 |
Glob Reg Health Technol Assess 2024; 11: 214-222 ISSN 2283-5733 | DOI: 10.33393/grhta.2024.3168 ORIGINAL RESEARCH ARTICLE |
 |
A cost-effectiveness analysis of Navina Smart on adult patients affected by neurogenic bowel dysfunction
ABSTRACT
Background and Objectives: The objective of this study is to evaluate the economic impact of the device Navina Smart on patients affected by neurogenic bowel dysfunction and dependent on transanal irrigation within the Italian context. This study employs the perspective of the Italian National Health Service.
Methods: The analysis was conducted through a Markov model, comparing two scenarios: standard bowel care vs. transanal irrigation. The model operates on a 30-year time period. The results were reported in terms of net monetary benefit.
Results: Transanal irrigation therapy was dominant in all scenarios with lower costs and higher effectiveness. The population was assumed to be composed of 1,000 subjects. Setting the willingness to pay at €35,000.00/QALYs (quality-adjusted life years), the analysis yielded a net monetary benefit of €81,087 and cost savings of €66,101 per patient over 30 years.
Conclusion: The results of this study substantiate that transanal irrigation therapy treatment employing the Navina Smart device can significantly benefit patients suffering from neurogenic bowel dysfunction by relieving their symptoms. In addition, this therapy offers important cost savings for the Italian National Health Service by reducing resource utilization.
Keywords: Cost-effectiveness analysis, Neurogenic bowel dysfunction, Transanal irrigation
Received: June 10, 2024
Accepted: October 25, 2024
Published online: November 18, 2024
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Neurogenic bowel dysfunction (NBD) is defined as the impairment of normal bowel functions due to a neurological disorder. Its occurrence is prevalent in patients with spinal cord injury (SCI) but can also stem from multiple sclerosis, spina bifida, congenital defects of the nervous system, and other central and peripheral nervous system diseases. SCI, the primary cause of NBD, has an estimated global annual incidence of approximately 375,000 cases, which primarily result from motor vehicle accidents, acts of violence and aggression, and sports-related activities (1). In Italy, the number of SCI cases is estimated at 85,330, with around 2,500 new cases occurring each year (2). Symptoms of NBD include constipation and fecal incontinence, with the most common complications of this condition encompassing urinary tract infections (UTIs), hemorrhoids, abdominal pain, rectal bleeding and prolapse, anal fissure, and autonomic dysreflexia (1,3-9). These symptoms are commonly associated with recurrent hospital admissions. Patients affected by NBD frequently suffer from severe psychological and social sequelae resulting from their conditions, as evidenced by extensive scholarly references (4,6,7,10), especially those in need of assistance with bowel management. NBD can negatively influence the patients’ working capacity and social relationships and lead to anxiety in social interactions, lack of self-esteem, and loss of personal independence (7). According to Musco et al (7), 50% of patients affected by NBD and requiring assistance spend more than 30 minutes per day managing their bowel-related management.
As mentioned earlier, the two main symptoms in patients with NBD are constipation and fecal incontinence (10). Emmanuel et al (3) have reported that 95% of patients with NBD suffer from constipation, whereas 75% of patients experience fecal incontinence at least once per year and 5% daily. Many people may not feel the need to evacuate, especially in the early stages of NBD, which sometimes causes inadvertent accidents and creates significant psychological disadvantages (10).
A careful evaluation of the patient’s medical history and an accurate clinical examination with analysis of the functionality of motor and sensory nerves are required to determine the most appropriate treatment for each patient (1). The diagnostic assessment should include the degree of bowel sensation and motor control, and testing of reflex activity in the anorectum, that is, anocutaneous reflex and bulbocavernosus reflex. In addition, it is imperative to evaluate the physical and cognitive capabilities of the patient (7). Hultling (10) and Emmanuel et al (11) have proposed a tiered, pyramid-stepped approach for NBD treatment. This method has been extensively used in clinical practice because it offers a flexible framework that can adapt to both the symptoms and the patient’s approach to treatment (7,10). The basal layer of the pyramid is referred to as the standard bowel care (SBC) and consists of dietary and fluid intake adjustments, lifestyle adaptations, laxative or constipation-alleviating drugs, digital rectal stimulation, suppositories, and biofeedback techniques. The objective of the protocol is the optimization of the patient’s bowel management regimen (7). The development of an effective and timely bowel management regimen is critical to enabling patients to avoid bowel accidents, regain control and privacy, and reduce or discontinue pharmacological treatments (11). This objective can be achieved through advanced and attentive planning on the part of patients and the establishment of a solid routine for all daily activities. The second layer of the pyramid consists of treatment options such as transanal irrigation (TAI) and neuromodulation techniques when SBC treatment is unsuccessful (3,7,12). It is recommended that clinical practitioners assess the second layer more frequently because they tend to skip it in many instances. Indeed, a 2019 survey conducted by the Danish Spinal Cord Injuries Association shows that 37% of the SCI patients interviewed were unaware of the TAI method (10). Subsequently, if these treatments prove inadequate, patients should move to the upper layer of the pyramid, which entails a more invasive course of action, such as nerve stimulation implants, surgical colonic irrigation, and the creation of a stoma (3). However, surgery should be regarded as a measure of last resort and be employed only when all other therapeutic procedures fail to improve the patient’s condition (7,12).
TAI is a therapeutic technology designed to facilitate the evacuation of feces from the bowel through the introduction of water via the anus (11). Several TAI devices are currently in use in many countries such as the United States, the United Kingdom, Germany, Italy, France, and the Netherlands (8). TAI has been shown to enhance the quality of life of patients by mitigating the symptoms and reducing chronic constipation and severe fecal incontinence (5,8,13). Most TAI devices use a manual pump, requiring caregivers and patients to carefully control the water flow. The Navina Smart is the only device currently available that features an electronic pump, which automatically regulates the water flow. Additionally, it allows for the storage of the patient’s technical data during the TAI procedure, which can be readily shared with clinical practitioners through a dedicated app (10,14). In the Italian market, Navina Smart held a 40% market share in 2023 compared to its competitors (14). Patients require an initial period of training in the proper use of the device, with support of a bowel care specialist (4,8,10,12,15).
This study sets out to investigate the cost-effectiveness of the Navina Smart TAI device, a product manufactured by Wellspect Healthcare, specifically for patients who have failed SBC versus continued SBC alone. Navina Smart is equipped with a software-controlled electronic pump that inflates a balloon and delivers water into the intestine, enabling the patient to perform the procedure independently. The device features built-in safety mechanisms to control the balloon’s maximum size, as well as the water volume and flow rate. The device also includes an app that tracks and records the patient’s bowel routine data. This information can be transmitted to the Navina Smart app via Bluetooth and easily shared with caregivers and healthcare professionals (HCPs) (14).
Methods
Review of the clinical literature on the Navina device and its comparators
In the Italian market, numerous TAI systems are available on prescription, produced by three different manufacturers. These individual systems offer similar and comparable benefits, indications, and contraindications. However, their specific mechanisms differ in many ways, such as the amount of water instilled (low or high volume), the delivery method (via a catheter or cone), and the type of system employed (manual pump, gravity, or electric pump).
Emmanuel et al (16) provided a practical decision-making guide for clinicians and concluded that the primary difference between devices is the volume of water used. Large-volume irrigation delivers water more proximally into the colon to promote peristalsis in the descending and sigmoid regions, while low-volume irrigation induces a localized washout.
Table 1 offers an overview of the high-volume devices currently available in Italy, emphasizing their key features.
It is important to note that most available academic evidence on TAI is not specific to any particular system and has broad applicability (17). At the time of writing, no recent studies were found that compared manual pumps, electric pumps, and/or gravity-fed systems. However, a study by Crawshaw et al (18) established that all methods were effective and resulted in similar levels of patient satisfaction. In this study, 75% of participants indicated a preference for electric pumps, although the reasons behind this preference were not clearly identified, suggesting a need for further investigation on the topic.
Model structure and hypothesis
A cost-effectiveness analysis was conducted by adopting the third-payer perspective (TPP), with a particular focus on the Italian National Health Service (NHS). To achieve this, a previously published (3) Markov model was tailored and adapted to the Italian healthcare context. This adaptation permitted to simulate patients’ transitions among various health states comparing two arms, as depicted in Figure 1.
| Company | Name of device | Volume | Tip | Pump |
|---|---|---|---|---|
| Coloplast | Peristeen | High | Balloon catheter | Constant flow |
| Qufora | Qufora IrriSedo Balloon | High | Balloon catheter | Gravity or manual |
| Qufora IrriSedo Bed | High | Catheter | Gravity or manual | |
| Qufora IrriSedo Cone | High | Cone | Gravity or manual | |
| Qufora IrriSedo Klick | High | Balloon catheter | Gravity or manual | |
| Aquaflush Lite | High | Cone | Gravity or manual | |
| Wellspect | Navina Classic | High | Catheter or cone | Manual |
| Navina Smart | High | Catheter or cone | Electric |
Adapted from Bardsley (17).
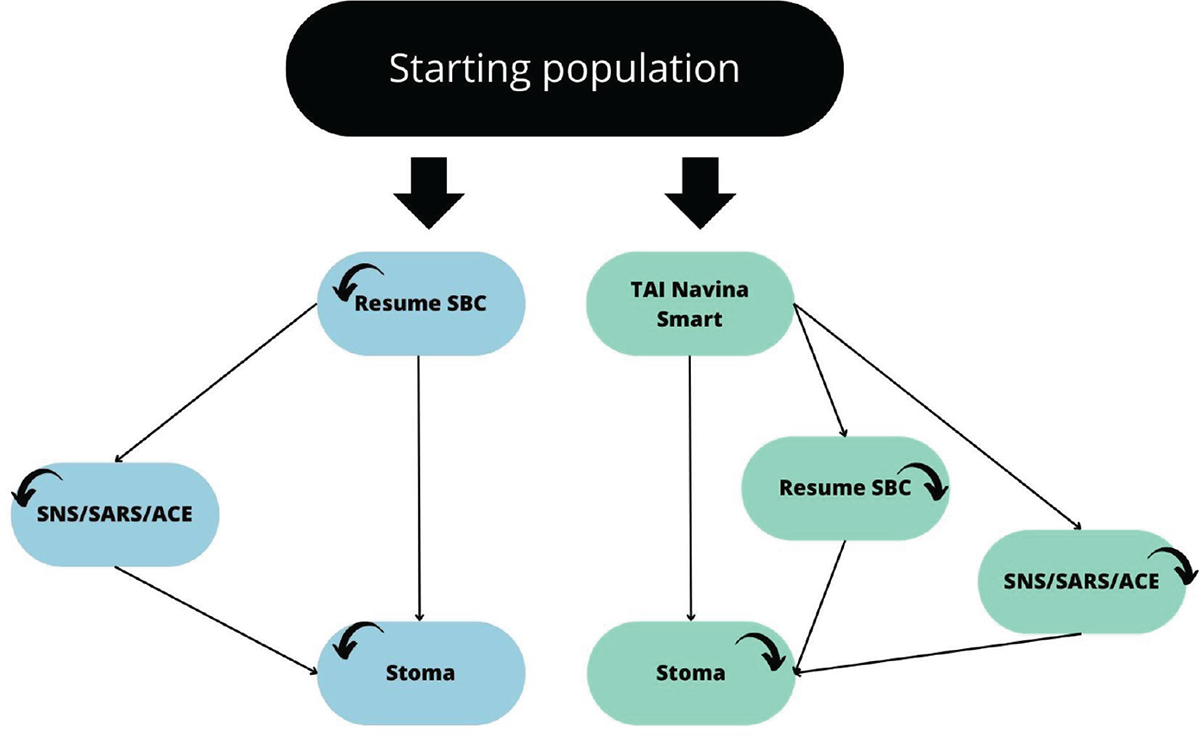
FIGURE 1 - Markov model: forecasting model of the patients’ health states. The figure is a graphic representation of a patient’s health state and how they can move from one state to another. The diagram illustrates the different health states that a neurogenic patient can transition between when SBC fails. For patients who have failed SBC for over 6 months, there are several options: (a) resuming SBC, (b) transitioning to SNS/SARS/ACE, or (c) transitioning to a stoma (absorbing state). When combining SBC with TAI, the options for patients who have failed SBC for over 6 months are: (a) initiating TAI, (b) resuming SBC, (c) transitioning to SNS/SARS/ACE, or (d) transitioning to a stoma. The model assumes that patients do not move directly from SBC/TAI to a stoma. ACE = antegrade continence enema; SARS = sacral anterior root stimulation; SBC = standard bowel care; SNS = sacral nerve stimulation; TAI = trans anal irrigation.
Only the valuation of the resources has changed according to the tariffs stipulated by the Italian jurisdiction. Additionally, a hypothetical population mimicked the baseline characteristics described by Emmanuel et al (3), consisting of 227 patients from three healthcare centers in the United Kingdom. The results of the study from the British patient population can be applied to the Italian context because the differences between the two populations appear not significant. Both the British and Italian healthcare systems are public, offering free or affordable access to care and quality of care. The treatments under investigation are available to the entire population through all national health centers. The demographic characteristics and the socioeconomic status of the two populations do not show any major difference, which also implies a homogeneous incidence and characteristics of SCIs. In addition, no major cultural differences are present that might influence treatment adherence and procedures (19). The first arm of the simulation involved the treatment of NBD using SBC under the assumption that the standard therapy was continued without any additional treatment options. The second arm of the simulation incorporated the treatment of NBD with SBC plus Navina Smart TAI in patients who showed no positive response to SBC alone. This model allows to systematically evaluate the evolution of patients’ conditions over time.
A Markov model is a mathematical framework employed to simulate the natural progression of a disease within a defined population. This is represented by different and mutually exclusive health states. A hypothetical cohort of patients can transition through different health states over successive Markov cycles, representing times for transition probabilities that can vary depending on the treatment administered. Each health state is characterized by a cost and a utility, which enables the presentation of the simulation results in terms of cost-effectiveness. Specifically, the patient health states represented by the model correspond to the previously mentioned pyramidal stepped approach (10,11) and are the following:
(1) patients currently receiving treatment;
(2) patients who have reverted to SBC;
(3) patients advancing to surgical interventions, which include sacral anterior root stimulation (SARS), sacral nerve stimulation (SNS), and antegrade continence enema (ACE);
(4) patients transitioning to stoma.
Transition probabilities were sourced from the literature (3) and are shown in Table 2. The model operates on 1-year cycles over a total period of 30 years. Frequencies of adverse events were estimated from the pertinent UK data and subsequently validated by a medical expert. Unit costs were derived considering the average cost of the healthcare services required for the treatment of each event.
Within the framework of cost-effectiveness analysis, it is important to adapt the decision models to different contexts to provide decision-makers with accurate and resilient information. More specifically, when compared to the UK jurisdiction, Italy faces systematically lower hospital and outpatient tariffs, alongside reduced technology costs. While this would suggest that TAI technology is cost-effective in the Italian context, decision-makers should possess accurate and contextual information, primarily for negotiation purposes.
Utilities, costs, and discount rate
Utility coefficients employed for calculating quality-adjusted life years (QALYs) gains were sourced from the academic literature (3). Costs related to the treatment of patients receiving SBC and its associated health states were obtained from the Italian National Pharmaceutical Formulary (Prestazioni Di Assistenza Specialistica Ambulatoriale, Ministero della Salute (20)). These costs include drugs, medical devices, surgical procedures, and adverse events, such as gastrointestinal infections, intestinal obstructions, and UTIs. Costs related to visits, diagnostic assessments, and adverse events are based on the Italian tariff formulary for outpatient services (21). Costs associated with treatment with TAI, specifically pertaining to the device Navina Smart, were provided by the manufacturer of the device Wellspect Healthcare. Costs associated with medical procedures that are not listed within the Italian National Pharmaceutical Formulary and are not included in the Italian tariff framework for outpatient medical services were replaced with corresponding British costs from different British databases, including online pharmaceutical retailers (12,19,22-24). Where multiple data sources were accessible, the resultant average value was employed. In addition, the economic evaluation applied a standard annual discount rate of 3% for both QALYs and incurred costs, in line with established practice in health economic analysis. Discount rates were applied according to the guidelines of the Italian Health Economics Association (25). A summary of the economic data employed to populate the model is provided in Tables 3 and 4.
Cost-effectiveness
The results of the study were conveyed in terms of incremental costs and incremental QALY, allowing for the estimation of incremental cost-effectiveness ratio (ICER) when incremental costs and QALYs were positive. Conversely, in scenarios characterized by negative incremental costs, the net monetary benefit (NMB) was employed. NMB quantifies the difference between monetary benefits and associated intervention costs.
The subsequent formula was employed to compute the NMB:
NMB = [WTP * (QALYTAI – QALYSBC)] – (CostsTAI – CostsSBC)
The willingness to pay (WTP) is defined as the maximum price or price range that a customer is willing to pay for a product or a service. In this analysis, the WTP was estimated to be €35,000.00/QALY (25).
| From/To | TAI + SBC | Resume SBC | SNS/SARS/ACE | Stoma |
|---|---|---|---|---|
| TAI+ SBC | 0.981 | 0.0069 | 0.0065 | 0.0057 |
| Resume SBC | 0 | 0.9692 | 0.0113 | 0.0195 |
| SNS/SARS/ACE | 0 | 0 | 0.9891 | 0.0109 |
| Stoma | 0 | 0 | 0 | 1 |
| SBC alone | ||||
| TAI | NA | NA | NA | NA |
| Resume SBC | NA | 0.9736 | 0.0097 | 0.0167 |
| SNS/SARS/ACE | NA | 0 | 0.9831 | 0.0116 |
| Stoma | NA | 0 | 0 | 1 |
ACE = antegrade continence enema; SARS = sacral anterior root stimulation; SBC = standard bowel care; SNS = sacral nerve stimulation; TAI = transanal irrigation.
| Resources | Costs (unit) | Frequency | Yearly costs |
|---|---|---|---|
| SBC | |||
| Bulking agent: Psyllogel (20 units) | €7.00 | Daily | €336.00 |
| Softener: Vaseline oil | €5.00 | Daily | €10.00 |
| Stimulant: Bisacodyl | €2.50 | Every other day | €30.00 |
| Osmotic: Movicol | €13.15 | Daily | €236.70 |
| Suppository glycerin | €1.50 | Every other day | €18.00 |
| Suppository bisacodyl | €7.90 | Every other day | €284.40 |
| Enema (1 unit) | €3.00 | Every other day | €540.00 |
| Anal plug | €43.68 | Daily and per FI episode | €1,572.48 |
| Incontinence pad | €11.44 | Daily and per FI episode | €274.56 |
| Surgical Interventions | |||
| SNS procedure | €2,700.00 | One-off | €2,700.00 |
| SNS follow-up | €12.91 | Every 7 years | €12.91 |
| SARS procedure | €9,172.00 | One-off | €9,172.00 |
| SARS follow-up | €12.91 | Two-monthly consultations | €309.84 |
| ACE procedure | €4,503.93 | One-off | €4,503.93 |
| ACE follow-up | €12.91 | Two-monthly consultations | €309.84 |
| Stoma Surgery | |||
| Surgery | €5,154.00 | One-off | €5,154.00 |
| Colostomy bag (pack of 30) | €60.00 | Twice daily | €1,440.00 |
| Belt (pack of 1) | €15.00 | Monthly | €180.00 |
| Skin barrier (pack of 30) | €25.88 | Twice daily | €621.12 |
| Adhesive remover (pack of 30) | €17.41 | Twice daily | €417.84 |
| HCP Visits | |||
| Consultant | €20.66 | 2 TAI; 3 SBC (annually) | €61.98 |
| Dietitian | €20.66 | 0.19 TAI; 0.57 SBC (annually) | €11.78 |
| General practitioner | €20.66 | 2.89 TAI; 3.75 SBC (annually) | €77.48 |
| Adverse Events | |||
| Hospitalizations | |||
| Gastrointestinal infections | €3,236.00 | 0.28 TAI; 1.37 SBC (annually) | |
| Pressure ulcer management | €4,290.00 | €4,433.32 | |
| Falls or other trauma | €2,707.16 | €5,877.30 | |
| Intestinal obstruction | €4,500.00 | €3,708.81 | |
| Abdominal pain | €1,666.53 | 4.5 SBC (annually) | €20,250.00 |
| €2,283.15 | |||
ACE = antegrade continence enema; FI = fecal incontinence; HCP = health care professional; SARS = sacral anterior root stimulation; SBC = standard bowel care; SNS = sacral nerve stimulation; TAI = transanal irrigation.
| Resources | Costs | Frequency | Yearly costs |
|---|---|---|---|
| TAI | |||
| Electronic Smart System (one device Navina Smart) | €434.37 | Irrigation every other day; replaced every 24 months | €217.17 |
| Rectal catheters (10 single use) | €14.18 | Irrigation every other day; 20 per year | €283.60 |
| Consumable set | €21.21 | Irrigation every other day; replaced every month (11) | €2,565.97 |
| Tubes set | €27.50 | 1 | €27.50 |
| Initial consultation | €20.66 | One-off | €20.66 |
| Follow-up phone call | €12.91 | 3 | €38.73 |
| HCP Visits | 2 TAI; 3 SBC (annually) | ||
| Consultant | €20.66 | 0.19 TAI; 0.57 SBC (annually) | € 41.32 |
| Dietitian | €20.66 | 2.89 TAI; 3.75 SBC (annually) | € 3.93 |
| General practitioner | €20.66 | € 59.71 | |
| Adverse Events | 0.28 TAI; 1.37 SBC (annually) | ||
| Hospitalizations | |||
| Gastrointestinal infections | €3,236.00 | €906.08 | |
| Pressure ulcer management | €4,290.00 | €1,201.20 | |
| Falls or other trauma | €2,707.16 | 4.5 SBC (annually) | €758.00 |
| Intestinal obstruction | €4,500.00 | €4,500.00 | |
| Abdominal pain | €1,666.53 | €466.63 | |
Electronic Smart system: 1 Navina Smart control unit (400 uses), 1 power adapter (multiple use), 1 cable (multiple use), 1 tube set (100 uses), 2 rectal catheters regular (single use), 1 Navina case (multiple use), 1 accessory set (multiple use), 1 water container 1.5 L (15 uses).
Tube set: 1 catheter/cone tube (100 uses), 1 water container tube (100 uses).
Consumable set: 1 water container 1.5 L (15 uses), 15 Navina catheters regular (single use).
HCP = health care professional; SBC = standard bowel care; TAI = transanal irrigation.
Sensitivity analysis
A multivariate sensitivity analysis was conducted to evaluate the impact of simultaneous variations of parameters on the model results. Two distributions were used, selected in accordance with the characteristics of the different parameters. Beta distributions were applied to effectiveness, utilities, and transition probabilities, while gamma distributions were employed to cost parameters. The sensitivity analysis was accomplished using a total of 1,000 Monte Carlo simulations.
Results
Cost-effectiveness
| SBC | TAI | Outcome | |
|---|---|---|---|
| Costs | €158,887.43 | €92,786.13 | – |
| QALYs | 10.95 | 11.38 | – |
| Incremental QALYs | – | – | 0.43 |
| Incremental costs | – | – | €66,101.30 |
| NMB | – | – | €81,087.06 |
NMB = net monetary benefit; QALY = quality-adjusted life year; SBC = standard bowel care; TAI = transanal irrigation.
Table 5 presents a comprehensive overview of the results obtained through a comparative analysis of the treatments with SBC and TAI. Treatment with TAI was dominant, implying that it was less expensive and resulted in enhanced health outcomes when considering a population of 1,000 individuals over a 30-year period. Notably, the costs associated with SBC exceeded those associated with TAI. Treatment with TAI has the potential to yield savings of €66,101 and 0.43 QALYs per patient over 30 years. In addition, treatment with TAI is correlated with a reduction in the probability of surgical interventions, stoma creation, adverse events, and HCP visits. The NMB was estimated to be €81,087 per patient over 30 years.
Sensitivity analysis
The results of the multivariate probabilistic sensitivity analysis comparing SBC to TAI are shown in the cost-effectiveness plane in Figure 2. This analysis illustrates that over 90% of the 1,000 Monte Carlo simulations fall in the first quadrant of the cost-effectiveness plane, demonstrating the dominance of Navina Smart TAI compared to SBC alone.
Conclusion
This analysis determined that the treatment with Navina Smart TAI is cost-effective when compared to SBC. Navina Smart TAI for the treatment of NBD can have significant positive effects on the Italian NHS, primarily in terms of cost savings. In Italy, the cost savings are estimated to be €66,101 per patient over a 30-year time horizon. This result is associated with the significant benefits that patients treated with Navina Smart TAI experience because of the lower probability of undergoing negative symptoms, visits, and surgical interventions.
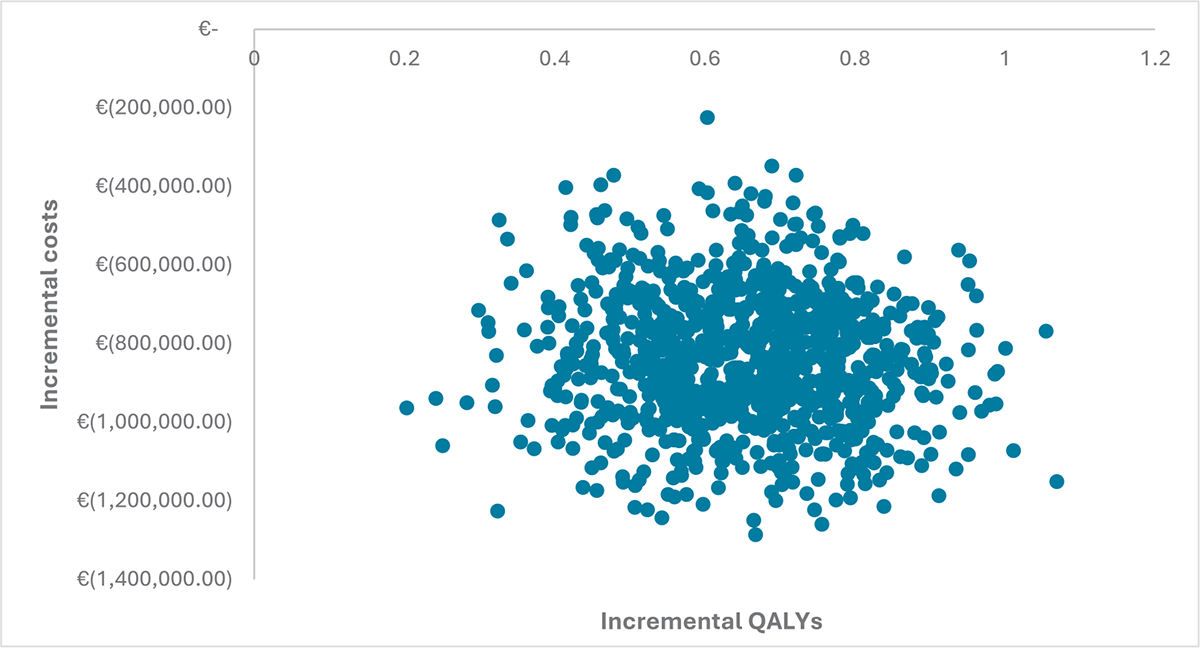
FIGURE 2 - CEAC plane. Results of the multivariate sensitivity analysis. The figure shows the results of the multivariate sensitivity analysis on a CEAC. The CEAC plane plots incremental costs on the y-axis and incremental qualities (or QALYs) on the x-axis. The negative values indicate that the intervention or treatment being analyzed is associated with cost savings compared to an alternative intervention or treatment. The x-axis represents incremental QALYs that are a measure of the effectiveness or health outcomes resulting from an intervention. CEAC = cost-effectiveness acceptability curve; QALYs = quality-adjusted life years.
The dominance of TAI has been confirmed in other studies (3,8,15). Emmanuel et al (3) reported that TAI was associated with significant reductions in fecal incontinence episodes, UTIs, and the likelihood of stoma surgery, improving QALYs and reducing lifetime costs per patient by GBP 21,768. Similar results were presented by Sengoku et al (8) and Christensen et al (15). Dale et al (5) confirmed the efficacy of Navina Smart TAI in improving patients’ conditions over time but did not find the device to be cost-effective. The difference in savings between Emmanuel et al (3) and the present analysis can be primarily explained by the lower tariffs for outpatient services set by the Italian NHS compared to the British healthcare system in the administration of TAI. In addition, Emmanuel et al (4) highlighted a 60% reduction in severe NBD symptoms with Navina Smart TAI. Nonetheless, these symptoms were not included in this analysis, potentially indicating a more favorable cost-effectiveness when considering symptom reductions.
The model used in the analysis is a modified version of a model previously developed and published in other research publications by the same authors (26-28). This adaptation accommodated specific variables or parameters relevant to the current study and adjusted its applicability to the context under investigation.
This model has certain limitations worth noting. First, it includes costs from the British context when the corresponding costs were unavailable in the Italian context. This can be problematic because the British system may use different suppliers and medical protocols that, in some cases, may not easily apply to the Italian system. Then, the model is simulation-based and provides a hypothetical scenario rather than being grounded on real data and real patients where unexpected adverse events may occur as opposed to statistical projections. In this context, our study should be considered as complementary to existing clinical studies. The availability of real-world data would certainly improve the quality of information used to populate the model. Specifically, data on the costs of the compared treatments should take into account elements of variability stemming from organizational factors related to the facilities where the treatments are administered. Personnel costs and technological equipment can have a significant impact on the outcomes. In this regard, it is relevant to note that the model can be easily adapted to accommodate future data availability. Also, the model employs a 30-year time horizon during which new technologies and medical procedures may emerge and fundamentally change the healthcare frame of reference for patients. In conclusion, an additional limitation of this study regards the pyramid treatment model as it exclusively compares one second-line alternative with SBC. Further research should focus on the comparison between different alternatives within the second line. In this way, it will be possible to gain a more comprehensive understanding of the cost-effectiveness of different treatment options at this stage. Moreover, this study did not include training costs associated with Navina Smart TAI for healthcare staff and patients due to a lack of robust information available for an accurate estimation.
The model of this study exhibits various strengths. The model allows a seamless integration as new data become available, with the possibility of readily exchanging real and simulated data. In addition, the model shows significant versatility across different medical contexts and scenarios, specifically when patients face linear fluctuations in their medical conditions. In this case, the probability of change in health status can be used to perform simulations. Finally, this model can be helpful for decision-makers in the healthcare regulatory, and governmental national authorities when allocating scarce resources in national health systems. Regulators tend to overlook the long-term cost savings that medical devices imply if immediate costs are significant. Public health regulators have faced growing pressure to control the costs because of policies aimed at containing public debt, a trend that has been common in Europe in recent years (29). Hence, regulators may have the propensity to maintain existing treatments, unless long-term savings are explicitly demonstrated and substantial. In these cases, the SBC can be erroneously favored because it maintains the costs unchanged; however, improvements in patients’ condition and cost savings can reduce the health system budgets over a longer period of time if cost savings are fully appreciated.
This study confirms that Navina Smart TAI, in comparison with SBC treatment, shows cost-saving benefits for the Italian NHS, with a potential cost reduction of €66,101 per patient and a corresponding QALY gain of 0.43. The findings of this study will encourage the treatment according to the individual patient’s specific condition.
Disclosures
Conflict of interest: The authors declare that there is no conflict of interest.
Financial support: This article was funded thanks to a partial unconditional grant from Wellspect AB. The views expressed in the article are those of the authors and do not necessarily represent the views of the funders or the authors’ institutions. Matteo Ruggeri received no fees or financial grants for participating in this project.
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Authors contribution: MR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology; AS: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing; SC: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft; GR: Conceptualization, Visualization, Validation
References
- 1. Bennett J, Das JM, Emmady PD. Spinal cord injuries. StatPearls Treasure Island, Florida: StatPearls Publishing; 2022. PubMed
- 2. Altems, Valutazione HTA del dispositivo medico Peristeen Plus®. Online. (Accessed June 2024)
- 3. Emmanuel A, Kumar G, Christensen P, et al. Long-term cost-effectiveness of transanal irrigation in patients with neurogenic bowel dysfunction. PLoS One. 2016;11(8):e0159394. CrossRef PubMed
- 4. Emmanuel A, Kurze I, Krogh K, et al. An open prospective study on the efficacy of Navina Smart, an electronic system for transanal irrigation, in neurogenic bowel dysfunction. PLoS One. 2021;16(1):e0245453. CrossRef PubMed
- 5. Dale M, Morgan H, Carter K, White J, Carolan-Rees G. Peristeen transanal irrigation system to manage bowel dysfunction: a NICE medical technology guidance. Appl Health Econ Health Policy. 2019;17(1):25-34. CrossRef PubMed
- 6. Knowles CH, Booth L, Brown SR, et al. Non-drug therapies for the management of chronic constipation in adults: the CapaCiTY research programme including three RCTs. Programme Grants for Applied Research. 2021;9(14):1-134. CrossRef PubMed
- 7. Musco S, Bazzocchi G, Martellucci J, et al. Treatments in neurogenic bowel dysfunctions: evidence reviews and clinical recommendations in adults. Eur J Phys Rehabil Med. 2020;56(6):741-755. PubMed
- 8. Sengoku A, Noto S, Nomi M, Emmanuel A, Murata T, Mimura T. Cost-effectiveness analysis of transanal irrigation for managing neurogenic bowel dysfunction in Japan. J Health Econ Outcomes Res. 2018;6(1):37-52. CrossRef PubMed
- 9. Sakakibara R. Gastrointestinal dysfunction in movement disorders. Neurol Sci. 2021;42(4):1355-1365. CrossRef PubMed
- 10. Hultling C. Neurogenic bowel management using transanal irrigation by persons with spinal cord injury. Phys Med Rehabil Clin N Am. 2020;31(3):305-318. CrossRef PubMed
- 11. Emmanuel AV, Krogh K, Bazzocchi G, et al; Members of working group on Trans Anal Irrigation from UK, Denmark, Italy, Germany, France and Netherlands. Consensus review of best practice of transanal irrigation in adults. Spinal Cord. 2013;51(10):732-738. CrossRef PubMed
- 12. National Institute for Health and Care Excellence (NICE). NICE Guidance. Peristeen transanal irrigation system for managing bowel dysfunction. Feb. 23, 2018. Online. (Accessed June 2024)
- 13. Juul T, Christensen P. Prospective evaluation of transanal irrigation for fecal incontinence and constipation. Tech Coloproctol. 2017;21(5):363-371. CrossRef PubMed
- 14. Wellspect Italia. Navina Medical Internal Documents. Wellspect Italia; 2024.
- 15. Christensen P, Andreasen J, Ehlers L. Cost-effectiveness of transanal irrigation versus conservative bowel management for spinal cord injury patients. Spinal Cord. 2009;47(2):138-143. CrossRef PubMed
- 16. Emmanuel A, Collins B, Henderson M, Lewis L, Stackhouse K. Development of a decision guide for transanal irrigation in bowel disorders. Gastrointest Nurs. 2019;17(7):24-30. CrossRef
- 17. Bardsley A. Transanal irrigation systems for managing bowel dysfunction: a review. Gastrointest Nurs. 2020;18(5):18-28. CrossRef
- 18. Crawshaw A, Marshall J, Bartolo DC, et al. Evaluating gravity versus pump irrigation for bowel dysfunction. Gastrointest Nurs. 2009;7(7):34-42. CrossRef
- 19. Association of the British Pharmaceutical Industry (ABPI). Our industry: facts, figures and industry data. Online. (Accessed June 2024)
- 20. Ministero della Salute. Gazzetta Ufficiale. Prestazioni Di Assistenza Specialistica Ambulatoriale – Allegato 3. Jan. 28, 2013. Online. (Accessed June 2024)
- 21. Ministero della Salute. Gazzetta Ufficiale. Tariffe Delle Prestazioni Di Assistenza Ospedaliera Per Acuti, Per Tipo Di Ricovero – Allegato 1. Jan. 28, 2013. Online. (Accessed June 2024)
- 22. UK Drug Database. UK Drug Information. Online. (Accessed June 2024)
- 23. Analytic Health. Data-driven actionable insights. Online. (Accessed June 2024)
- 24. National Health System Business Service Authority (NHS BSA). NHS Prescription Services, Drug Tariff. Online. (Accessed June 2024)
- 25. Fattore G. A proposal for guidelines for the economic evaluation of health interventions in Italy. PharmacoEcon Ital Res Artic. 2009;11(2):83-93. CrossRef
- 26. Ruggeri M, Signorini A, Caravaggio S, et al. Estimation model for healthcare costs and intensive care units access for Covid-19 patients and evaluation of the effects of remdesivir in the Portuguese context: hypothetical study. Clin Drug Investig. 2022;42(4):345-354. CrossRef PubMed
- 27. Ruggeri M, Signorini A, Caravaggio S, et al. Modeling the potential impact of remdesivir treatment for hospitalized patients with COVID-19 in Saudi Arabia on healthcare resource use and direct hospital costs: a hypothetical study. Clin Drug Investig. 2022;42(8):669-678. CrossRef PubMed
- 28. Ruggeri M, Signorini A, Drago C, Rosiello F, Marchetti M. Model for estimating the healthcare costs and capacity of intensive care units in Italy in the treatment of patients with COVID-19: remdesivir impact assessment. AboutOpen. 2020;7(1):95-102. CrossRef
- 29. Rechel B. Funding for public health in Europe in decline? Health Policy. 2019;123(1):21-26. CrossRef PubMed