrizione di farmaci a base di vitamina D; Agenzia Italiana del Farmaco: Rome, Italy, 2021. Online (Accessed July 2024)
- 24. Rea F, Biffi A, Ronco R, et al. Cardiovascular outcomes and mortality associated with discontinuing statins in older patients receiving polypharmacy. JAMA Netw Open. 2021;4(6):e2113186. CrossRef PubMed
- 25. Gulliford M, Ravindrarajah R, Hamada S, et al. Inception and deprescribing of statins in people aged over 80 years: cohort study. Age Ageing. 2017;46(6):1001-1005. CrossRef PubMed
- 26. Odden MC, et al. The population impact and cost-effectiveness of statins for primary prevention in adults 75 and older in the United States. Ann Intern Med. 2015;162(8):533. CrossRef PubMed
- 27. Bonnet F, Bénard A, Poulizac P, et al. Discontinuing statins or not in the elderly? Study protocol for a randomized controlled trial. Trials. 2020;21(1):342. CrossRef PubMed
- 28. Chadha M, Jain SM, Chawla R, Dharmalingam M, Chaudhury T, Talwalkar PG, Tripathi S, Singh SK, Gutch M, Dasgupta A. Evolution of Guideline Recommendations on Insulin Therapy in Type 2 Diabetes Mellitus Over the Last Two Decades: A Narrative Review. Curr Diabetes Rev. 2023;19(8):e160123212777. CrossRef PubMed
- 29. Ashkenazi I, Schermann H, Gold A, et al. Is continuation of anti-platelet treatment safe for elective total hip arthroplasty patients? Arch Orthop Trauma Surg. 2020;140(12):2101-2107. CrossRef PubMed
- 30. Kaatz S, Mahan CE, Nakhle A, et al. Management of elective surgery and emergent bleeding with direct oral anticoagulants. Curr Cardiol Rep 2017; 19, 124 CrossRef
- 31. Avery AJ, Bell BG. Rationalizing medications through deprescribing. BMJ. 2019;364:l570. CrossRef PubMed
- 32. Procacci C, Degli Esposti L, Furno C, et al. The economic impact of different blood glucose monitoring systems in the diabetic patient: analysis of real-world data from an Italian local health authority. Ethics Med Public Health. 2023;29:100912. CrossRef
- 33. Karapinar-Çarkit F, Borgsteede SD, Zoer J, et al. Effect of medication reconciliation on medication costs after hospital discharge in relation to hospital pharmacy labor costs. Ann Pharmacother. 2012;46(3):329-338. CrossRef PubMed