 |
Glob Reg Health Technol Assess 2024; 11: 239-247 ISSN 2283-5733 | DOI: 10.33393/grhta.2024.3170 ORIGINAL RESEARCH ARTICLE |
 |
A 1-year per-patient cost of therapy administration analysis of mosunetuzumab and tisagenlecleucel in relapsed or refractory follicular lymphoma patients receiving two or more lines of systemic therapy
ABSTRACT
Objective: A per-patient cost of therapy administration model was developed to estimate the cost of mosunetuzumab vs. tisagenlecleucel in patients with relapsing or refractory follicular lymphoma (R/R FL) receiving two or more lines of systemic therapy (3L+) from both the Italian hospital and societal perspectives.
Methods: A per-patient total cost of therapy administration model was developed to compare the resource consumption of two treatments – mosunetuzumab and tisagenlecleucel. The model considered direct costs such as healthcare labor costs for drug preparation and administration, non-drug consumable costs, and drug purchase. Indirect costs such as patient and caregiver’s loss of productivity, transportation, and relocation were also considered. The unit costs and resource use data were retrieved from literature and standard Italian tariffs. To appraise the impact of patients’ residency on access-to-care and out-of-pocket expenses, three scenario analyses were conducted.
Results: Over 1 year, mosunetuzumab costs approximately one-fourth of tisagenlecleucel per patient. The base-case scenario showed a hospital cost reduction of €158,870 per patient with mosunetuzumab, increasing to €161,974 when including societal costs. Scenario analyses for the societal perspective estimated cost differences of −€161,170, −€166,507, and −€166,811 for scenarios A, B, and C, respectively. Sensitivity analysis indicated that tisagenlecleucel’s price had the greatest impact on cost differences, followed by mosunetuzumab’s price.
Conclusions: This analysis identifies mosunetuzumab as an accessible therapeutic option for 3L+ R/R FL patients in Italy. Future research should collect real-time data and evaluate long-term outcomes.
Keywords: Economic evaluation, Healthcare costs, Micro-costing, Oncology
Received: July 4, 2024
Accepted: November 17, 2024
Published online: December 9, 2024
This article includes supplementary materials
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Follicular lymphoma (FL) is the most commonly occurring type of indolent lymphoma in Western countries, accounting for 30% of all lymphomas. It is characterized by a variable and heterogeneous course, as it tends to relapse and remit over time, posing significant therapeutic challenges (1-5).
Median survival of FL patients is about 20 years, yet it significantly decreases in the case of early progression and with the increase in treatment lines (1-5). There was a high unmet medical need for relapsed/refractory (R/R) heavily treated patients, considering that approximately 20% of FL patients treated with chemo-immunotherapy relapsed within 2 years (6). In this clinical setting, there was a lack of new effective treatments, which often resulted in the rechallenge with previously administered drug combinations (7).
An advancement in the field of precision medicine has been the approval of tisagenlecleucel, a chimeric antigen receptor T-cell therapy (CAR-T) (8,9) for R/R FL patients who have received at least two prior systemic therapies (3L+). Although Advanced Therapy Medicinal Products (ATMPs) have high therapeutic potential, their implementation is hindered by accessibility constraints, the need for close monitoring of adverse events (AEs) (5,6,10), and the necessity of a 3-4 week timeline for manufacturing and administration. In this context, mosunetuzumab – the first bispecific monoclonal antibody approved by the Italian National Medicines Agency in the hematology-oncology setting – could represent a therapeutic option. Mosunetuzumab has demonstrated a high rate of complete responses (CRs) achieved rapidly and sustained over time (CR: 60%, objective response rate (ORR): 80%) (11). As shown in the relevant clinical studies, both alternatives lead to comparable rates of 24-month overall survival (87% for both mosunetuzumab and tisagenlecleucel) and slightly different progression-free survival (48% for mosunetuzumab vs. 57% for tisagenlecleucel), although the clinical differences of the patients enrolled in the respective clinical trials must be taken into account (12,13). Mosunetuzumab is an intravenous (IV) treatment administered for 8 cycles for patients that achieve CR, scalable up to a maximum of 17 cycles for those who achieve a partial response (PR) or maintain a stable disease unless there are concerns of unacceptable toxicity or disease progression. It has a manageable safety profile and can be administered on an outpatient basis, obviating the need for hospitalization (13).
Drawing on data from the ELARA and GO29781 (11,12) trials and corroborating from a recent US source showing similar clinical efficacy for both treatments (14), this study aims to evaluate their relative economic outcomes. The present simulation study compares the economic impact of therapeutic administration of tisagenlecleucel and mosunetuzumab in 3L+ R/R FL patients. The investigation, aimed at providing a monetary evaluation of resources and time during the administration period of 1 year of either therapy, adopts the Italian hospital and societal perspectives.
Methods
Model description
A per-patient total cost of therapy model was developed to evaluate the economic implications of mosunetuzumab and tisagenlecleucel (CAR-T) therapy administration in R/R FL patients. The model was designed to calculate each cost incurred by the hospital and society by simulating the patient’s journey throughout a 1-year horizon. The selection of a 1-year horizon aligns with the analytical framework of the per-patient cost-of-care analysis, focusing on the administration phase of both treatments. This period is consistent with the standard administration protocol for mosunetuzumab, which can extend up to 17 cycles depending on patient response (13). This timeframe also comprehensively captures tisagenlecleucel’s pathway, spanning approximately 130 days, as detailed by Jagannath et al (15), allowing for a thorough comparative analysis of the administration costs between CAR-T and mosunetuzumab.
The adopted costing approach was bottom-up (micro-costing), and accounted for the time spent by the patient, the caregiver, and the healthcare workers (HCWs) to either receive, support, prepare, or administer each specific therapy.
The logical flow of the model development may be summarized in a few steps:
- outlining of the planned treatment pathway for each therapy;
- identification of pharmaceutical consumption and acquisition costs;
- identification of resource consumption and costs associated with inpatient activities;
- comparison of resource consumptions and costs.
The costing process for the administration of mosunetuzumab and tisagenlecleucel comprises three main phases: (i) the patient’s and caregiver’s commute (intended as traveling) from and to the hospital (16), (ii) drug preparation, and (iii) drug administration. Within the drug preparation and administration steps, both resource usage and the time spent by the HCW to perform specific tasks were taken into account. This approach allows to estimate costs accounting for healthcare resources consumed, such as HCWs, drug, non-drug consumable, and the time spent with each resource as a patient moves along the entire care pathway.
Since the model adopts a societal perspective, costs related to productivity loss, and the time invested by patients and caregivers were additionally considered (Tab. 1).
| Phase | Activity | Resources | Perspective |
|---|---|---|---|
| Patient and caregiver commute (I) | Patient and caregiver transfer to the hospital | Time spent by the patient and the caregiver commuting from their accommodation to the hospital to receive the therapy | S |
| Drug preparation | Drug preparation of the clinical pharmacist: • employment of consumable hospital resources (i.e., medical devices) |
Time spent by the pharmacist for the preparation of the drug Costs of the consumable hospital resources |
H |
| Drug administration | Pre-infusion | ||
• patient preparation • administration resources preparation |
Time spent by the clinical specialist nurse (CSN) to perform pre-infusion activities | H | |
• patient and caregiver waiting time for the completion of the pre-infusion activities performed by the CSN |
Time spent by the patient and caregiver to wait for the completion of the pre-infusion activities | S | |
| Infusion | |||
• active monitoring performed by the CSN • employment of consumable hospital resources for infusion |
Time spent by the CSN actively monitoring the patient while the drug is being administered Costs of the consumable hospital resources |
H | |
• time spent by the patient while being administered the drug • waiting time spent by the caregiver during the infusion session |
Drug administration time Waiting time spent by the caregiver for the completion of the drug infusion |
S | |
| Post-infusion | |||
The CSN performs: • post-infusion operations • post-infusion cleaning • post-infusion patient monitoring |
Time spent by the CSN to perform all the post-infusion operations | H | |
• time taken to wait for the completion of post-infusion operations by the caregiver • the patient undergoes post-infusion observation |
Monitoring time during post-infusion time Caregiver waiting time for the completion of post-infusion activities on the patient |
S | |
| Visit time | Patient and caregiver time spent for: • attending visits due to AEs • attending monitoring visit (CAR-T only) |
Time spent by the patient for being visited at AEs occurrence. Time spent by the caregiver for waiting for the patient visit completion CAR-T only: time spent by the patient to be visited in the post-infusion monitoring phases of CAR-T. Waiting time for the caregiver to accompany the patient to monitoring visits |
S |
| Patient and caregiver commute (II) | Transport of the patient and caregiver: • the patient returns home |
Time spent by the patient and caregiver for transportation from the hospital to their residence to undergo therapy | S |
| Accommodation and housing | Hotel or rental for the accommodation during the initial 130 days of gene therapy (CAR-T) | Only patients coming from regions lacking ATMP-specialized centers must reside near the reference hospital center | S |
H = hospital perspective; S = societal perspective.
Mosunetuzumab
The therapeutic pathway for patients with R/R FL receiving mosunetuzumab was modeled to reflect the therapeutic pathway framework of another monoclonal antibody used in a similar setting (Tab. 2), including the main activities and sub-activities, such as drug preparation and infusion procedures in the treatment room (17).
The time to off-treatment curve was employed to estimate the patient’s persistence in therapy for 1 year (Study GO29781; cut-off date August 27, 2021) (11).
Tisagenlecleucel
The patient’s pathway for gene therapy was based on the description by Jagannath and colleagues (Fig. S1). Patients, before proceeding to CAR-T infusion, go through the phases of apheresis, bridging, and conditioning therapy, followed by 7 days of hospitalization and 93 days of post-infusion monitoring (15). The patient flow entering the infusion phase is derived from the ELARA study (Tab. S4) (18,19).
Main inputs
Costs, whether direct or indirect, were applied according to the adopted perspective. The societal perspective accounted for both direct and indirect non-healthcare costs, while the hospital perspective considered direct medical costs only (i.e., drugs, medical consumable resources, hospital overheads, HCW time, AEs).
Direct healthcare costs
Drug costs were calculated by applying ex-factory net prices with confidential rebates to achieve maximum hospital tender price (data on file). The dose consumed was determined based on the summary of product characteristics’ indications, applied to weight (70 kg, assumption), where pertinent (20). The costs associated with serious AEs were also considered for both alternatives. AE management was based on their severity and duration, as well as the employment of specific drugs and monitoring clinical and lab exams (21). For high-grade, life-threatening AEs, it was assumed that each occurrence would require admission to the intensive care unit (ICU). The clinical and lab exam costs were estimated based on their frequencies of use, applying specific tariffs (22) (more details on supplemental material Tabs. S1 and S2 (23-25) Tabs. S6 and S7 (18,26-28)).
| Phase | Activity | Personnel | Time spent (minutes) | Source | Notes |
|---|---|---|---|---|---|
|
Drug preparation PRE-INFUSION |
Drug preparation | Pharmacist | 11.00 | (36) | – |
|
Drug administration PRE-INFUSION |
Patient preparation | Clinical specialist nurse (CSN) | 8.50 | – | |
| Consumables preparation | 8.90 | – | |||
| Non-specified | Patient | 17.40 | Calculated | Sum of the time spent by the CSN for preparing the patient and the instruments/consumable resources for the infusion | |
| Caregiver | 17.40 | Calculated | |||
Drug administration INFUSION |
Drug administration | CSN | 9.20 | (36) | Share on active time EASIER (data on file) |
| Patient | 240.00 | (13) | Infusion time from Summary of Product Characteristics (SPC) | ||
| Caregiver | 240.00 | ||||
|
Drug administration POST-INFUSION |
Post-infusion activities | CSN | 3.50 | (36) | – |
| Patient | 3.50 | ||||
| Caregiver | 3.50 | ||||
| Clearing and tidying the operational site | CSN | 6.10 | – | ||
| Active monitoring | CSN | 3.90 | |||
| Patient | 30.00 | – | Assumption | ||
| Caregiver | 30.00 | – | Assumption |
In Italy, healthcare resources for CAR-T administration are comparable to the autologous stem cell transplantation (ASCT), due to overlapping clinical management aspects, such as apheresis procedures, conditioning therapies, and inpatient infusions. An American study compared resource utilization between ASCT and CAR-T treatment in patients with R/R diffuse large B-cell lymphoma (29). The study found that CAR-T treatment required approximately 30% less HCWs’ time than ASCT, particularly among nursing staff, due to fewer chemotherapy cycles, outpatient visits, and shorter hospital stays. This observed difference in time associated with CAR-T treatment has been used to adjust the cost related to resource consumption of ASCT from the perspective of an Italian hospital (29,30). The remainder of CAR-T therapy-related costs consisted of bridging therapy, tisagenlecleucel drug costs, and post-infusion monitoring costs. Hospital-attributable costs for the bridging therapy were estimated based on the percentage of use of specific drugs, as described in the ELARA study (45% of patients received optional antineoplastic bridging chemotherapy for stabilization) (Tabs. S3 and S4) (31). The drugs employed in this phase, which last approximately 4 weeks, include rituximab, gemcitabine, oxaliplatin, etoposide, cyclophosphamide, and vincristine (Tab. S3). Patients undergoing CAR-T are usually monitored post-infusion, these costs are modeled according to the resource use frequency (Tab. S5).
Acknowledging the existence of Italian studies on the topic, we included one supplemental analysis including estimates retrieved from Cavallo et al (32). This secondary analysis replaces the estimated hospitalization costs for the base case with the average hospitalization cost derived from the work of Cavallo and colleagues, which considered the resource consumption of only CAR-T-eligible patients who received infusion.
Healthcare workers
The time HCWs spend on the activities and sub-activities was valued by average hourly gross wages to quantify the hospital cost of HCW, as defined in ARAN and ISTAT (Tab. S8) (33-35). The costs attributable to the time spent by HCWs during mosunetuzumab and bridging therapy administration were calculated on the time measurements available in literature in similar settings (36,37).
For mosunetuzumab, we employed time estimates from the EASIER study (36) (data on file). These estimates pertain to the active time for pharmacists in drug preparation and for nurses in the treatment room during the administration of a comparable IV drug. Additionally, the active time for nurses administering mosunetuzumab was adjusted to its specific summary of product characteristic (SPC) (Tab. 2).
Conversely, the active-time estimates observed by De Cock and colleagues (37) – which measured drug preparation and chair time for IV rituximab – were used as reference for the bridging therapy phase of the CAR-T pathway. The same time estimates, adjusted as necessary to maintain the ratio between nurse active time and rituximab infusion time, were applied to concurrent chemotherapies or those administered without rituximab (Tab. S4).
All non-drug consumables for preparation and administration of inpatient IV therapies were applied as one-off costs and their cost was set as per specific public tender price (Tab. S9). The overhead costs were also considered, valued according to an Italian survey: in particular, these costs were estimated as 25% of the full costs of health service – that is, in-hospital IV administration, HCW wage, excluding drug acquisition cost (38).
Direct non-healthcare and indirect costs
The model employs the human capital and Proxy Good approaches to assess the indirect costs associated with patients receiving either mosunetuzumab or tisagenlecleucel. The productivity losses resulting from patients undergoing therapy, including both paid and unpaid work, were calculated. Whereas the value of paid activities was based on the Italian Time Use Survey – which provides data, grouping by age and sex, on time dedicated to paid and unpaid activities of the general population – the monetary value of unpaid work was valued by applying minimum wage rates (34). The demographic characteristics employed as proxies for Italian patients with FL included a mean age of 60 years and a distribution of male and female patients of 61% and 39%, respectively, as observed in the intention-to-treat population of the GO29781 study (11). Productivity losses were calculated by considering the time to undergo infusions related to both treatments and to manage any AEs’ occurrence. In the CAR-T pathway, productivity losses also included the average hospital visit duration for monitoring, which is assumed to be of 1 hour as it comprises several tests (i.e., electrocardiogram, full blood count, biopsy, etc.) (15). In the case of in-ward hospitalizations for severe AEs in both treatments, the patient’s productivity loss excluded the time dedicated to daily sleep, and considered the absence of the caregiver during this period (39).
The expenses incurred by caregivers were modeled according to their distribution across the Italian population needing care, that is, 80% (40), of which 91% consists of informal caregivers (36) (data on file). Formal care costs were based on standard wages for domestic workers. Correspondingly, the monetary value of informal care was calculated by proportionally deducting caregiving time from daily routines (Tab. S8).
Transportation and Rent Accommodation
Additional patient-borne costs are divided into two categories: (i) commuting time-related costs and (ii) relocation and rent accommodation costs.
Commuting costs were estimated based on the average travel time from the patient’s residence to the hospital – assumed to be 30 minutes – multiplied by the average cost of transport (Tab. S11). The average transportation cost was calculated based on usage estimates of specific means of transportation collected during the EASIER observational study (unpublished data) (41-47). Commuting costs were applied each time the patient and, consequently, their caregiver went to the hospital, whether for the administration of either therapy, any AE occurrence, or monitoring visits.
Relocation and accommodation costs were exclusively considered for patients undergoing tisagenlecleucel administration. CAR-T therapy is a specialized medical intervention with limited accessibility in Italy. Consequently, patients receiving this treatment may incur significant relocation expenses that are worth being appraised. These costs were calculated based on the period a patient (130 days) is required to relocate close to the ATMP-specialized healthcare center (within 2 hours’ distance), if necessary, and the various types of accommodations available (15). The patient is expected to either use a rented flat or stay in a standard hotel room (Tab. S12) (48). The base-case analysis takes into account the unavailability of specialized healthcare centers across Italy, which forces 14.90% of patients to relocate to the nearest region for treatment. Given the Italian geographical barriers, 2.68% of the Italian population may necessitate air travel, as could be the case for residents in Sardinia (49) (Tab. S13).
To address the potential costs associated with relocation across the Italian population, three distinct scenarios were hypothesized. Scenario A presumes that all (100%) patients reside in an Italian region with an ATMP-specialized healthcare center. In this scenario, patients can reach a specialized center within 2 hours and only incur commuting costs. Scenario B assumes patients must travel to a neighboring region only by car, and they must rely on local accommodation. Instead, scenario C applies to the sole patients (such as Sardinian) who must rely on air transportation to reach a specialized healthcare center. Patients in scenario C bear the highest average transportation expenses and incur local accommodation costs. Transportation costs associated with each scenario are calculated based on the average kilometrical distance that patients must travel to reach the nearest ATMP-specialized center, adjusted for the mode of transportation expected to cover that distance. Distances were assumed to be the straight-line kilometers from the regional capital of residence to the closest ATMP center.
Univariate sensitivity analysis
To assess parameter uncertainty, a one-way sensitivity analysis was conducted. This type of analysis addresses the relative importance of the model’s variables, by varying each parameter by ±20% one at a time. A tornado diagram is built on the cost difference between mosunetuzumab and tisagenlecleucel pathways from the societal perspective. The varied parameters are sorted in descending order by their relative impact on the model outcome
Results
Hospital perspective
Based on the considered timeframe, which includes eight cycles for CR and up to 17 cycles for PR, the estimated mean cost of administering mosunetuzumab is €52,619 in the base-case analysis. This cost includes the drug cost, as well as the cost of consumables, HCWs, and the management of serious AEs related to the therapy (Tab. 3).
On the other hand, the estimated mean cost for administering tisagenlecleucel-based therapy is €211,489, which includes the cost of engineered lymphocytes, peri-infusional costs (apheresis, conditioning, and hospitalization), bridging therapy, managing serious AEs, and post-infusion monitoring. Overall, the base-case use of mosunetuzumab results in a reduction of hospital costs by €158,870 per patient along the considered time horizon (Tab. 3).
| Base-case results, hospital perspective (total direct healthcare costs) | Cost per patient (€) |
|---|---|
| Mosunetuzumab* | 52,619.42 |
| Tisagenlecleucel* | 211,489.46 |
| Difference (mosunetuzumab vs. tisagenlecleucel) | –158,870.04 |
* Total cost of therapy administration includes all direct healthcare costs, such as drugs, administration, monitoring, and AE management cost. Drug prices net of mandatory confidential discounts were applied to achieve the maximum tender hospital prices of mosunetuzumab, tisagenlecleucel, bridging therapy, and drugs for managing AEs.
As far as the secondary analysis incorporating Italian patient-level estimates (32) is concerned, similar results were obtained. This analysis revealed that the estimated mean cost difference between administering mosunetuzumab and tisagenlecleucel is −€157,342 (Tab. S10), which aligns with the base-case results.
Societal perspective
The estimated cost difference between mosunetuzumab and tisagenlecleucel, including hospital and social costs, is €161,974 in the base case (Tab. 4).
For mosunetuzumab, the impact of indirect and non-healthcare direct costs is broken down into €491 for administration, €410 for AE-related expenses, and €306 for transportation. In the case of tisagenlecleucel, the indirect costs are 70% higher, totaling roughly €3,000 (Fig. 1). Tisagenlecleucel costs borne by the patient and caregiver amount to €1,196 for administration, €101 at the bridging therapy stage, €1,189 for AE-related expenses, €374 for monitoring, and €1,450 for transportation and relocation (Tab. 4).
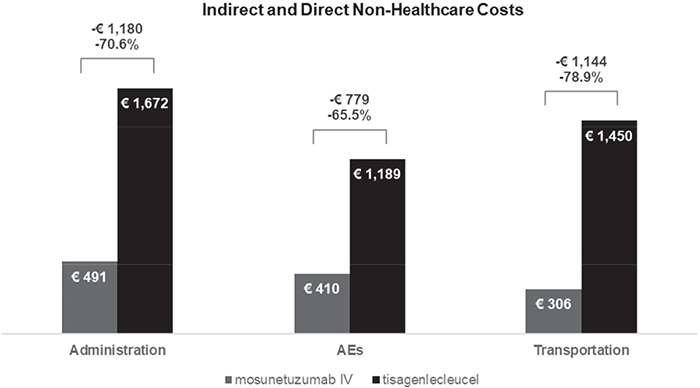
FIGURE 1 - Base-case results, detail on indirect and direct non-healthcare costs, societal perspective.
| Cost of therapy administration, mosunetuzumab | Cost per patient (€) |
|---|---|
| Mosunetuzumab, direct healthcare costs* | 52,619.42 |
| Indirect (patient and caregiver) and direct costs (formal caregiver) for administration | 491.42 |
| AEs indirect and direct non-healthcare costs (patient and caregiver) | 410.18 |
| Indirect (patient and caregiver) and direct costs (formal caregiver) for transportation | 306.15 |
| Total cost | 53,827.17 |
| Cost of therapy administration, tisagenlecleucel | Total cost per patient (€) |
| Tisagenlecleucel, direct healthcare costs* | 211,489.46 |
| Tisagenlecleucel infusion indirect and direct non-healthcare costs (patient and caregiver) | 1,196.15 |
| Bridging therapy indirect and direct non-healthcare costs (patient and caregiver) | 101.35 |
| Monitoring indirect and direct non-healthcare costs (patient and caregiver) | 374.06 |
| AE indirect and direct non-healthcare costs (patient and caregiver) | 1,189.32 |
| Indirect (patient and caregiver) and direct costs (formal caregiver) for transportation and relocation | 1,450.33 |
| Total cost | 215,800.67 |
| Difference (mosunetuzumab vs. tisagenlecleucel) | –161,973.50 |
* Total cost of therapy administration includes all direct healthcare costs, such as drugs, administration, monitoring and AE management cost. Drug prices net of law mandatory discounts are shown. For the analyses, mandatory confidential discounts were applied to achieve the maximum tender hospital prices of mosunetuzumab, tisagenlecleucel, bridging therapy, and drugs for managing AEs.
From the societal perspective, three scenario analyses were hypothesized to address that some patients are required to move from their original region to undergo gene therapy. The associated savings for mosunetuzumab amount to €161,170, €166,507, and €166,811, respectively for the scenarios A, B, and C (Tab. 5).
Univariate sensitivity analysis
The tornado diagram resulting from the sensitivity analysis summarizes the effect of variation in key model parameters on the cost difference between the two therapeutic pathways from the societal perspective (Fig. S2). Ranking first, the price of tisagenlecleucel is the most impactful variable, followed by mosunetuzumab drug price.
| Scenario | Main assumption | Difference (mosunetuzumab vs. tisagenlecleucel) (€) |
|---|---|---|
| A | 100% of patients come from a region with an ATMP-specialized center. | –161,170.05 |
| B | 100% of patients have to travel and relocate to a neighboring region by car. | –166,506.61 |
| C | 100% of patients have to use air travel services to reach an ATMP-specialized center. | –166,810.84 |
Discussion
According to recently published studies, mosunetuzumab and tisagenlecleucel offer similar clinical efficacy for patients with R/R FL who have received three or more lines of therapy (14). Yet – as shown by the results of the present per-patient cost analysis – from both societal and hospital perspectives, mosunetuzumab’s therapeutic costs are approximately one-quarter of that of its comparator. According to the conducted univariate sensitivity analysis, drug prices are the main drivers behind the cost difference between these two therapeutic pathways.
The limited availability of centers – specialized in ATMP – in addition to the difficult management of patient referral is a significant barrier for patients seeking access to this treatment option in Italy. These hurdles can disproportionately affect patients residing in certain regions, posing a great inequality burden on them (10,50,51). To address these societal issues, three scenario analyses were conducted. Our results consider the impact of indirect and non-healthcare direct costs associated with the two therapies: the further away the patients live from the nearest ATMP-specialized center the higher the expected supplementary disbursement. Patients dwelling in regions equipped with ATMP centers show the lowest cost difference vs. mosunetuzumab, €161,170 (scenario A). Conversely, patients living in Sardinia, whose location was used as a proxy for the most unfavorable scenario (scenario C), bear higher additional expenses, up to approximately €5,600 per patient as against mosunetuzumab.
Mosunetuzumab follows the typical outpatient administration pathway as any other IV drug, whereas CAR-T therapies involve several inpatient sub-steps that contribute to the difference in savings in both perspectives (15,32). This resulted in the optimization of HCW efficiency and minimization of direct healthcare resources when compared to the complex and resource-intensive pathway typically involved in CAR-T therapy.
To the best of our knowledge, the present study is accompanied by only two literature resources for the cost estimation of the CAR-T therapeutic pathway in the Italian setting (32,52). The common ground these studies lay on is the great challenge for the Italian NHS in addressing the implementation costs associated with managing CAR-T patients. Among others, the augmentation of medical staff alongside hospitalization capacity constitutes a prominent concern. Furthermore, the research strategy in both studies relies on expert opinion, resulting in a highly variable spectrum of estimates for resource expenditures entailed in CAR-T administration. The deficit of validated information on resource spending suggests the need for time and motion studies on this topic and constitutes one of the limitations of the analyses conducted thus far, including one of the present study. Nevertheless, the resource consumption associated with the CAR-T therapeutic pathway reported in the most recent Italian study (32) supports the current analysis’ estimation, as shown in the conducted secondary analysis.
Due to the short timeframe of 1 year, it was not possible to assess the progression of patients after either therapy, who allegedly undergo further lines of treatment, rehospitalizations, or therapeutic changes. Strictly linked to this, the model assumes that resources are consumed linearly in both therapeutic routes, meaning that there are no usage deviations during the 1-year time horizon, underestimating certain additional costs that the healthcare center may incur in a real-life setting. A further major limitation of our research is its reliance on aspecific literature data (15,36,37) as model inputs. Our approach was driven by the absence of detailed Italian- and drug-specific time and motion studies. Consequently, the data used may not fully capture actual healthcare practices, potentially leading to generalizability issues. Despite this, considerable efforts were made to adapt the non-specific estimates to better align with the context of interest (29,30). In this study, it is also crucial to acknowledge two assumptions. First – due to the lack of therapy-differential data on the role of informal (36,40) and formal caregivers in providing patient support – it was assumed that either caregiver contribution would be the same for both CAR-T and mosunetuzumab pathways. This may affect the accuracy of our cost and resource utilization estimates. Secondly, monitoring visits for tisagenlecleucel (15) are assumed to last 1 hour. Such duration may not fully represent real-world practice but it was deemed an acceptable estimate, in the absence of more precise data or expert opinion.
According to our 1-year analysis, mosunetuzumab has proven to be a cost-saving alternative from both the societal and hospital perspectives in the Italian context. The use of this novel bispecific monoclonal antibody for R/R FL 3L+ patients could enable local hospitals to reallocate saved resources and address inequality and access-to-therapy constraints. Notwithstanding the innovative clinical efficacy of tisagenlecleucel, enduring a CAR-T inpatient journey may affect patients’ quality of life, additional costs, and hospital disbursements. The scenario analyses displayed that a closer residence to an ATMP-specialized center does not pledge striking savings vs. mosunetuzumab. In the societal base-case scenario, the cost difference of €161,975 between mosunetuzumab and tisagenlecleucel underscores the substantial impact of indirect costs on patients and caregivers. The time required from both patients and their caregivers results in productivity losses. Coupled with relocation and transportation expenses, these factors contribute to approximately a 70% increase in financial strain due to supplementary disbursements in our analysis. In the broader context of socioeconomics, these factors emphasize the importance of considering the holistic implications of gene therapies that are often overlooked and hardly estimated (50,53).
Some key considerations are worth mentioning for future research. Besides methodological refinement that should incorporate a more comprehensive and dynamic approach, it would be beneficial to invest in collecting real-time data on resource utilization, time consumption, and costs associated with the administration of mosunetuzumab and tisagenlecleucel. This could be attained by conducting time and motion observational studies capturing patient’s therapeutic course and treatment patterns. Furthermore, efforts should be directed toward bridging the gap in evaluating the long-term outcomes of patients receiving either therapy. Comprehensive assessments should also consider the progression of patients over an extended period, including subsequent lines of treatments or resource use changes past the 1-year time horizon. Finally, model validation against real-world data, when available, is essential to enhance the credibility of the findings. This validation process could involve comparing the model predictions with real-life observed outcomes.
Conclusion
Mosunetuzumab and tisagenlecleucel offer similar clinical efficacy for patients with R/R FL 3L+. The two administration pathways largely differ, seeing mosunetuzumab as a valid outpatient alternative to the complex CAR-T patient journey. These major differences in administration, tied to the suboptimal availability of ATMP-specialized centers in Italy, resulted in 75% monetary and significant time resource savings for patients who undergo infusions of the bispecific antibody from both the Italian hospital and societal perspective.
Authors’ contribution
DG established the overall direction and objectives, developed the initial ideas and framework for the study. AS and MB managed the data collection and ensured the integrity and accuracy of the datasets used in the research. Data analysis (statistical evaluations and interpretation) was conducted by AS and MB. The core research efforts (i.e., data collection) were conducted by AS and MB. MB designed the methods and was also responsible for the development and maintenance of software tools. The project was supervised by LP, who managed timelines and resources, offering guidance and oversight to ensure the research was conducted appropriately. ADA, EOS, and DG performed the validation of results and data verification. AS and MB were responsible for creating visual representations of the data, to aid in interpretation and presentation. The initial drafting and writing of the manuscript were done by AS and MB, who compiled the research findings into a coherent document. AS and MB reviewed and edited the manuscript, making revisions and improvements to ensure clarity and quality of the final publication.
Disclosures
Conflict of interest: MB and AS are employees of AdRes srl, which has received project funding from Roche SpA for the development of this research. LP is a co-owner and employee of AdRes srl, which has received project funding from Roche SpA for the development of this research. DG is an employee of Roche SpA. EOS and ADA have received consulting fee from AdRes srl for manuscript writing.
Financial support: This study was funded by Roche SpA
Data availability statement: The data presented in this study are available as supplementary material to this article.
References
- 1. Kanas G, Ge W, Quek RGW, Keeven K, Nersesyan K, Jon E Arnason. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020-2025. Leuk Lymphoma. 2022 Jan;63(1):54-63. CrossRef. PubMed
- 2. Kaseb H, Ali MA, Koshy NV. Follicular lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Online. (Accessed July 2024)
- 3. Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol. 2019 Feb;184(4):660-663. CrossRef PubMed
- 4. Mounier M, Bossard N, Remontet L, et al; EUROCARE-5 Working Group; CENSUR Working Survival Group. Changes in dynamics of excess mortality rates and net survival after diagnosis of follicular lymphoma or diffuse large B-cell lymphoma: comparison between European population-based data (EUROCARE-5). Lancet Haematol. 2015;2(11):e481-e491. CrossRef PubMed
- 5. Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. July 2020;10(7):1-12. CrossRef
- 6. Rodgers TD, Casulo C, Boissard F, Launonen A, Parreira J, Cartron G. Early relapse in first-line follicular lymphoma: a review of the clinical implications and available mitigation and management strategies. Oncol Ther. 2021 Dec;9(2):329-346. CrossRef
- 7. Wagner-Johnston ND, Schuster SJ, deVos S, et al. Outcomes of patients with up to 6 years of follow-up from a phase 2 study of idelalisib for relapsed indolent lymphomas. Leuk Lymphoma. 2021;62(5):1077-1087. CrossRef PubMed
- 8. Gazzetta ufficiale dell’Unione europea. Sintesi delle decisioni dell’Unione europea relative alle autorizzazioni all’immissione in commercio di medicinali dal 1 o agosto 2018 al 31 agosto 2018. Online. (Accessed July 2024)
- 9. European Medicines Agency (EMA). Kymriah. Online. (Accessed July 2024)
- 10. Canales Albendea MÁ, Canonico PL, Cartron G, et al. Comparative analysis of CAR T-cell therapy access for DLBCL patients: associated challenges and solutions in the four largest EU countries. Front Med (Lausanne). 2023;10:1128295. CrossRef PubMed
- 11. Genentech, Inc. An open-label, multicenter, phase I/II trial evaluating the safety, efficacy, and pharmacokinetics of escalating doses of mosunetuzumab (BTCT4465A) as a single agent and combined with atezolizumab in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. clinicaltrials.gov; 2023 Nov. Report No.: NCT02500407. Online. (Accessed July 2024)
- 12. Dreyling M, Dickinson M, Martinez Lopez J, et al. Long-term clinical outcomes and correlative efficacy analyses in patients (pts) with relapsed/refractory follicular lymphoma (r/r FL) treated with tisagenlecleucel in the ELARA trial. Blood. 2022;140(suppl 1):1459-1463. Online. (Accessed July 2024)
- 13. European Medicines Agency (EMA). RCP. Mosunetuzumab. Online. (Accessed July 2024)
- 14. Matasar M, Sanchez Alvarez J, Parisé H, et al. Cost-effectiveness analysis of mosunetuzumab for treatment of relapsed or refractory follicular lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2024;0(ja):1-36. CrossRef
- 15. Jagannath S, Joseph N, Crivera C, et al. Component costs of CAR-T therapy in addition to treatment acquisition costs in patients with multiple myeloma. Oncol Ther. 2023 June;11(2):263-275. CrossRef
- 16. Cheng AC, Levy MA. Determining burden of commuting for treatment using online mapping services – a study of breast cancer patients. AMIA Annual Symposium Proceedings. 16 aprile 2018;2017:555. PubMed
- 17. Bellone M, Pradelli L, Tavarozzi R, et al. Economic consequences of administering obinutuzumab as a short duration infusion in Italian patients with advanced follicular lymphoma: a cost analysis. Farmeconomia. Health Economics and Therapeutic Pathways. 2022 Nov; 23(1). CrossRef
- 18. Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022 Feb;28(2):325-332. CrossRef
- 19. Fowler NH, Dickinson M, Ghosh M, et al. Assessment of healthcare resource utilization and costs in patients with relapsed or refractory follicular lymphoma undergoing CAR-T cell therapy with tisagenlecleucel: results from the Elara study. Blood. 2021;138(suppl 1):3533. CrossRef
- 20. AIFA. Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale. 2020. Online (Accessed July 2024)
- 21. CODIFA. L’Informatore Farmaceutico. 2023. Online. (Accessed July 2024)
- 22. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. CrossRef PubMed
- 23. Tarricone R, Torbica A, Franzetti F, Rosenthal VD. Hospital costs of central line-associated bloodstream infections and cost-effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010 May;8:8. CrossRef PubMed
- 24. Ministero della Salute. Ministero della Salute. Ricoveri Ospedalieri, SDO. Rapporti Annuali. 2020. Online. (Accessed July 2024)
- 25. Budde LE, Sehn LH, Matasar M, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055-1065. CrossRef PubMed
- 26. Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. IJMS. 2021 July;22(14):7652. CrossRef PubMed
- 27. Thieblemont C, Dickinson M, Martinez-Lopez J, et al. Efficacy of tisagenlecleucel in adult patients (Pts) with high-risk relapsed/refractory follicular lymphoma (r/r FL): subgroup analysis of the phase II Elara study. Blood. 2021;138(suppl 1):131. CrossRef
- 28. Adkins S. CAR T-cell therapy: adverse events and management. J Adv Pract Oncol. 2019;10(suppl 3):21-28. PubMed
- 29. Ring A, Grob B, Aerts E, et al. Resource utilization for chimeric antigen receptor T cell therapy versus autologous hematopoietic cell transplantation in patients with B cell lymphoma. Ann Hematol. 2022;101(8):1755-1767. CrossRef PubMed
- 30. Martino M, Console G, Russo L, et al. Autologous stem cell transplantation in patients with multiple myeloma: an activity-based costing analysis, comparing a total inpatient model versus an early discharge model. Clin Lymphoma Myeloma Leuk. 2017;17(8):506-512. CrossRef PubMed
- 31. SORESA. 2023. Online. (Accessed July 2024)
- 32. Cavallo MC, Cavazza M, Bonifazi F, et al. Cost of implementing CAR-T activity and managing CAR-T patients: an exploratory study. BMC Health Serv Res. 2024 Jan;24(1):121. CrossRef
- 33. ARAN. Retribuzioni medie pro-capite nella pubblica amministrazione per tipologia di personale. 2021. Online. (Accessed July 2024)
- 34. ISTAT. Uso del tempo. Online. (Accessed July 2024)
- 35. ISTAT. Struttura delle retribuzioni. 2013. Online. (Accessed July 2024)
- 36. Pradelli L, Massaia M, Todisco E, et al. Improved efficiency of daratumumab treatment of multiple myeloma adopting the subcutaneous route: a micro-costing analysis in three Italian hematology centers. Cancer Med. 2023 Dec;12(23):21480-21489. CrossRef
- 37. De Cock E, Kritikou P, Sandoval M, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One. 2016;11(6):e0157957. CrossRef PubMed
- 38. Mantellini P, Lippi G. I costi dello screening. Online. (Accessed July 2024)
- 39. AGENAS. Agenzia Nazionale per i Servizi Sanitari Regionali per ricoveri personale e spesa delle aziende ospedaliere. 2003. Online. (Accessed July 2024).
- 40. Il Sole 24ore. Quotidiano Sanità. Going lean in oncoematologia. L’ottimizzazione dei day hospital. 2016. Online. (Accessed July 2024)
- 41. Automobile Club d’Italia – Sito ufficiale. Online. (Accessed July 2024)
- 42. FormazioneTurismo.com. Voli, le tariffe europee per chilometro: dove conviene acquistare i biglietti. Online. (Accessed July 2024)
- 43. Bologna-guide.com. Taxi Bologna. Numeri di Telefono – Tariffe – Costi – Prenotazione. Online. (Accessed July 2024)
- 44. InTaxi – Prenotazione Taxi Online a Milano. Tariffe taxi Milano. 2021. Online. (Accessed July 2024)
- 45. Transfer Milano. Taxi Milano Linate Aeroporto. Online. (Accessed July 2024)
- 46. Aeroporti di Roma. Benvenuti all’aeroporto di Roma Fiumicino «Leonardo da Vinci». Online. (Accessed July 2024)
- 47. Comune di Milano. Servizio taxi e tariffe applicate. Online. (Accessed July 2024)
- 48. Tremolada L. Info Data. 2022.Quanto costa in Italia vivere in una casa? Quasi un terzo del reddito per chi è in affitto. Online. (Accessed July 2024)
- 49. Associazione Italiana contro le Leucemie – linfomi e mieloma (AIL). Accesso alle CAR-T. Online. (Accessed July 2024)
- 50. Jommi C, Bramanti S, Pani M, Ghirardini A, Santoro A. CAR T-cell therapies in Italy: patient access barriers and recommendations for health system solutions. Front Pharmacol. 2022 June;13:915342. CrossRef PubMed
- 51. ATPM. Forum 2022. Online. (Accessed July 2024).
- 52. Foglia E, Garagiola E, Ladisa V, et al. Multidimensional results and reflections on CAR-T: the Italian evidence. Int J Environ Res Public Health. 2023 Feb;20(5):3830. CrossRef PubMed
- 53. Odstrcil MS, Lee CJ, Sobieski C, Weisdorf D, Couriel D. Access to CAR T-cell therapy: focus on diversity, equity and inclusion. Blood Rev. 2024 Jan;63:101136. CrossRef PubMed