 |
Glob Reg Health Technol Assess 2024; 11 (Suppl. 2): 18-21 ISSN 2283-5733 | DOI: 10.33393/grhta.2024.3071 ORIGINAL RESEARCH ARTICLE |
 |
Usefulness of dalbavancin in early discharge and nonhospitalization. It’s time to throw your heart over the obstacle?
ABSTRACT
Introduction: Dalbavancin is a semisynthetic lipoglycopeptide long-acting antibiotic approved for the treatment of acute bacterial skin and skin structure infections (ABSSSIs). Its features can be useful in the current healthcare scenario characterized by the shortage of available hospital beds.
Materials, methods and results: We implemented several actions in order to optimize the use of dalbavancin allowing an improvement strategy both from the healthcare system and the patient’s perspective in two hospital settings. In the Emergency Department we hospitalized only patients who met the clinical criteria and not the logistic criteria (i.e., the need for antibiotic therapy infusion). During the years 2017-2023, this strategy was applied in 40 cases, thus avoiding 40 hospitalizations for a total saving of 280 days of hospitalization.
In the Internal Medicine ward and surgery department when there was no longer any need for hospitalization, we discharged the patient as early as possible. During the years 2017-2023, this strategy was applied in 189 cases, saving at least 1,134 days of hospitalization. The outcome of the treated patients was favorable in 228 out of 229 patients (99.5%).
Conclusions: Our experience using dalbavancin in ABSSSI has been very satisfactory overall. The efficacy was close to 100%. Minor adverse events of slight severity occurred rarely. At the same time, this strategy allowed a more efficient allocation of hospital beds. Dalbavancin presents an ideal pharmacodynamic/pharmacokinetic profile for the management of ABSSSI especially in settings where shortage of hospital beds is critical.
Keywords: ABSSSI, Antibiotic, Health technology assessment (HTA), Long acting
Received: March 18, 2024
Accepted: April 29, 2024
Published online: July 29, 2024
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Dalbavancin is a semisynthetic lipoglycopeptide antibiotic with activity against all gram-positive pathogens including methicillin-resistant Streptococcus aureus (MRSA), which has been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of acute bacterial skin and skin structure infections (ABSSSIs) (1). Dalbavancin has a long lipophilic side chain that confers it two innovative properties: a faster and more potent bactericidal activity than the traditional glycopeptides and a long terminal half-life ranging from 149 to 250 hours in human subjects, allowing for a weekly dose (2). Its favorable pharmacokinetic profile and the long elimination half-life, which is longer in tissues (e.g., skin, bone) than plasma, represent a key advantage over other intravenous drugs requiring multiple daily doses or oral antibiotics, which require patients’ adherence and may be encumbered by adverse events. These factors facilitated the development of single-dose or once-weekly dosing regimens to treat ABSSSIs. Despite being highly protein bound (93%), dalbavancin has a steady-state volume of distribution >10 L and distributes widely into the skin, bone, peritoneal space, and epithelial lining fluid, but not cerebrospinal fluid (3).
The current healthcare context is characterized by significant management challenges due to the scarcity of hospital beds compared to the real needs of the population. It is therefore essential to correctly frame the patient’s clinical conditions in order to be able to identify patients who really require hospitalization from those who can be managed safely outside the hospital.
In this context, the possibility of using a reliable but at the same time manageable therapeutic option that allows the patient not to be hospitalized or discharged early appears to be very relevant.
Obviously, the aim must be not to waste an option that can play a pivotal role in difficult-to-treat infections.
In fact, off-label use is increasingly widespread for the treatment of not only osteoarticular infections such as osteomyelitis (4), joint prosthesis infections (5), spondylodiscitis (6), but also endocarditis (7) and central venous catheter (CVC)-related bacteremia (8). In most cases, dalbavancin is used as sequential treatment. This use appears rational considering numerous clinical and laboratory evidence that highlights excellent tissue penetration compared to the minimum inhibitory concentrations (MICs) detected (9) and an equally adequate anti-biofilm action of dalbavancin (10,11).
Most of the literature analyzes the use of dalbavancin from the point of view of economic savings. In fact, a health technology assessment (HTA) study had assessed that the high cost of the drug compared to most antibiotics was largely compensated by the economic savings in terms of hospitalization costs (12). These preliminary and theoretical data were subsequently confirmed by real-life studies.
These observations are supported by a systematic review, network meta-analysis and cost analysis comparing the newer lipoglycopeptides to standard care and to each other for the treatment of complicated skin and soft tissue infections (SSTIs), estimating that using dalbavancin could save healthcare system $1,442 to $4,803 per case (13). Another recent study aimed to evaluate the direct costs associated with the management of severe ABSSSI patients from a national healthcare provider’s perspective in three countries (Italy, Romania and Spain). The hypothetical administration of dalbavancin rather than the Standard of Care (SoC) therapy (based on vancomycin, teicoplanin, or linezolid) resulted in an estimated mean reduction in hospital stay of 3.3 days per ABSSSI patient, with no significant incremental costs from a National Health System perspective (14).
These studies highlight some key points. However, we believe that the most captivating topic is the decrease in hospitalization, and the higher availability of hospital beds from a healthcare system perspective, and above all allowing the patients to be protected from the “side effects” of hospitalization. In particular, intravenous antibiotic infusion needs vascular access insertion, such as, for example, the middle-term venous access devices like peripherally inserted central catheter (PICC), which poses an average cost per patient of $873 for placement and $205 for complications (e.g., infection, thrombophlebitis, malposition, malfunction). If systemic and serious complications occur (e.g., bacteremia, endocarditis, sepsis), PICC-related costs markedly increase (15) without considering the risk to patient’s health. Daily intravenous antibiotics require nursing time for drug dilution, positioning, removing infusion line and patient observation, which has variable costs across different hospitals/regions.
It should also be remembered that the hospitalization of patients, especially the elderly, can lead to problems related to disorientation and/or psychological decompensation of the patient, which can lead to a further prolongation of hospitalization (16,17).
Further evidence suggests that the use of dalbavancin determines improvements in work productivity and ability to complete daily activities, in addition to patient satisfaction, thus saving social costs as well (18).
Materials, methods and results
We implemented several actions in order to optimize the use of dalbavancin allowing an improvement strategy both from the healthcare system perspective and the patient’s perspective in two hospital settings. Our infectious diseases consultancy unit manages three hospitals, one hub and two spokes, with a total number of 671 beds serving a population of approximately 310,000 inhabitants. We adopt a restrictive policy toward the use of new antibiotics. In addition, the prescription of dalbavancin is carefully monitored and limited to specific infectious disease prescription.
We evaluated two settings where multidisciplinary collaboration is key.
The first work, with the involvement of Emergency Room colleagues, is focused on the attempt to hospitalize only those patients who meet the clinical criteria and not the logistic criteria stemming from the need for antibiotic therapy infusion.
In the period between 2017 and 2023, this strategy was applied in 40 cases and therefore 40 hospitalizations were avoided. This led to a net saving of at least 280 days of hospitalization.
The second work aimed to discharge the patient as early as possible as soon as there was no longer any need for hospitalization. In this case, we involved our Internal Medicine specialists and surgeons.
The multidisciplinary approach consisted of carrying out training meetings with the various specialists potentially involved in order to share the “inclusion criteria” of patients, without however neglecting the role of pharmacists and microbiologists.
In the period between 2017 and 2023, this strategy was applied in 189 cases and therefore at least 1,134 days of hospitalization were saved.
The results obtained in each year are illustrated in Figure 1.
The outcome of the treated patients was favorable in 228 out of 229 patients (99.5%). Only in one case there was a recurrence of ABSSSI within 30 days, which also responded to a new administration of dalbavancin, probably because the infection had not been completely eradicated.
Among the adverse events observed, we noted an episode of facial flushing during the infusion, which however was terminated by slowing down the infusion without further skin manifestations in the following 14 days.
In two cases (0.8%), skin reactions characterized by urticarial rash without serious manifestations resolved with symptomatic therapy within a total of 10 days. No serious adverse events and no alterations in renal or hepatic function were noted.
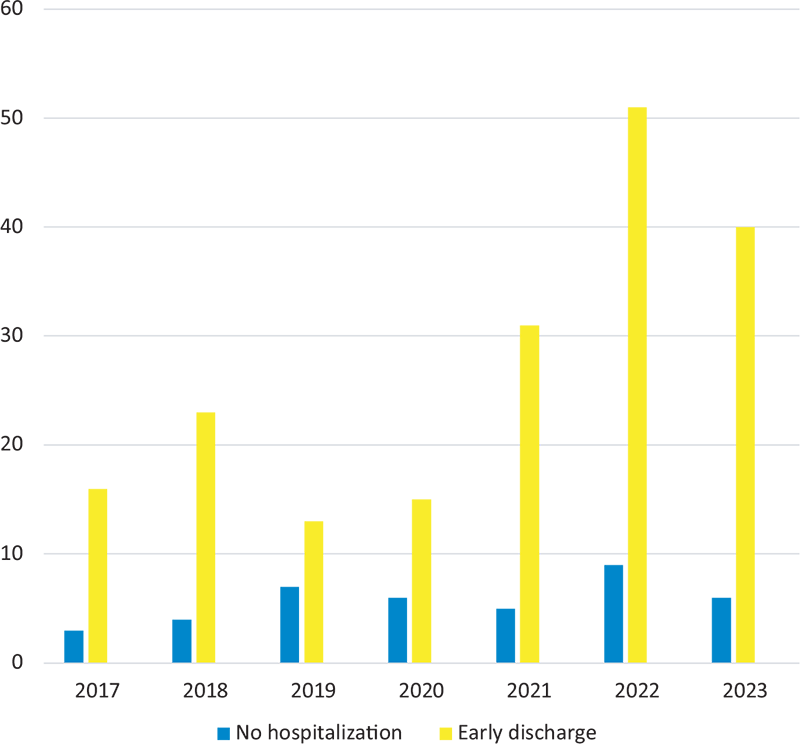
FIGURE 1 - Patients who were not hospitalized (light blue bar) or discharged early (yellow bar) for each year between 2017 and 2023.
Discussion
Our experience using dalbavancin in ABSSSI has been overall very satisfactory. The treatment was associated with an efficacy close to 100% and also rarely associated with minor adverse events.
At first glance, ABSSSIs, for which dalbavancin is indicated, are infections in which the use of dalbavancin may seem excessive, especially considering the numerous therapeutic options available. However, our experience indicates that dalbavancin is a very effective therapeutic option, which at the same time allows the patient to be managed totally or mainly out of hospital, ensuring not only a better allocation of hospital beds, with its economic implications but, most importantly, a reduced incidence of the possible complications related to hospitalization. Precisely for this reason, dalbavancin’s true potential could be expressed in the use, which currently is off-label, of infections such as osteomyelitis, arthroplasty infections, endocarditis and vascular prosthesis infections, which usually require long-term antibiotic therapy.
As far as ABSSSI is concerned, the real potential lies precisely in the opportunity to completely avoid hospitalization. This is also what is suggested by the work of Oliva et al (19). In this regard, the hurdle that we are still encountering is of a “psychological” nature on the part of Emergency Room doctors who often still prefer to hospitalize the patient or keep them under observation for at least 48 hours.
Exactly for this reason the best management strategy, in most cases, may be represented by a short observation in the Emergency Room of 24-48 hours after the administration of dalbavancin. With this approach, hospitalization can be avoided, and, at the same time, the doctor can discharge the patient reassured by the improvement in the patient’s clinical conditions.
Conclusions
Dalbavancin presents an ideal pharmacodynamic/pharmacokinetic profile for the management of ABSSSI.
Our experience, gathered in a hospital where proper allocation of hospital beds is a major priority, could contribute to a more effective use of dalbavancin for the out-of-hospital management of ABSSSI. The drug has proven to be reliable both in terms of efficacy and in terms of safety, contributing, at the same time, to the long-term sustainability of the healthcare system.
Acknowledgment
The authors thank their colleagues for their constant clinical collaboration.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Payment of medical writing and publication fees was supported by an unconditional grant by Angelini Pharma S.p.A.
References
- 1. Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169-2179. CrossRef PubMed
- 2. Barberán J, de la Cuerda A, Barberán LC. Dalbavancin. Rev Esp Quimioter. 2021Sep;34 (Suppl 1):26-28. CrossRef PubMed
- 3. Molina KC, Miller MA, Mueller SW, Van Matre ET, Krsak M, Kiser TH. Clinical pharmacokinetics and pharmacodynamics of dalbavancin. Clin Pharmacokinet. 2022;61(3):363-374. CrossRef PubMed
- 4. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2019;6(1):ofy331. CrossRef PubMed
- 5. Lovatti S, Tiecco G, Mulé A, et al. Dalbavancin in bone and joint infections: a systematic review. Pharmaceuticals (Basel). 2023;16(7):1005. CrossRef PubMed
- 6. Ramadan MS, Gallo R, Lugarà M, et al. Dalbavancin treatment for spondylodiscitis: multi-center clinical experience and literature review. J Chemother. 2022;34(6):360-366. CrossRef PubMed
- 7. Fazili T, Bansal E, Garner D, Gomez M, Stornelli N. Dalbavancin as sequential therapy for infective endocarditis due to Gram-positive organisms: a review. Int J Antimicrob Agents. 2023;61(4):106749. CrossRef PubMed
- 8. Venturini S, Reffo I, Avolio M, et al. Dalbavancin in catheter-related bloodstream infections: a pilot study. Infez Med. 2023;31(2):250-256. CrossRef.
- 9. Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128-136. CrossRef PubMed
- 10. Žiemytė M, Rodríguez-Díaz JC, Ventero MP, Mira A, Ferrer MD. Effect of dalbavancin on staphylococcal biofilms when administered alone or in combination with biofilm-detaching compounds. Front Microbiol. 2020;11:553. CrossRef PubMed
- 11. Oliva A, Stefani S, Venditti M, et al. Biofilm-related infections in gram-positive bacteria and the potential role of the long-acting agent dalbavancin. Front Microbiol. 2021;12:749685. CrossRef PubMed
- 12. Favaretti C, Kheiraoui F, di Pietro ML, et al. Valutazione dell’impatto clinico, organizzativo, economico ed etico dell’introduzione di una nuova tecnologia sanitaria, Dalbavancina, per il trattamento delle infezioni batteriche acute di cute e struttura cutanea in Italia. Int J Public Health. 2016;5(4). Online. Accessed April 2024.
- 13. Agarwal R, Bartsch SM, Kelly BJ, et al. Newer glycopeptide antibiotics for treatment of complicated skin and soft tissue infections: systematic review, network meta-analysis and cost analysis. Clin Microbiol Infect. 2018;24(4):361-368. CrossRef PubMed
- 14. Marcellusi A, Viti R, Sciattella P, et al. Economic evaluation of the treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) from the national payer perspective: introduction of a new treatment to the patient journey. A simulation of three European countries. Expert Rev Pharmacoecon Outcomes Res. 2019;19(5):581-599. CrossRef PubMed
- 15. Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram+ acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. CrossRef PubMed
- 16. Rieck KM, Pagali S, Miller DM. Delirium in hospitalized older adults. Hosp Pract (1995). 2020 Mar;48(suppl 1):3-16. CrossRef PubMed
- 17. Mei X, Liu YH, Han YQ, Zheng CY. Risk factors, preventive interventions, overlapping symptoms, and clinical measures of delirium in elderly patients. World J Psychiatry. 2023;13(12):973-984. CrossRef PubMed
- 18. McCarthy MW, Keyloun KR, Gillard P, et al. Dalbavancin reduces hospital stay and improves productivity for patients with acute bacterial skin and skin structure infections: the ENHANCE trial. Infect Dis Ther. 2020;9(1):53-67. CrossRef PubMed
- 19. Oliva A, Carbonara S, Cianci V, et al. Direct or early discharge of acute bacterial skin and skin structure infection patients from the emergency department/unit: place in therapy of dalbavancin. Expert Rev Anti Infect Ther. 2023;21(7):703-721. CrossRef PubMed