 |
Glob Reg Health Technol Assess 2025; 12: 16-28 ISSN 2283-5733 | DOI: 10.33393/grhta.2025.3182 ORIGINAL RESEARCH ARTICLE |
 |
Economic and clinical burden associated with respiratory syncytial virus and impact of universal immunization with nirsevimab in Italy
ABSTRACT
Objectives: To describe the seasonal respiratory syncytial virus (RSV) burden in Italy considering the current prophylaxis strategy with palivizumab recommended only for high-risk infants (representing only 4.4% of an estimated birth cohort) and to evaluate the potential benefits of a new prophylaxis strategy targeting all infants with nirsevimab.
Methods: A static decision analytic model previously used in the US was adapted to evaluate the RSV-related health and cost outcomes associated with nirsevimab versus standard of care (SoC) for the prevention of RSV medically attended lower respiratory tract infections (RSV-MA-LRTIs). Monthly probabilities of RSV infections, health events, mortality, and complications associated with RSV infections were obtained from the literature. Costs associated with each event were obtained using the available literature and through real-world data analysis of National Hospital Discharge Records.
Results: For one RSV season, in the current SoC, the model estimated 216,100 RSV-MA-LRTIs, 15,121 associated complications, and 16 RSV-deaths–corresponding to an economic burden of approximately €50.5 million related to RSV-MA-LRTIs management, €10.9 million associated with potential complications due to RSV and €3 million in lost productivity due to RSV-deaths. Nirsevimab is expected to prevent 100,208 RSV-MA-LRTIs, 6,969 complications, and 6 deaths due to RSV infections, corresponding to an economic saving of about €23.3, €5, and €1.2 million, respectively.
Conclusion: Nirsevimab is a new prophylaxis strategy that helps to protect all infants against RSV disease and could substantially reduce the clinical and economic burden of RSV in Italy in infants experiencing their first RSV season.
Keywords: Cost of Illness, Prevention strategy, Respiratory syncytial virus, Universal immunization
Received: June 27, 2024
Accepted: November 11, 2025
Published online:January 29, 2025
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2025 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Respiratory syncytial virus (RSV) is one of the global leading causes of respiratory infections during childhood, and it is associated with a high level of morbidity and mortality. RSV has been estimated to be the first cause of hospitalization and death among respiratory infections in children aged ≤1 year (1-3). Age below one year and birth before or during the endemic season are among the main risk factors for RSV infections (4). Additional risk factors are prematurity and the presence of comorbidities, such as congenital heart disease, chronic respiratory diseases, and immunodeficiency (5,6). However, most of the burden of disease occurs in healthy full-term infants. In fact, as confirmed by recent national studies, at least 87% of infants hospitalized for RSV did not present underlying morbidities and were born at term or late preterm (>34 weeks gestational age—wGA) (7). Even >90% of infants with lower respiratory tract infection (LRTI) visited by family pediatricians were full-term born or otherwise healthy (8). RSV infections are also associated with high hospitalization costs (9); a recent national study included children aged 1 month to 1 year admitted to IRCCS Bambino Gesù Children Hospital in Rome has estimated that hospitalization for bronchiolitis caused by RSV is associated with a higher mean cost per patient (€ 5,753.4 ± € 2,041.6) compared to hospitalization for bronchiolitis caused by etiologies that differ from RSV (€ 5,395.2 ± € 2,040.9) (p = 0.04) (10). Currently, the only prophylaxis available at the national level is palivizumab, recommended for infants with a higher risk of severe RSV-associated LRTIs [infants of ≤35 wGa and with age below 6 months at the beginning of the RSV season, infants with age <2 years who have been treated for bronchopulmonary dysplasia in the last 6 months and infants with age <2 years with hemodynamically significant congenital cardiac disease (11)]. For late preterm and term infants (>35 weeks gestational age), there is no prophylaxis option officially recommended or approved in Italy.
In the United States, a decision analytic model developed to track the US birth cohort during its first RSV season estimated that an all-infant immunization strategy with nirsevimab against RSV infections could significantly reduce the number of RSV cases, hospitalizations and costs related to RSV (12). This study aimed to describe the seasonal RSV epidemiological and economic burden in Italy in terms of health events and associated costs considering the current prophylaxis strategy (only palivizumab eligible infants) compared with an alternative scenario where a universal immunization strategy with nirsevimab is adopted.
Methods
A static decision analytic model previously published from the US perspective was adapted to evaluate the health and cost outcomes associated with nirsevimab introduction versus standard of care (SoC) for the prevention of RSV medically attended lower respiratory tract infections (RSV-MA-LRTIs). The time horizon was the first RSV season (5 months) for all health events associated with RSV-MA-LRTIs except for premature deaths, which were considered lifetime and for the risk of developing long-term complications, recurrent wheezing, and asthma, which were considered up to 3 and 18 years of age respectively. The time horizon of a season was selected to capture the resources and related costs associated with RSV infection, considering the typical duration of the infection period.
The cohort of newborns in Italy in 2023 (395,348 infants) (13) was divided into two subpopulations to account for the differential risks of RSV-MA-LRTI. The subpopulations were defined as following: (1) palivizumab-eligible infants, representing preterm newborns ≤35 weeks gestational age (wGA) or infants with congenital heart or chronic lung disease (14); (2) late preterm and term infants, representing newborns with >35 wGA. The preterm infants’ proportion (≤35 weeks wGA) was estimated at 3.9% of the birth cohort based on the CeDAP (Certificato di assistenza al parto—Certificate of Birth Attendance) report of the Ministry of Health published in 2021 (15), while the proportion of infants with congenital heart or chronic lung disease was estimated considering the prevalence of bronchopulmonary dysplasia [1-5/10,000, from ORPHANET (16)] and the prevalence of congenital heart disease (5-10/1,000 live births, from Italian Society of Pediatrics [SIP]) (17). Hence, the proportion of palivizumab-eligible infants equal to 4.4%; this proportion was also confirmed by Italian clinicians involved in the study (Table 1).
The two subpopulations were followed through the model during the first endemic RSV season. In line with the work of Barbati et al. 2020 (7) and supported by clinicians’ opinions, the model considered that the epidemic season of RSV starts in late autumn (November), a peak in winter (January), and has a variable end in early spring (April).
The seasonal immunization with nirsevimab consisted of a single administration occurring at the beginning or during the epidemic season, respectively, for infants born out or during the RSV season. Seasonal immunization with palivizumab was considered a monthly administration during the entire epidemic season, meaning 5 doses for those born before the season and a variable number of doses for those born during the season, depending on the date of birth. The model compared the current immunization strategy with palivizumab indicated only in infants with a higher risk of severe RSV-associated LRTIs, with the new immunization strategy using nirsevimab protecting all infants against RSV considering different coverage rates based on immunization strategy and subpopulations. For the SoC, the coverage rate was assumed equal to 75% based on reported sales of palivizumab for the season 2022-2023 and considering an average of 4.2 doses per immunized infant, while the immunization strategy with nirsevimab, for palivizumab-eligible infants was assumed equal to palivizumab (conservative assumption related to the likely better compliance for nirsevimab can be expected), while for late preterm and term infants was assumed a coverage rate equal to 60% (Table 1). The impact of each immunization strategy in terms of health events and costs was based on coverage rate on efficacy, the latter defining a reduction of RSV-MA-LRTIs.
The efficacy of nirsevimab was obtained from the pooled analysis of phase III (MELODY) and phase 2b clinical trials (18); since within the clinical trials it was also demonstrated that pharmacokinetic data from the phase II-III MEDLEY study (19) support the extrapolation of the estimated efficacy for preterm and term infants to infants eligible for palivizumab (18), the same efficacy was also applied to the palivizumab-eligible infants (Table 1), considering a non-inferiority in terms of protection against RSV MA-LRTIs for nirsevimab versus palivizumab (19).
The efficacy of palivizumab was obtained from a meta-analysis conducted to evaluate the efficacy and safety of palivizumab compared to placebo (20) (Table 1).
Based on clinical and pharmacokinetic data, and as reported in the summary of product characteristics, the duration of protection offered by nirsevimab is at least 5 months, and this duration of efficacy was also assumed in the model. Monthly per-patient risks of RSV-event were obtained from the literature (7,21-26) and were validated by clinicians involved in the analysis. The number of RSV health events for each month of age was calculated considering the seasonality of births [ISTAT (27)] and the probability of contracting RSV infection per month [Barbati et al. 2020 (7)].
Costs associated with each RSV- related health event were obtained using the available literature and conducting a real-world data analysis of National Hospital Discharge Records.
In the base case, the National Health Service (NHS) perspective was considered. The productivity loss for premature mortality due to RSV infections (societal perspective) was evaluated in a scenario analysis.
The analysis also provided an estimate of the number of infants needed to be immunized to prevent one RSV-related event per RSV season.
All inputs considered in the model have been validated by clinicians involved in the development of the analysis (who are co-authors of this study). They are healthcare professionals working in public hospitals specialized in pediatric healthcare or specilists in Public Health. Several advisory boards were organized to collect their opinions to define which input was the most appropriate to include in the model, considering both available data in the literature and their experience.
Finally, a deterministic sensitivity analysis was conducted to assess the impact of each input parameter on the results of the analysis. For each parameter, a variability of 10% was assumed.
Table 1 reports a comprehensive list of the parameters included in the model.
Model structure
The two subpopulations were followed during its first RSV season using the model shown in Figure 1.
Monthly cycles allowed us to evaluate the exposure to the risk of contracting RSV-MA-LRTIs about the infants’ age (expressed in months) and the month of birth (out or in epidemic season).
For each subpopulation, the model described the RSV-MA-LRTI cases in terms of primary care visits, emergency room (ER) visits, and hospital admissions (including admissions in the intensive care unit—ICU) that occur during the RSV season. RSV-MA-LRTI cases were estimated considering the birth month of infants (28) and the probability of RSV-MA infection per month obtained from Barbati et al. 2020 (7) (Table 1).
Among infants hospitalized due to RSV, the model also considered the probability of developing mid-long-term complications such as recurrent wheezing and asthma.
Lastly, the model included all-cause mortality rates among infants by age and the mortality rate among RSV inpatient hospitalizations.
Risk of RSV-related health events
Where it was possible, and national data was available, the per-patient risk of RSV-related health events was stratified by subpopulation and per month of age (0-11 months). In the absence of local data, RSV-LRTI hospitalization rates per month of infant’s age for palivizumab-eligible infants were obtained from the palivizumab clinical trial [21], while for the late preterm and term infants subgroup, data from a retrospective observational study conducted in Spain was used to estimate the total hospitalizations and costs associated with severe bronchiolitis cases in children aged below 2 years of age (22) (Table 1).
The proportion of infants hospitalized for RSV infections that will require an intensive care unit (ICU) admission was obtained from the study by Barbati et al. 2020 (7), estimating that 16.5% of infants hospitalized for RSV infections required admission to ICU. This estimation referred to the overall proportion of ICU due to RSV infections (without any specification about the chronological and gestational age of the infant); therefore, to obtain a specific proportion of ICU admission for each subpopulation of the analysis, the overall estimate was re stratified applying a distribution of ICU admission estimated from Spain in Sanchez-Luna et al. 2017 study (23) (Table 1).
The risks of emergency room visits due to RSV- LRTIs per month of infant’s age were obtained from the Spanish study BARI (Burden of Acute Respiratory Infections), a retrospective observational study conducted on the administrative database to estimate costs associated with RSV infection cases among infants aged <5 years during the epidemic season 2017-2018 (24).
Since these ER visit rates were related to the overall cases of RSV infection, the difference between subpopulations found for hospitalization risk was also applied to ER visit rates (Table 1) to obtain a distribution of risk specific to each subpopulation.
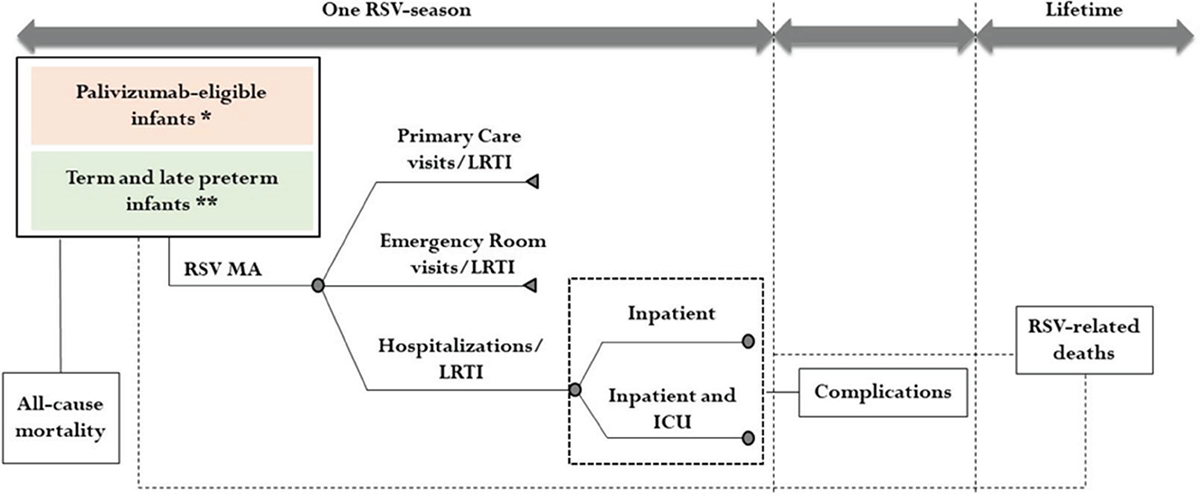
FIGURE 1 - Model structure
* Infants with wGA ≤35 weeks and infants with congenital heart or chronic lung disease
** Infants with wGA >35 weeks
RSV = Respiratory Syncytial Virus; MA = Medically Attended; LRTI = Lower Respiratory Tract Infection; ICU = Intensive Care Unit
Lastly, the RSV-LRTIs primary care visit rates were obtained and stratified by infant’s month of age using the Spanish study BARI (24). According to the clinicians involved in this study, they were assumed to be equal between the two subpopulations (Table 1).
Concerning complications following an RSV LRTI hospitalization, international literature was used as well in the absence of specific national data to document these risks.
The risk of developing recurrent wheezing following an RSV hospitalization was obtained from Li et al. 2022 (25) study, which reported a risk of recurrent wheezing during the first year of life following an RSV hospitalization equals 31% (29), while the risk of recurrent wheezing in the second and third year of age was respectively equal to 27% and 17% (30) (Table 1).
The probability of developing asthma after an RSV LRTI hospitalization was informed by a case-control study conducted in Scotland to evaluate the association between hospitalization due to RSV infection and asthma among infants who had an RSV hospitalization during their first two years of life [26]. Within the model, the risk of contracting asthma was considered as a one-off probability of being hospitalized for asthma following an RSV hospitalization (Table 1); this one-off probability was calculated as the sum of the annual probabilities of being hospitalized for asthma up to 18 years of age following hospitalization for RSV infection within the first two years of life (26).
Finally, the analysis considered both all-cause mortality rates among infants and in-hospital mortality due to RSV infection. The all-cause mortality rates among infants by age were obtained from the mortality table published by ISTAT and relating to the year 2021 (31), while the risk of in-hospital mortality due to RSV infection by subpopulation was obtained from the Spanish study conducted by Sanchez-Luna et al. 2017 (23) (Table 1) as no local data was available.
Cost parameters
In terms of direct costs, the economic model considered the cost associated with RSV-related health events and the management costs of the complications related to RSV hospitalization.
Indirect costs associated with premature death due to RSV infection were evaluated in the scenario analysis.
The cost associated with each health event was conservatively assumed to be equal for both subpopulations in the analysis. The hospitalization and ICU admission-associated costs were estimated through a retrospective analysis conducted on the National Hospital Discharge Records for RSV admissions among infants aged <2 years for the period 2016-2019 (diagnosis codes are reported in the Appendix) (Table 1). The cost of an emergency room visit was obtained from Mattoni’s project by the Ministry of Health (32), and it was actualized in 2021 when the cost of a primary care visit was estimated using the national tariff associated with the outpatient general visit [Code 89.7 (33)]) (Table 1). Concerning the cost associated with mid- to long-term complications resulting from an RSV-related hospitalization, the cost of recurrent wheezing management was estimated considering the hospitalization cost for bronchitis and asthma [national tariff associated with DRG 98 - Bronchitis and asthma age <18 years (33)] for the first year of age and assuming the cost of 5.5 primary care visits [as considered in the economic analysis conducted by Li et al. (25)] for the second and third year of age (Table 1). Wheezing management costs related to the second and third year of age were also discounted, considering an annual discount of 3% (34).
Asthma management cost was obtained from the work conducted by Calabria et al., 2021 [35], a national observational study based on another Italian administrative database to evaluate the health resource utilization and costs associated with patients with asthma, BPCO and mixed disorders asthma/BPCO (Table 1). This cost was then projected for 18 years considering an annual discount rate of 3%; the one-off cost associated with the asthma management was obtained as a weighted average of the annual costs related to the asthma management (discounted and estimated for 18 years) for the respective probabilities of an asthma-related hospitalization following RSV hospitalization stratified by age. This one-off cost was then associated with the one-off probability of being hospitalized for asthma following a hospitalization for RSV infection (26).
Direct total costs associated with RSV related health events reported above were calculated for each subpopulation. The estimates of direct total costs were influenced by the coverage rates and the efficacy of immunization strategies measured in terms of reduction in RSV-MA-LRTIs events (2,3).
In the scenario analysis, the productivity loss due to RSV-related premature deaths was also considered as indirect costs. The productivity loss was evaluated in terms of income lost due to premature death and considering an average age of exit from the labour market equal to 62 years (36). The average annual income was calculated considering the average hourly income [€11.40) (37)], the number of working hours per day (6.7 hours) (calculated considering 36.9 hours per week (38) and assuming 5.5 working days per week), the number of working days per year (255) and the employment rate [58.1% (39)].
Results
Under the current standard of practice, the disease burden of an epidemic RSV season in Italy is estimated at 216,100 RSV-MA-LRTIs, 15,121 complications (asthma and recurrent wheezing), and 16 deaths due to RSV-MALRTIs. In particular, the model estimated a total of 171,941 primary care visits due to RSV infections, 29,068 ER visits, and 15,091 hospitalizations (of which 2,073 ICU admissions) (Figure 2). Regarding hospitalizations, the model also estimated 5,118 (39.3%) and 7,900 (60.7%) RSV hospitalizations (including admissions in ICU) for infants born before the RSV season and infants born during the RSV season, respectively (Figure 3). Regarding mid-long-term sequelae following an RSV-related hospitalization, the model estimated a total of 11,318 recurrent wheezing events and a total of 3,803 asthma events (Figure 2).
| Input | Palivizumab-eligible infants | Late preterm and term infants | Source |
|---|---|---|---|
| Population size | 4.4% | 95.6% | CeDAP 2021 (15), ORPHANET (16), SIP (17) |
| Proportion of annual births by month (%) | |||
| April | 7.6 | ISTAT - Births by month (year 2021) (27) | |
| May | 7.8 | ||
| June | 7.8 | ||
| July | 8.6 | ||
| August | 8.8 | ||
| September | 9.3 | ||
| October | 9.1 | ||
| November | 8.5 | ||
| December | 8.7 | ||
| January | 7.8 | ||
| February | 7.4 | ||
| March | 8.4 | ||
| Monthly probability of RSV infections (%) | |||
| April | 3.4 | Barbati et al. 2020 (7) | |
| May | 0.0 | ||
| June | 0.2 | ||
| July | 0.2 | ||
| August | 0.0 | ||
| September | 0.0 | ||
| October | 0.2 | ||
| November | 2.3 | ||
| December | 22.8 | ||
| January | 29.4 | ||
| February | 28.5 | ||
| March | 13.2 | ||
| Coverage rate (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| Palivizumab | 75.0 | - | Data for season 2022-2023 |
| Nirsevimab | 75.0 | 60.0 | Data for season 2022-2023 and assumption |
| Efficacy (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| Nirsevimab (inpatient) | 79.5 | 79.5 | Andabaka et al. 2013 (20), Simoes et al. 2023 (18) |
| Nirsevimab (outpatient) | 79.5 | 79.5 | |
| Palivizumab (inpatient) | 51 | ||
| Palivizumab (outpatient) | 51 | ||
| Inpatient hospitalization risk per month (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| 0 months | 11.4 | 6.9 | Feltes et al. 2003 (21) and Heppe Montero et al. 2022 (22) |
| 1 month | 21.9 | 10.2 | |
| 2 months | 12.1 | 6.8 | |
| 3 months | 8.7 | 3.9 | |
| 4 months | 7.5 | 3.1 | |
| 5 months | 4.0 | 2.2 | |
| 6 months | 3.5 | 1.9 | |
| 7 months | 4.7 | 1.5 | |
| 8 months | 2.9 | 1.1 | |
| 9 months | 3.2 | 0.9 | |
| 10 months | 3.1 | 0.7 | |
| 11 months | 2.4 | 0.6 | |
| ICU hospitalization risk per month (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| 0 months | 2.8 | 1.1 | Data from Barbati et al. 2020 (7) has been reproportioned with Spanish data from Sanchez Luna et al. 2017 (23) |
| 1 month | 5.4 | 1.6 | |
| 2 months | 2.9 | 1.0 | |
| 3 months | 2.1 | 0.6 | |
| 4 months | 1.8 | 0.5 | |
| 5 months | 1.0 | 0.3 | |
| 6 months | 0.9 | 0.3 | |
| 7 months | 1.2 | 0.2 | |
| 8 months | 0.7 | 0.2 | |
| 9 months | 0.8 | 0.1 | |
| 10 months | 0.8 | 0.1 | |
| 11 months | 0.6 | 0.1 | |
| ER visit risk per month (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| 0 months | 30.1 | 17.0 | Martinon-Torres et al. 2022 (BARI study) (24) → with application of gradient observed for hospitalizations |
| 1 month | 50.8 | 22.0 | |
| 2 months | 28.9 | 15.0 | |
| 3 months | 16.8 | 7.0 | |
| 4 months | 17.3 | 6.5 | |
| 5 months | 15.9 | 7.9 | |
| 6 months | 6.3 | 3.2 | |
| 7 months | 7.4 | 2.1 | |
| 8 months | 7.1 | 2.5 | |
| 9 months | 7.6 | 2.0 | |
| 10 months | 7.9 | 1.7 | |
| 11 months | 7.8 | 1.8 | |
| Primary care visit risk per month (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| 0 months | 89.4 | 89.4 | Martinon-Torres et al. 2022 (BARI study) (24) |
| 1 month | 144.1 | 144.1 | |
| 2 months | 79.5 | 79.5 | |
| 3 months | 48.1 | 48.1 | |
| 4 months | 48.1 | 48.1 | |
| 5 months | 48.1 | 48.1 | |
| 6 months | 15.6 | 15.6 | |
| 7 months | 15.6 | 15.6 | |
| 8 months | 15.6 | 15.6 | |
| 9 months | 15.6 | 15.6 | |
| 10 months | 15.6 | 15.6 | |
| 11 months | 15.6 | 15.6 | |
| Complications due to RSV hospitalisation (%) | Palivizumab-eligible infants | Late preterm and term infants | Source |
| Risk of recurrent wheezing in the first year of life following a hospitalization due to RSV in the first year of life | 31.0 | 31.0 | Li et al. 2022 (25) |
| Risk of recurrent wheezing in the second year of life following a hospitalization due to RSV in the first year of life | 27.0 | 27.0 | Li et al. 2022 (25) |
| Risk of recurrent wheezing in the third year of life following a hospitalization due to RSV in the first year of life | 17.0 | 17.0 | Li et al. 2022 (25) |
| Risk of hospitalization due to asthma following a hospitalization due to RSV infection in the first two years of life (one-off probability) | 25.2 | 25.2 | Coutts et al. 2019 (26) |
| All-cause mortality among infants by age | Palivizumab-eligible infants | Late preterm and term infants | Source |
| 0-5 months | 0.0002 | 0.0002 | Mortality Table 2021 ISTAT (31) |
| 6-11 months | 0.0002 | 0.0002 | |
| Risk of death among RSV inpatient hospitalizations by age | Palivizumab-eligible infants | Late preterm and term infants | Source |
| 0-5 months | 0.0095 | 0.0005 | Sanchez Luna et al. 2016 (23) |
| 6-11 months | 0.0095 | 0.0005 | |
| Health event costs (€) | Cost per event | Fonte | |
| Inpatient hospitalization | 2,050.9 | SDO Italia 2016-2019 | |
| ICU | 5,484.2 | SDO Italia 2016-2019 | |
| ER visit | 305.6 | Progetto Mattoni (32) actualized at 2021 | |
| Primary care visit | 20.7 | National tariff of outpatient visits (33) | |
| Cost for the management of recurrent wheezing (€) | Annual cost | Source | |
| Recurrent wheezing (year 1) | 1,538.0 | - | National tariff of acute hospital care services (33) (DRG 98) |
| Recurrent wheezing (year 2) | 110.3 | - | 5.5 outpatient visits discounted in the second year (Li et al. 2022 (25); National tariff of outpatient visits (33) |
| Recurrent wheezing (year 3) | 107.1 | - | 5.5 outpatient visits discounted in the third year (Li et al. 2022 (25); National tariff of outpatient visits (33) |
| Cost for the management of asthma (€) | One-off cost | Source | |
| Hospitalization due to asthma | 787.4 | - | Calabria et al. 2021 (35) and Coutts et al. 2020 (26). Annual costs up to 18 years of age, discounted considering an annual rate of 3% |
The economic burden associated with the RSV infection management and the associated events was estimated at a total cost incurred by NHS equal to € 61.4 million. The costs associated with hospitalizations, ER visits, and primary care visits were estimated to equal €38.1 million (of which €11.4 million for ICU for admissions), €8.9 million, and €3.5 million, respectively (Figure 2). The cost associated with the management of recurrent wheezing following a hospitalization due to RSV infection was estimated at €7.9 million, while asthma management cost was estimated at €3 million (Figure 2). Considering also indirect costs, the cost associated with premature death among RSV inpatient hospitalizations was estimated at €3 million, leading to a total cost from the societal perspective estimated at € 64.4 million.
The model estimated that an immunization strategy with nirsevimab could lead to a reduction of 100,208 RSV-MA-LRTIs, 6,969 associated complications, and could avoid 6 deaths following an RSV-related hospitalization. To be more specific, the model estimated a reduction of 6,955 hospitalizations (including 948 ICU admissions), 13,382 ER visits, and 79,871 primary care visits due to RSV (Figure 4). These reductions of RSV-MA-LRTIs also might generate a reduction of 5,216 events of recurrent wheezing following an RSV hospitalization and a reduction of 1,753 asthma events (Figure 4). The reduction of RSV-related health events following nirsevimab introduction can be translated into economic savings by reducing direct costs to €28.3 million (excluding the cost of prophylaxis). The model estimated savings of up to €17.5 million, €4.1 million, and €1.7 million, respectively, associated with hospitalizations, ER visits, and primary care visits reduction (Figure 5); the consequent reduction of complications could also generate a decrease in the management costs for recurrent wheezing and asthma equal to €5 million (Figure 5).
Regarding the productivity loss due to premature mortality in RSV hospitalized patients, the immunization strategy with nirsevimab could generate a reduction of indirect costs equal to €1.2 million (Figure 5).
The deterministic sensitivity analysis shows that the parameter associated with the greatest impact on economic savings generated by the use of the immunization strategy with nirsevimab (€28.3 million) is the risk of RSV-related health events for the term infants population (Figure 6). Table A in the Appendix also reported the epidemiological and economic impact of nirsevimab introduction estimated by the model for each region considering the resident population and keeping all other parameters constant.
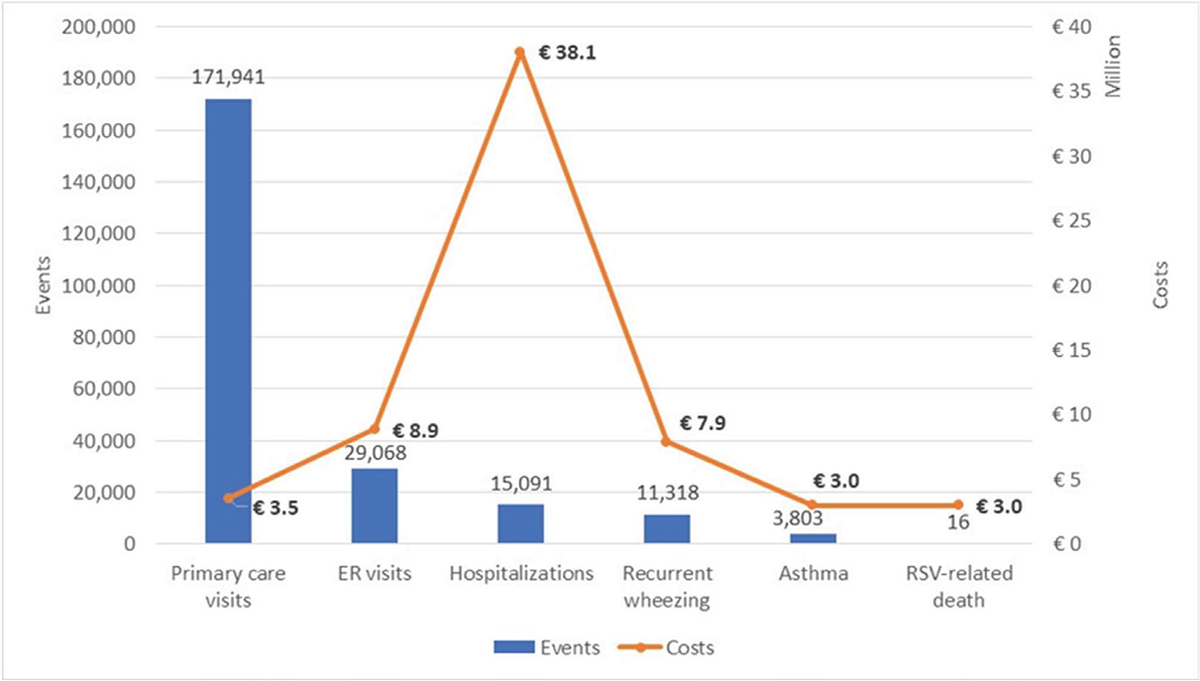
FIGURE 2 - Disease burden of an epidemic RSV season under the current standard of practice
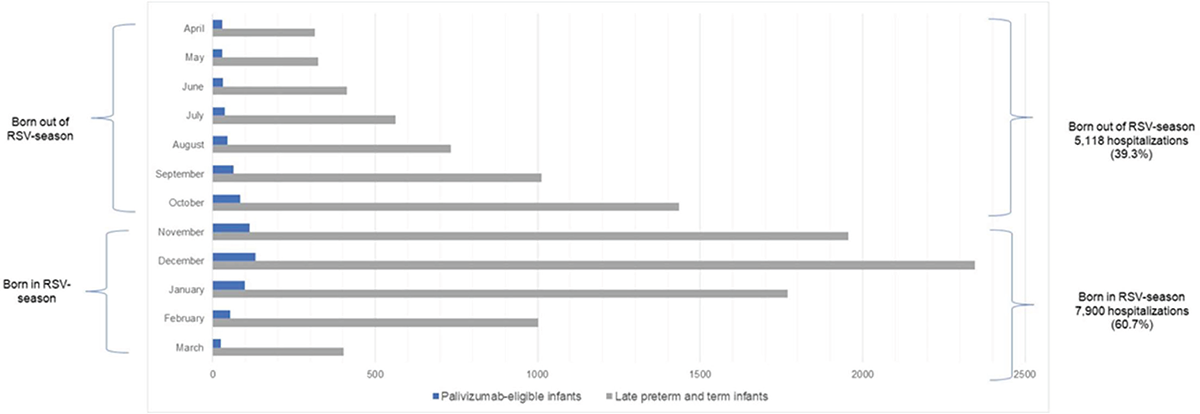
FIGURE 3 - Number of Inpatient Hospitalizations per month—SoC
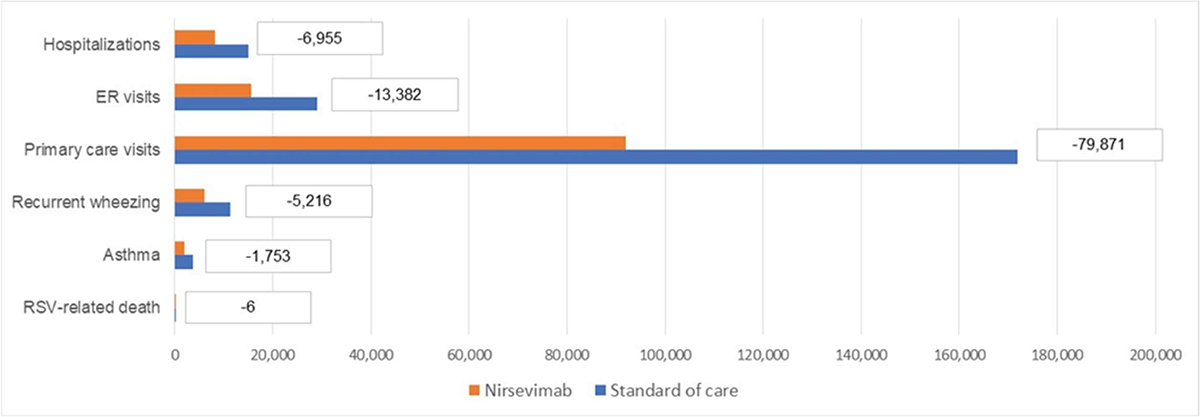
FIGURE 4 - Health events due to RSV-MA-LRTIs for each immunization strategy
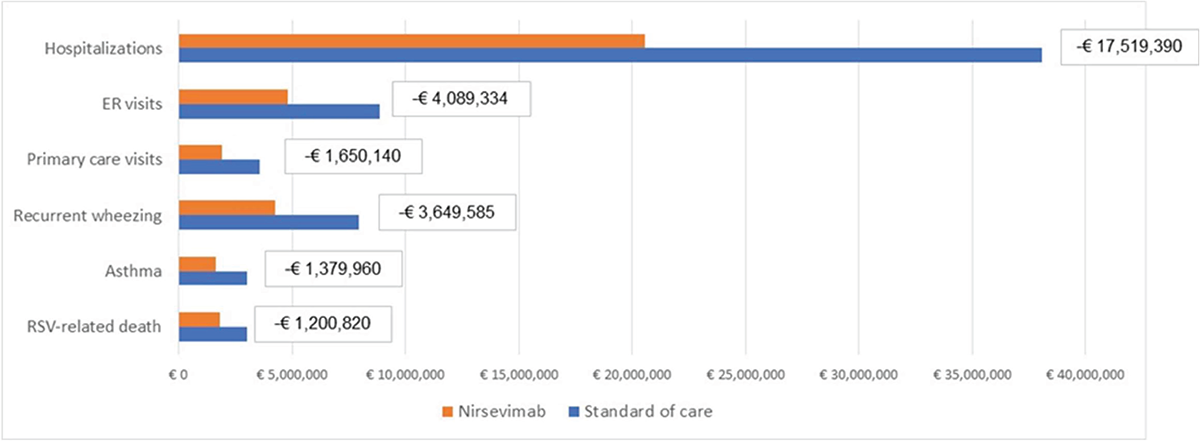
FIGURE 5 - Costs associated with the management of health events due to RSV-MA-LRTIs for each immunization strategy.
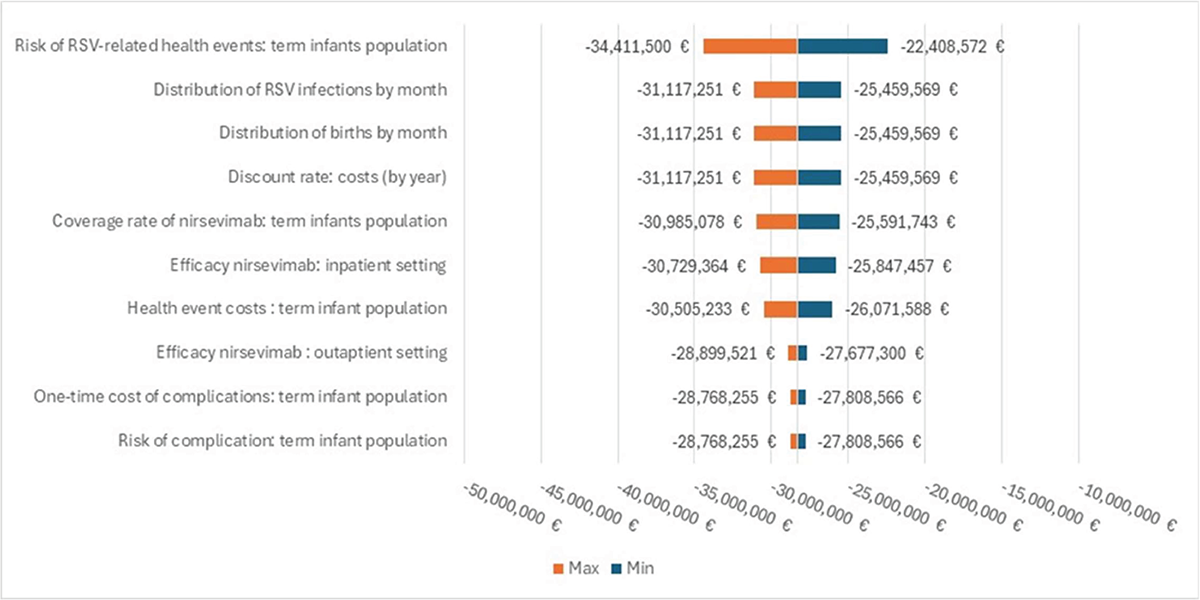
FIGURE 6 - Results of the deterministic sensitivity analysis—Tornado graph
Lastly, the model provided an estimate of the number needed to immunize (NNI) to prevent one RSV-related event each season. Considering an efficacy for nirsevimab equal to 79.50% against RSV MA-LRTI for both subpopulations, among newborns, 3 infants need to be immunized to prevent one RSV MA-LRTI, 35 infants need to be immunized to prevent one RSV-LRTI hospitalization, 241 infants need to be immunized to prevent one ICU visit due to RSV- LRTIs, 16 infants need to be immunized to prevent one ER visit due to RSV- LRTIs, 3 infants need to be immunized to prevent one primary care visit due to RSV- LRTIs and 24,942 infants need to be immunized to prevent one inpatient death.
Discussion
This work is the first study to describe the seasonal RSV disease and economic burden in Italy in terms of health events and associated costs considering the current prophylaxis strategy protecting only palivizumab-eligible infants, including preterm infants ≤35 weeks gestational age (wGA) or infants with congenital heart or chronic lung disease. A cost-consequence analysis was conducted to evaluate the potential benefits of nirsevimab, a new prophylaxis strategy protecting all infants, in terms of RSV-related health events reduction and associated costs. Currently, the coverage rate associated with palivizumab is assumed at 75%. Using this coverage rate, the model estimated a clinical burden associated with RSV equal to 231,237 events, of which 93% were RSV infections and 7% were complications and death due to RSV infections.
More specifically, the model estimated 216,100 cases of RSV-MALRTIs (15,091 hospitalizations (≈13.7% ICU), 29,068 ER visits, and 171,941 PC visits), 11,318 cases of recurrent wheezing, 3,803 asthma events and 16 deaths due to RSV infection. With the current immunization strategy, the economic burden associated with RSV was estimated to equal €61.4 million (€38.1 million for hospitalization, €8.9 million for ER visits, €3.5 million for PC visits, and €10.9 million for complications). The immunization strategy with nirsevimab, applying the conservative 75% coverage rate for palivizumab-eligible infants and a 60% coverage rate for late preterm and term infants, could lead to a reduction of 107,183 RSV-related health events (6,955 hospitalizations, 13,382 ER visits, 79,871 PC visits, 6,969 complications, and 6 death). The immunization strategy with nirsevimab could reduce the costs associated with the management of RSV events, saving €28.3 million (€17.5 million for hospitalizations, €4.1 million for ER visits, €1.7 million for PC visits, and €5 million for complications).
At the time of the analysis, the price of nirsevimab was not known; hence, it was decided to focus on the public health and economic impact associated with the different immunization strategies, similar to what has been done in a recently published study conducted in Spain (40).
The study conducted in Spain using data from a longitudinal electronic medical records database from two Spanish regions has estimated a healthcare cost associated with 3,460 children aged <5 years old who have been diagnosed with RSV-specific and acute lower respiratory infection (ALRI) in season 2017/18 equal to €3.8 million, mainly driven by hospitalizations (45.8%) (24). Also, in our study, the main cost driver was hospitalization costs, which correspond to 62% of the total economic burden associated with RSV-MA-LRTIs in the current immunization strategy. Moreover, in the Spanish study, most of the healthcare costs were generated by children aged <1 year old (who contribute to 78.7% of the overall healthcare costs of medically attended RSV-specific cases) and by healthy children, regardless of age (who contribute to 86.1% of the direct healthcare costs of medically attended RSV-specific cases); this data confirms that most of the burden of disease occurs in healthy full-term infants (24).
This study also considered indirect costs to better define the economic burden of RSV infections.
For the SoC, the model estimated a productivity loss equal to €3 million due to premature mortality following an RSV-LRTI hospitalization. The introduction of nirsevimab could generate €1.2 million in savings associated with the reduction of indirect health events.
With the current standard of practice, in addition to the management costs associated with RSV, the NHS sustains an expenditure of about €44 million for palivizumab doses purchase (about 56,000 doses) to protect palivizumab-eligible infants (41).
The model also attempts to estimate the public health benefit of nirsevimab using the number needed to immunize (NNI). Considering an efficacy for nirsevimab equal to 79.50% against RSV-MA-LRTI for the entire cohort of newborns, 35 infants need to be immunized to prevent one hospitalization due to RSV-LRTI and 3 infants need to be immunized to prevent one primary care visit due to RSV-LRTI. A recently published study estimated that for extended half-life RSV monoclonal antibodies (EHL-mAb) with 70% efficacy, 37-280 infants would need to be immunized to prevent one RSV-associated hospitalization in the first year of life, and 13-33 infants would need to be immunized to prevent one RSV associated LRTI outpatient visit in the first year of life (42).
The estimated NNI for nirsevimab was lower than the number needed to vaccinate (NNVs) for vaccines against other pathogens included in the routine childhood immunization schedule in Italy. For an influenza vaccine with 50% efficacy, 1,000–3,000 children 6–23 months of age would need to be vaccinated to prevent one influenza-attributable hospitalization, and 12–42 children vaccinated to prevent one influenza-attributable outpatient visit (43). Another study estimated that 671 infants would need to be immunized with a pneumococcal conjugate vaccine to prevent one case of invasive pneumococcal disease (44).
This study presents several limitations, mainly due to the absence of local data referring to the national Italian context. For instance, data on costs for RSV health events were assumed to be equal for the analyzed subgroups. The hospitalization risks for both subpopulations were obtained from a Spanish study conducted by Heppe Montero et al. 2022 (22) for late preterm and term infants and from the clinical trial of palivizumab for preterm and palivizumab-eligible infants (21). The overall intensive care hospitalization risk rates obtained from the Barbati et al. publication (7) were reproportioned according to the distribution of intensive care admission estimated for Spain in the study conducted by Sanchez-Luna et al. 2017 to obtain the proportion of intensive care admission for both subpopulations (23); the RSV emergency room risks per infant’s month of age were obtained by the Spanish study BARI (24). To obtain the distribution rates of these risks also for each subpopulation, the gradient observed for hospitalization risks was applied; the RSV primary assistance rate per child’s month of age was obtained from the Spanish study BARI (24), and these rates, agreeing with clinicians involved in the analysis, were considered equal for both subpopulation. The risk of contracting recurrent wheezing and asthma due to RSV infection was obtained from international literature (25,26) and assumptions validated by experts. In particular, with reference to asthma, the evidence present in the literature shows that demonstrating an association between asthma and RSV-LRTI is challenging (45). Some studies suggest no clear evidence of a definitive association with RSV-LRTI (46,47), while others report a significantly increased risk throughout childhood (48-50). According to clinicians involved in the study, it was considered appropriate to attempt to provide an estimate of the possible clinical and economic impact of developing asthma among infants hospitalized due to RSV.
Another limitation related to the lack of national data regarding the in-hospital mortality probabilities; these estimates were obtained from the Spanish study done by Sanchez-Luna et al. 2017 (23).
In addition, it has been assumed that each event is mutually exclusive as the total RSV-MA-LRTIs was calculated as the sum of hospitalizations, ER visits, and primary care visits due to RSV infections.
Furthermore, as a conservative approach, the model did not consider the effect of the waning efficacy of nirsevimab after 5 months of protection. However, as reported in the US study that used the same assumption (12), given that the analyses were performed for a single RSV season, waning would only affect the final month for infants born outside of the season and was not expected to impact the study conclusions.
Another limitation consists in the same efficacy applied to the palivizumab-eligible infants for both prophylaxis strategies since the clinical trials conducted for nirsevimab support the extrapolation of the estimated efficacy for preterm and term infants to the infants eligible for palivizumab (18,19), the same efficacy was assumed (19).
Moreover, the coverage rate for nirsevimab for palivizumab-eligible infants was assumed to be equal to palivizumab (75%), while for late preterm and term infants, nirsevimab coverage rate was assumed to be similar to those associated with recommended non-mandatory vaccines in early childhood in Italy (60%).
Those assumptions were considered conservative considering the high acceptance rate achieved in the countries or regions that have implemented a prevention program during the season 2023/2024, such as Galicia or Cataluna in Spain (51,52), Portugal (53), France (54) and Luxembourg (55).
Lastly, due to the absence of more specific data, costs associated with each health event were conservatively assumed equal for both subpopulations in the analysis.
Conclusion
In the current standard of practice, the immunization strategy with palivizumab restricted to palivizumab-eligible infants (4.4 % of the full birth cohort) leaves a substantial RSV burden on the healthcare system. A new immunization strategy with nirsevimab targeting all infants could substantially reduce RSV-related health events (hospitalizations, ER visits, PC visits, complications, and inpatient deaths), with an important reduction in the costs associated with the management of RSV infections and associated events. Since the new prophylaxis strategy is intended to protect all infants, it represents a possible solution to prevent the increase of the clinical and economic burden of RSV. In this perspective, as already recommended by several Italian Scientific Societies (56), the inclusion of nirsevimab in the national immunization calendar for a newborn cohort prevention strategy against RSV may support the implementation and the equity of RSV prevention for all infants during their first RSV season.
Disclosures
Financial Support: Study funded by Sanofi and AstraZeneca.
Conflict of Interest: Muzii B, Soudani S, Kieffer A, and Beuvelet M are employees of Sanofi and may have shares and/or stock options of the Company. Mennini FS, Marcellusi A, Bini C, Bozzola E, Midulla F, Baraldi E, Bonanni P, Boccalini S, and Orfeo L declare that they have no conflicts of interest.
References
- 1. Cutrera R, Wolfler A, Picone S, et al. Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016-2017 epidemic season: a systematic review of seven Italian reports. Ital J Pediatr. 2019;45(1):139. CrossRef PubMed
- 2. Janet S, Broad J, Snape MD. Respiratory syncytial virus seasonality and its implications on prevention strategies. Hum Vaccin Immunother. 2018;14(1):234-244. CrossRef PubMed
- 3. Mazur NI, Martinón-Torres F, Baraldi E, et al. Respiratory Syncytial Virus Network (ReSViNET). Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015;3(11):888-900. CrossRef PubMed
- 4. Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in Western countries. Infect Dis Ther. 2016;5(3):271-298. CrossRef PubMed
- 5. Azzari C, Baraldi E, Bonanni P, et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47(1):198. CrossRef PubMed
- 6. Manti S, Staiano A, Orfeo L, et al. UPDATE - 2022 Italian guidelines on the management of bronchiolitis in infants. Ital J Pediatr. 2023;49(1):19. CrossRef PubMed
- 7. Barbati F, Moriondo M, Pisano L, et al. Epidemiology of respiratory syncytial virus-related hospitalization over a 5-year period in Italy: evaluation of seasonality and age distribution before vaccine introduction. Vaccines (Basel). 2020;8(1):15. CrossRef PubMed
- 8. Barbieri E, Cavagnis S, Scamarcia A, et al. Assessing the burden of bronchiolitis and lower respiratory tract infections in children ≤24 months of age in Italy, 2012-2019. Front Pediatr. 2023;11:1143735. CrossRef PubMed
- 9. Bechini A, Salvati C, Bonito B, et al. Costs and healthcare utilization due to respiratory syncytial virus disease in paediatric patients in Italy: a systematic review. Public Health. 2024;227:103-111. CrossRef PubMed
- 10. Bozzola E, Ciarlitto C, Guolo S, et al. Respiratory syncytial virus bronchiolitis in infancy: the acute hospitalization cost. Front Pediatr. 2021;8:594898. CrossRef PubMed
- 11. Kobelt G, Woronoff AS, Richard B, et al. Disease status, costs and quality of life of patients with rheumatoid arthritis in France: the ECO-PR Study. Joint Bone Spine. 2008;75(4):408-415. CrossRef PubMed
- 12. Kieffer A, Beuvelet M, Sardesai A, et al. Expected impact of universal immunization with nirsevimab against rsv-related outcomes and costs among all US infants in their first rsv season: a static model. J Infect Dis. 2022;226(suppl 2):S282-S292. CrossRef PubMed
- 13. ISTAT. Popolazione residente al 1° gennaio 2023. Online (Accessed June 2024)
- 14. Associazione Nazionale Malati Reumatici (ANMAR), Società Italiana di Reumatologia (SIR), and Fondazione Censis. Un percorso ad ostacoli. Primo rapporto sociale sull’artrite reumatoide 2008. Online. (Accessed June 2024)
- 15. Ministero della Salute. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita - Anno 2021. Online. (Accessed June 2024)
- 16. ORPHANET, Displasia broncopolmonare. Online.
- 17. SIP. Cardiopatie congenite, 5 cose da sapere. Online. (Accessed June 2024)
- 18. Simões EAF, Madhi SA, Muller WJ, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomized controlled trials. Lancet Child Adolesc Health. 2023;7(3):180-189. CrossRef PubMed
- 19. Domachowske J, Madhi SA, Simões EAF, et al; MEDLEY Study Group. Safety of nirsevimab for rsv in infants with heart or lung disease or prematurity. N Engl J Med. 2022;386(9):892-894. CrossRef PubMed
- 20. Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;(4):CD006602. PubMed
- 21. Feltes TF, Cabalka AK, Meissner HC, et al. Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532-540. CrossRef PubMed
- 22. Heppe Montero M, Gil-Prieto R, Walter S, et al. Burden of severe bronchiolitis in children up to 2 years of age in Spain from 2012 to 2017. Hum Vaccin Immunother. 2022;18(1):1883379. CrossRef PubMed
- 23. Sanchez-Luna M, Burgos-Pol R, Oyagüez I, et al. Cost-utility analysis of Palivizumab for Respiratory Syncytial Virus infection prophylaxis in preterm infants: update based on the clinical evidence in Spain. BMC Infect Dis. 2017;17(1):687. CrossRef PubMed
- 24. Martinón-Torres F, Carmo M, Platero L, et al. Clinical and economic burden of respiratory syncytial virus in Spanish children: the BARI study. BMC Infect Dis. 2022;22(1):759. CrossRef PubMed
- 25. Li X, Bilcke J, Vázquez Fernández L, et al; REspiratory Syncytial virus Consortium in EUrope (RESCEU) Investigators. Cost-effectiveness of respiratory syncytial virus disease prevention strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226(suppl 1):S95-S101. CrossRef PubMed
- 26. Coutts J, Fullarton J, Morris C, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol. 2020;55(5):1104-1110. CrossRef PubMed
- 27. ISTAT. Nati per giorno del mese e della settimana e per mese dell’anno - Anno di iscrizione 2021. Online. (Accessed June 2024)
- 28. Schauer U, Hoffjan S, Bittscheidt J, et al. RSV bronchiolitis and risk of wheeze and allergic sensitization in the first year of life. Eur Respir J. 2002;20(5):1277-1283. CrossRef PubMed
- 29. Escobar GJ, Masaquel AS, Li SX, et al. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13(1):97. CrossRef PubMed
- 30. ISTAT. Tavole di mortalità: singole età. 2021. Online. (Accessed June 2024)
- 31. Ministero della salute. Progetto Mattoni SSN. Pronto Soccorso e sistema 118. Proposta metodologica per la valutazione dei costi dell’emergenza. Online. (Accessed June 2024)
- 32. Decreto del Ministero della Salute 18 ottobre 2012. Tariffe delle prestazioni di assistenza specialistica ambulatoriale. Online. (Accessed June 2024)
- 33. AIFA. Linee guida per la compilazione del Dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale ai sensi del D.M. 2 agosto 2019.; Online. (Accessed June 2024)
- 34. Calabria S, Ronconi G, Dondi L, et al. Analisi real-world dei disturbi ostruttivi delle vie respiratorie: caratterizzazione, assistenza sanitaria e costi. Recenti Prog Med. 2021;112(4):285-293. PubMed
- 35. OECD. Online. (Accessed June 2024)
- 36. ISTAT. Average hourly earnings for employee jobs in the private sector in 2019. Online. (Accessed June 2024)
- 37. OECD. Average usual weekly hours worked on the main job. Total age. Total employment. Total declared employment.; Online. (Accessed June 2024)
- 38. ISTAT. Employment rate 2020. Online. (Accessed June 2024)
- 39. Gil-Prieto R, Pérez JJ, Drago G, et al. Modelling the potential clinical and economic impact of universal immunization with nirsevimab versus standard of practice for protecting all neonates and infants in their first respiratory syncytial virus season in Spain. BMC Infect Dis. 2024;24(1):924. CrossRef PubMed
- 40. Kobelt G, Jönsson L, Lindgren P, et al. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2310-2319. CrossRef PubMed
- 41. Finelli L, Choi Y, Goldstein E. Number needed to immunize to prevent RSV with extended half-life monoclonal antibody. Vaccine. 2020;38(34):5474-5479. CrossRef PubMed
- 42. Lewis EN, Griffin MR, Szilagyi PG, et al. Childhood influenza: number needed to vaccinate to prevent 1 hospitalization or outpatient visit. Pediatrics. 2007;120(3):467-472. CrossRef PubMed
- 43. Palmu AA, Jokinen J, Nieminen H, et al. Vaccine-preventable disease incidence of pneumococcal conjugate vaccine in the Finnish invasive pneumococcal disease vaccine trial. Vaccine. 2018;36(14):1816-1822. CrossRef PubMed
- 44. Fauroux B, Simões EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther. 2017;6(2):173-197. CrossRef PubMed
- 45. Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomized controlled trial. Lancet Respir Med. 2018;6(4):257-264. CrossRef PubMed
- 46. Korppi M, Piippo-Savolainen E, Korhonen K, et al. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol. 2004;38(2):155-160. CrossRef PubMed
- 47. Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045-1052. CrossRef PubMed
- 48. Green CA, Yeates D, Goldacre A, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child. 2016;101(2):140-146. CrossRef PubMed
- 49. Henderson J, Hilliard TN, Sherriff A, et al. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386-392. CrossRef PubMed
- 50. López-Lacort M, Muñoz-Quiles C, Mira-Iglesias A, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Euro Surveill. 2024;29(6):2400046. CrossRef PubMed
- 51. Dirección Xeral de Saúde Pública. FOLLOW-UP REPORT ON IMMUNIZATION WITH NIRSEVIMAB IN GALICIA. Data up to week 52, 2024 (31-12-2023). Online. (Accessed June 2024)
- 52. Região Autónoma da Madeira. Boletim de Imunização contra VSR - N.14. Online. (Accessed June 2024)
- 53. Marcellusi AVR, Mecozzi A, et al. The direct and indirect cost of diabetes in Italy: a prevalence probabilistic approach. The European journal of health economics. HEPAC Health Econ Prev Care. 2014. CrossRef
- 54. Ernst C, Bejko D, Gaasch L, et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalizations during the initial 2023/24 season in Luxembourg. Euro Surveill. 2024;29(4):2400033. CrossRef PubMed
- 55. SIP. et al. Calendario vaccinale per la vita. 4° edizione 2019. Online. (Accessed June 2024)