 |
Glob Reg Health Technol Assess 2024; 11: 207-213 ISSN 2283-5733 | DOI: 10.33393/grhta.2024.3055 ORIGINAL RESEARCH ARTICLE |
 |
Survival, treatment duration and costs of patients with prostate cancer treated with triptorelin in Italy: a study of administrative databases
ABSTRACT
Background: Several data support the efficacy/effectiveness, safety and favorable impact on quality of life of triptorelin treatment in patients with prostate cancer. However, little evidence is available concerning triptorelin use in the long term.
Methods: We analyzed data on triptorelin treatment in patients with prostate cancer in an integrated Italian administrative database, covering around 6 million health-assisted subjects throughout the country. Patients with at least one prescription for triptorelin in the period 2010-2020 and with no evidence of metastasis were included and followed up until 2021. Overall survival (OS) and duration of treatment were analyzed using Kaplan-Meier curves, starting from the date of first prescription.
Results: The cohort included a total of 3,411 patients (mean age: 76.8 ± 8.7 years), of whom 1,326 (38.9%) were treated with triptorelin only and 2,085 (61.1%) with triptorelin combined with an anti-androgen. Overall, 847 (24.8%) patients with prostate cancer died and 1,037 (30.4%) had a treatment switch during the follow-up period, and both the median OS and median duration of treatment were not reached in both groups. The mean annual total cost per patient was estimated as 5,574 €, with almost half of the costs related to medication expenses (2,737 €).
Conclusions: We found a long survival and duration of triptorelin treatment in this population of Italian patients with prostate cancer. This study with a long follow-up period further highlights the usefulness of healthcare utilization databases to integrate results obtained from clinical studies with those from everyday clinical practice.
Keywords: Administrative databases, Prostate cancer, Real-world studies, Treatment, Triptorelin
Received: February 29, 2024
Accepted: October 14, 2024
Published online: November 11, 2024
This article includes supplementary material
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Prostate cancer (PC) is the second most common neoplasm in men worldwide, with an estimated number of over 1.4 million new cases and 375,000 deaths in year 2020 (1). Italy has a high life expectancy at birth (i.e., about 80 years) and a large proportion of elderly subjects (i.e., over one-fifth of the population is aged 65 years or older) (2). Since PC is typically a tumor of the elderly, in Italy it ranks (by far) first in terms of male cancer incidence (with 35,000 to 40,000 estimated cases per year) and third in terms of cancer mortality (with about 7000 deaths per year) (1,3).
The proportion of patients achieving 5-year survival after PC diagnosis is high and highly heterogeneous depending on age and disease stage at diagnosis (4-6). The treatment approach selected is also dependent on clinical tumor stage, as well as on other clinicopathologic factors, such as Gleason score and prostate-specific antigen concentration (7).
PC cells’ growth is stimulated by androgens (8). Historically, surgical castration represented the first therapeutic approach to control the PC growth. Then, androgen deprivation therapy (ADT), through most widely used gonadotropin-releasing hormone (GnRH) analogs, became the backbone of the treatment of PC. It is used in patients with localized disease in combination with local radical treatments (surgery or radiotherapy), with duration of treatment generally varying between 6 and 36 months, as well as in patients with biochemical relapse and/or metastatic spread. If metastasized, ADT remains the mainstay of the treatment alone or in combination with other agents (7,9). Triptorelin is one of the GnRH agonists and it is indicated in patients who require an ADT as therapeutic strategy to control their PC. The literature, including both clinical trials and real-world data (RWD), clearly supported the efficacy/effectiveness, the safety profile and the impact on patients’ quality of life of this agent (10-13). However, little evidence is available concerning treatment change in the long term (14). The analysis of administrative databases can provide information on the use of triptorelin in a large population of patients and on long-term use, in a clinical practice setting.
The aim of this study is, therefore, to describe the main characteristics of a large population of patients with PC treated with triptorelin, and to provide data on patients’ survival outcomes, duration of treatment, as well as the costs and health resources utilization in this patient setting in Italy.
Methods
The Italian National Health Service is a “universalistic” system, which aims to provide care to the whole reference population on any type of disease. About two decades ago, the Italian Health Ministry encouraged Italian regions to collect administrative data on the utilization of healthcare services and their costs (15).
This is a retrospective study of patients with PC based on an integrated set of administrative databases for healthcare resources consumption from a sample of Italian Local Health Units (LHUs), covering around 6 million health-assisted individuals throughout Italy. The population comprised patients with at least one ADT prescription between January 2009 and June 2021.
For the aims of the present study, inclusion criteria were: (i) patients with at least one prescription for triptorelin (Decapeptyl®) (Anatomical Therapeutic Chemical [ATC] Classification System code L02AE04, and marketing authorization code [AIC] 026999021, 026999058, 026999060) during the period January 2010-June 2020 (i.e., inclusion period); (ii) with a characterization period of at least 12 months available before the index date (i.e., date of first triptorelin prescription during the inclusion period); and (iii) with at least 12 months of potential observation after the index date. Exclusion criteria were: (i) patients aged ≤18 years; (ii) female patients; (iii) patients with any reported presence of metastases. Supplementary Figure 1 shows the study periods in detail.
Data sources
Data were extracted from the following databases: (i) the beneficiaries’ database which contains all demographic data for patients in analysis; (ii) pharmaceutical database that provides information on the drugs supplied for patients in analysis, as ATC, AIC, prescription date, number of packages, cost per package; (iii) hospitalization database, which comprises all hospitalization data for patients in analysis, such as date of hospitalization, main and secondary diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), Diagnosis-Related Group (DRG) and DRG-related charge (provided by the National Health System, NHS); (iv) outpatient specialist database, which incorporates all information about specialist visits and laboratory tests for patients under analysis (including the date of prescription).
An anonymous univocal numeric code was assigned to each health-assisted individuals by the LHUs to guarantee patients’ privacy, in full conformity with UE Data Privacy Regulation 2016/679 (“GDPR”) and Italian D.lgs. n. 196/2003, as amended by D.lgs. n. 101/2018. All the results were produced as aggregated summaries, which could never be connected, either directly or indirectly, to specific subjects. In accordance with the pronouncement of the Data Privacy Guarantor Authority, General Authorization for personal data treatment for scientific research purposes – n.9/2014, informed consent was not required, since it was not possible, for clear organizational reasons, to collect it. The project from which these analyses were drawn was approved by the Ethics Committee of the LHUs involved in the analysis, reported in detail in Supplementary Table 1.
| Overall (N = 3,411) | Triptorelin alone (N = 1,326) | Triptorelin + ADT (N = 2,085) | |
| n (%) | n (%) | n (%) | |
| Age (years), mean (SD) | 76.8 (8.7) | 77.1 (9.6) | 76.7 (8.1) |
| 18-44 | 13 (0.4) | 13 (1.0) | - |
| 45-54 | 32 (0.9) | 15 (1.1) | 17 (0.8) |
| 55-64 | 227 (6.7) | 79 (6.0) | 148 (7.1) |
| 65-74 | 906 (26.6) | 328 (24.8) | 578 (27.8) |
| 75-84 | 1628 (47.8) | 627 (47.4) | 1001 (48.1) |
| ≥85 | 605 (17.8) | 264 (19.9) | 341 (16.4) |
| Charlson index, mean (SD) | 0.9 (0.9) | 0.9 (0.9) | 0.9 (0.9) |
| 0 | 1273 (37.3) | 515 (38.8) | 758 (36.4) |
| 1 | 1377 (40.4) | 518 (39.1) | 859 (41.2) |
| ≥2 | 761 (22.3) | 293 (22.1) | 468 (22.4) |
| Comorbidity profile | |||
| Hypertension | 2500 (73.3) | 969 (73.1) | 1531 (73.4) |
| Hyperlipidemia | 1139 (33.4) | 440 (33.2) | 699 (33.5) |
| COPD | 730 (21.4) | 295 (22.2) | 435 (20.9) |
| Cardiovascular disease | 523 (15.3) | 208 (15.7) | 315 (15.1) |
| Diabetes | 595 (17.4) | 231 (17.4) | 364 (17.5) |
| Depression | 402 (11.8) | 169 (12.7) | 233 (11.2) |
ADT = androgen deprivation therapy; COPD = chronic obstructive pulmonary disease; SD = standard deviation.
| Overall (N = 3,201) | Triptorelin alone (N = 1,251) | Triptorelin + ADT (N = 1,950) | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Number of triptorelin and ADT prescriptions | 6.9 (4.5) | 5.0 (3.3) | 8.2 (4.7) |
| Number of other drug prescriptions | 17.5 (11.6) | 17.3 (11.6) | 17.6 (11.6) |
| Number of hospitalizations | 0.3 (0.6) | 0.2 (0.6) | 0.3 (0.7) |
| Number of outpatient specialist services | 12.3 (11.9) | 12.5 (12.5) | 12.1 (11.5) |
ADT = androgen deprivation therapy; SD = standard deviation.
A number of baseline patient characteristics were derived from the administrative databases, including age at index date and clinical characteristics, through the Charlson Comorbidity Index (16), that assigns a score to each concomitant disease. Specifically, a modified version of the Charlson Index not accounting for cancer was applied. Several comorbidities, assessed during the 12 months characterization period using medication prescriptions and diagnoses recorded in hospital discharge data, were considered (17): hypertension; hyperlipidemia; diabetes; chronic obstructive pulmonary disease; depression; cardiovascular events, including ischemic heart disease, cardiac dysrhythmias, heart failure, cerebrovascular disease, atherosclerosis and aneurysm; and other peripheral vascular disease. Radical prostatectomy occurring during the 12 months characterization period was identified through ICD-9-CM codes. The presence of reported metastases was evaluated during all available period, through the exploration of information present in the hospitalization database. Patients with reported metastases were excluded. Supplementary Table 2 reports the rules and ICD-9-CM codes used to identify all the above comorbidities, as well as radical prostatectomy and reported metastases.
Patient stratification
The analyses were performed considering all patients treated with triptorelin together, as well as according to strata defined by type of treatment. Specifically, the subgroups considered were: triptorelin as only treatment (i.e., no other drug observed during the inclusion period; reported as “triptorelin alone”); and triptorelin and an anti-androgen (identified by: cyproterone, ATC code: G03HA01; flutamide, ATC code: L02BB01; nilutamide, ATC code: L02BB02; bicalutamide, ATC code: L02BB03; reported as “triptorelin + ADT”).
During the inclusion period considered in this study, which extends over about 10 years, several changes in the treatment options for patients with non-metastatic PC occurred, with consequent updating of the management guidelines (18-21). The introduction of watchful waiting and active surveillance strategies into clinical practice around 2015 led to the reduction of active treatments for some subpopulations of low-risk patients. Stratification for period of index date was thus also performed (i.e., 2010-2014 vs. 2015-2020, Supplementary Materials).
Healthcare costs and resources utilization analysis
In alive patients, during the first year of follow-up, the healthcare costs related to overall drug prescriptions (evaluated for those drugs reimbursed by the Italian NHS, and using the Italian NHS purchase price), hospitalizations (determined by using the DRG tariffs) and outpatient specialist services (according to regional tariffs, for laboratory tests and specialist visits) were estimated. Data were reported as the mean annual overall healthcare cost per patient, and as the mean annual cost and number of drug prescriptions, hospitalizations and outpatient specialist services per patient.
Statistical analysis
A number of descriptive analyses were conducted. Continuous variables were reported as mean ± standard deviation (SD), and categorical variables were expressed as numbers and percentages. Overall survival (OS) and duration of treatment were analyzed using Kaplan-Meier product-limit survival curve estimates (22), starting from the date of first prescription. Median survival times and the corresponding 95% confidence intervals were computed. For the analysis of OS, patients were observed until death (event) or end of follow-up period with the patient being still alive (censored). For the analysis of treatment duration, patients were observed until change of PC treatment (toward a drug of the same or of a different class) (event) or end of follow-up period with the patient being still on the same treatment (censored). In the latter analysis, deaths were considered as censored data, with censoring occurring at the date of death. All analyses were performed using Stata SE version 12.0 (StataCorp, College Station, TX, USA). According to the “Opinion 05/2014 on Anonymisation Techniques” drafted by the “European Commission Article 29 Working Party,” the analyses involving fewer than three patients were not reported, as they were potentially traceable to single individuals. Therefore, results referred to ≤3 patients were described as “not reported” (NR).
Results
Table 1 reports the main characteristics at baseline of patients treated with triptorelin, overall and according to different therapy combinations. The cohort included a total of 3,411 patients. The mean (SD) age of the population included was 76.8 ± 8.7 years. Most patients (92.2%) were aged 65 years or more, with 47.8% of the population aged 75-84 years. A total of 1,273 (37.3%) PC patients had Charlson Comorbidity Index equal to 0, while 761 (22.3%) subjects had an index ≥2. The most frequently observed comorbidities were hypertension (in 73.3% of patients with PC), hyperlipidemia (33.4%), chronic obstructive pulmonary disease (21.4%) and diabetes (17.4%). A total of 1,326 out of 3,411 patients (38.9%) were treated with triptorelin alone, and 2,085 (61.1%) received triptorelin combined with an anti-androgen.
Figure 1 describes the OS curves in all patients (Panel A) and according to the different treatment groups (Panel B). Overall, 847 patients died (24.8%, median OS not reached). When the analyses were stratified by treatment type, patients treated with triptorelin alone and those with combination treatment showed similar survival curves. Corresponding analyses of OS in subgroups of period of patient inclusion (Supplementary Figure 2), presence of comorbidities (Supplementary Figure 4) and previous prostatectomy (Supplementary Figure 6) are reported in the supplementary materials.
Figure 2 reports Kaplan-Meier curves of duration of treatment, overall (n=3,411, Panel A) and in subgroups of type of treatment combination at initiation (Panel B). In the overall analysis, a total of 1,037 (30.4%) patients with PC changed treatment during the follow-up period, and median was not reached. In subgroup analyses, broadly similar curves were observed for triptorelin as monotherapy (median: not reached) and for triptorelin combined with an anti-androgen (median: not reached). Corresponding analyses of duration of treatment in subgroups of period of patient inclusion (Supplementary Figure 3), presence of comorbidities (Supplementary Figure 5) and previous prostatectomy (Supplementary Figure 7) are reported in the supplementary materials.
Figure 3 shows the average healthcare cost per patient (€) during the first year of follow-up, overall and according to different types of healthcare costs. The mean annual total cost per patient was estimated as 5,574 €. Almost half of the mean annual total cost per patient was related to medication expenses (total of 2,737 €, i.e., 1,727 € of triptorelin and ADT costs, and 1,010 € of costs associated with other drugs), followed by outpatient specialist services costs (1,961 €) and costs associated with hospitalizations (877 €).
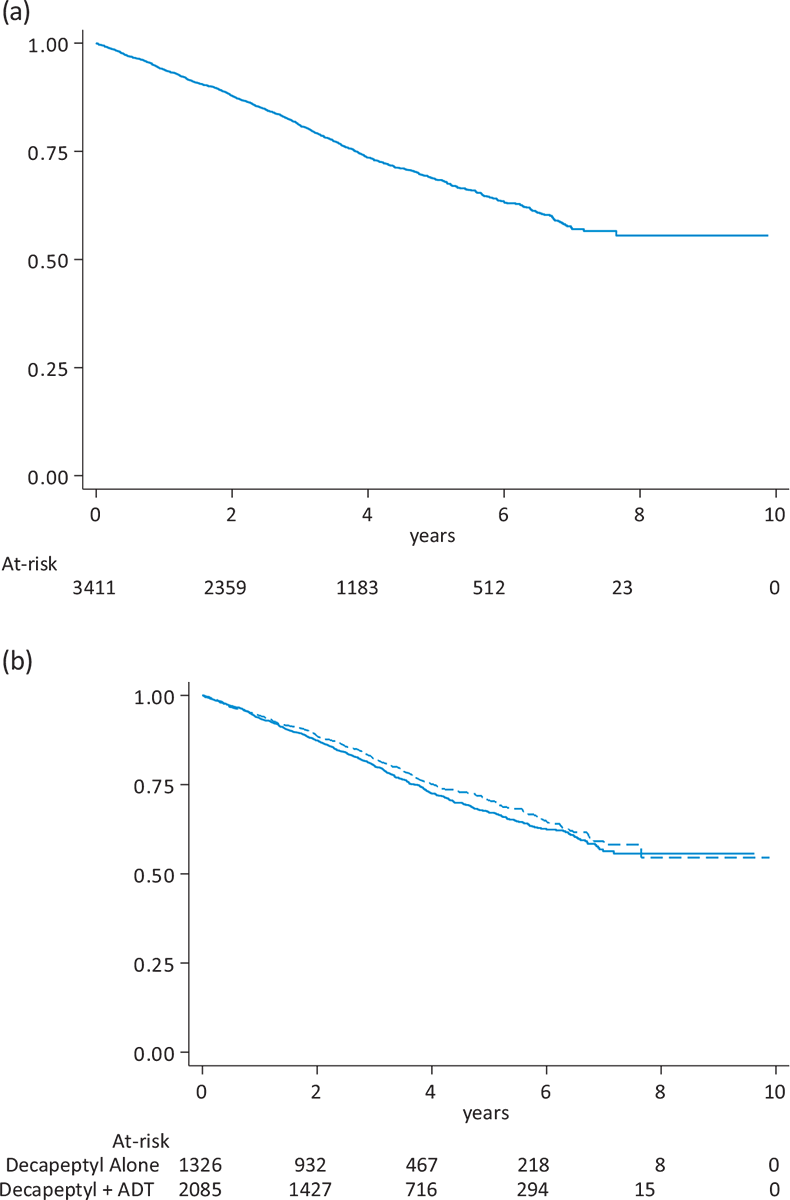
FIGURE 1 - Overall survival in 3,411 patients with prostate cancer treated with triptorelin (Panel A) and according to type of treatment combination at initiation (Panel B). ADT = androgen deprivation therapy.
Table 2 reports the average utilization of health resources per patient during the first year of follow-up. Overall, a mean of 6.9 (SD: 4.5) prescriptions of triptorelin and ADT treatments, 17.5 (SD: 11.6) prescriptions of other drugs, 12.3 (SD: 11.9) outpatient specialist services and 0.3 (SD: 0.6) hospitalizations per patient per year were described. With reference to different combinations of triptorelin treatment, the average annual use of healthcare resources was generally comparable in patients treated with triptorelin alone and triptorelin + anti-androgen.
Discussion
This study provides new relevant descriptive information on triptorelin use alone or in combination with anti-androgens from a RWD study based on administrative databases, covering about 10% of the Italian population. At initiation of triptorelin use, almost 40% of patients were treated with monotherapy and about 60% with combination therapy (with another androgen antagonist). About two-thirds of patients treated with triptorelin were aged 75 or older – the mean age at baseline was around 77 years – and had at least one comorbidity. A long duration of survival was observed in the whole cohort, because median OS was not reached. A similar pattern emerged for the analyses of triptorelin duration of use. Besides the clinical impact of PC, the high burden of disease in terms of both average annual costs and healthcare resources utilization per patient was confirmed and further quantified in this Italian population.
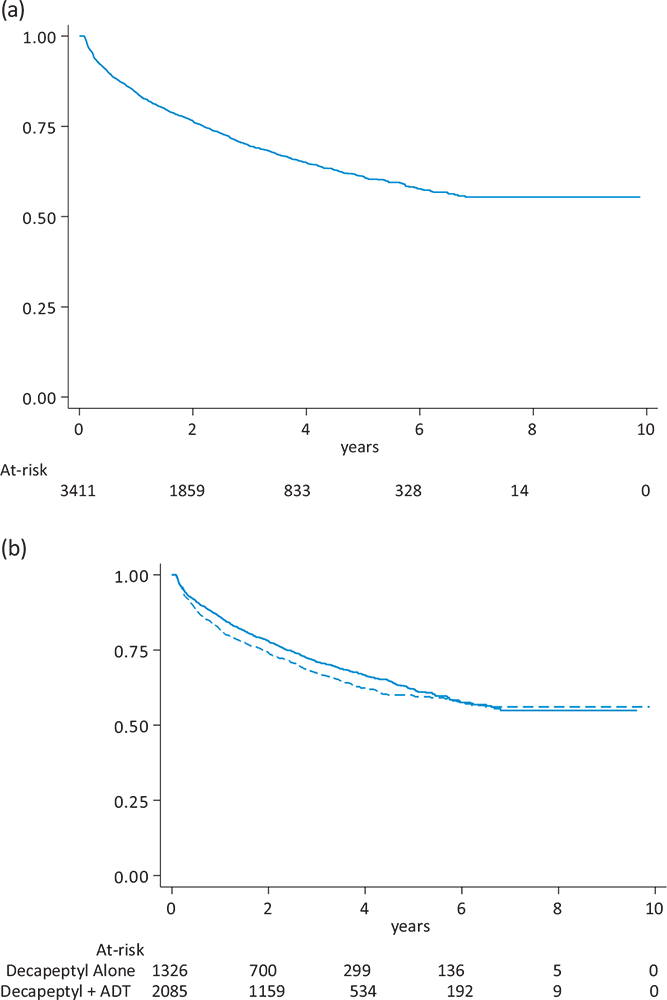
FIGURE 2 - Duration of treatment in 3,411 patients with prostate cancer treated with triptorelin (Panel A) and according to type of treatment combination at initiation (Panel B). ADT = androgen deprivation therapy.
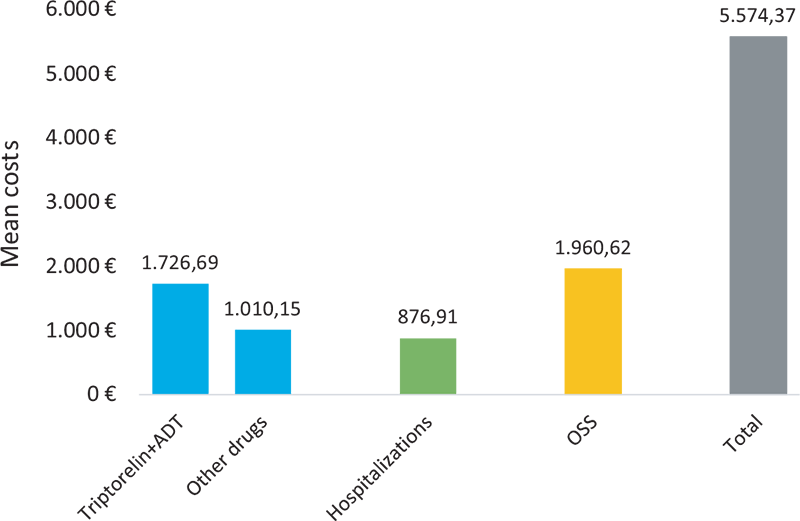
FIGURE 3 - Healthcare costs per patient during the first year of follow-up. ADT = androgen deprivation therapy; OSS = outpatient specialist services.
The efficacy and safety of slow-release triptorelin formulations and of other GnRH analogs in PC treatment have long been established (23,24). This notwithstanding, information on the characteristics of patients treated in real life and on long-term use of triptorelin is still relatively limited. In the ongoing French TALISMAN study, the mean age at baseline of patients with histologically confirmed PC initiating triptorelin treatment was 73.9 years, and 71% of patients had one or more comorbidities (14). In a Belgian study investigating changes in body image in 98 patients with PC treated with triptorelin, enrolled subjects were mostly elderly, with a mean age at baseline of 77.8 years (25). In another RWD study from Germany that included – among 2,382 subjects treated with GnRH analogs – 308 patients treated with triptorelin for advanced PC (17), 78.6% of cases were aged >70 years (mean age: 75) and comorbidities were common (although separate data for triptorelin-treated patients were not given). In line with these studies, and consistently with the good tolerability profile of triptorelin (9), in this Italian population we found that PC triptorelin users are frequently – i.e., about two-thirds of patients – aged ≥75 years (mean age of 76.8 years), and present at least one combined disease. RWD thus confirm the expectation that triptorelin is widely used in elderly and/or comorbid patients.
Data on long-term OS and duration of treatment specific to triptorelin use in real life are limited. In the German study already described (17), over an observation period of about 4 years, the median OS in 308 patients with locally advanced or metastatic PC treated with triptorelin was 477 days (i.e., 1.3 years), and the median time to treatment switch was 495 days (i.e., 1.4 years). Our study reported much longer median OS and duration of treatment (median not reached in both analyses), this being at least in part explained by two factors: (i) the long duration of the observation period, ranging from 2010 to mid-2021 (data maturity); and (ii) differences between the two studies in the stage of patients with PC included, because advanced or metastatic PCs only were examined in the German analysis, whereas we excluded patients with reported metastases. A relevant fraction of patients (i.e., about one-third) was treated with triptorelin monotherapy. This likely reflects the large study period considered, starting from 2010. Adherence to EAU Guidelines on ADT treatment was reported to be low in Italy during 2010-2012 (26), and this might have influenced our results. Further, a subgroup of about 8% of patients was reportedly treated with radical prostatectomy before starting (adjuvant) triptorelin use (although this proportion may be affected by limitations of administrative data), and it is possible that at least some other patients were first treated with radiotherapy.
The estimated overall cost per patient with PC during the first year of follow-up was around 5,600 € in this Italian study. Medication expenses accounted for about half of the total cost per patient. An earlier analysis conducted in five European countries, including Italy, reported an average direct cost per patient in the first year after diagnosis ranging between 3,256 € in Spain and 5,851 € in France (27). The estimated cost per patient in Italy, based on data of 2004-2006, was 5,226 €. A higher spending estimate emerged in another Italian study, valued in year 2000, reporting a mean cost per patient in the first year after PC diagnosis of 6,575 € (28). Similar to our findings, medication costs accounted for 43% of total expenses, that is, about 2,830 €. Again, according to a recent survey of Italian clinical experts, the mean direct cost of each patient with non-metastatic castration-resistant PC was 4,710 € per year (29). Costs were much higher in another study, which was however focused on a different population, that is, metastatic castration-resistant PC alone (30). In line with this observation, a Swedish analysis highlighted a sevenfold increase in total mean costs per patient per year from the non-metastatic hormone-sensitive to the metastatic castration-resistant PC state (31). Thus, our results provide additional, up-to-date quantification of the economic burden of PC in Italy at initiation of (triptorelin) treatment. Although it is difficult to compare studies conducted in different periods and patient populations, our cost estimates tended to be in line with those from previous Italian analyses.
RWD studies conducted through the use of administrative databases suffer from typical limitations (32). Our analysis may thus be affected by bias, particularly those deriving from the peculiar type of retrieval of information. Administrative data lack several useful clinical information (e.g., disease stage, reason for treatment switch, etc.), thus limiting the interpretability of the descriptive analyses and the possibility to perform in-depth evaluations, including the calculation of progression-free survival and the exploration of potential safety/tolerability issues. The availability of information related to a restricted time period may also increase the risk of information bias, for example, if interruptions in triptorelin treatment (i.e., intermittent ADT use) had occurred before the start of the observation period. Further, the study had mainly descriptive aims, and no formal comparisons were performed. Conversely, strengths of this investigation are the long period of observation, the inclusion of an unselected population (based on clinical practice, and including both hospitalized and non-hospitalized cases) and the availability of data covering an important fraction of the Italian population, allowing the examination of several clinical and economic aspects related to PC. Italy is an optimal setting to explore this topic, given the high incidence of PC, the adequate coverage of administrative databases and the modest risk of patient selection (given the universal care system and the low proportion of patients with a private insurance in Italy compared with several other high-income countries).
Conclusions
In conclusion, we reported a long survival and duration of triptorelin treatment in this population of Italian patients with PC. Considering the general long survival and limited information available in this setting of patients, this study provided new important RWD findings to address the knowledge gap on long-term use of triptorelin in everyday clinical practice. This study further highlights the usefulness of healthcare utilization databases to provide additional evidence integrating results obtained from clinical studies.
Acknowledgments
The authors thank Statinfo (Italy) for providing medical writing and editorial support, which was industry sponsored in accordance with Good Publication Practice guidelines (GPP22).
Disclosures
Conflict of interest: G.Fa.: consulting or advisory role, travel from Astellas, IPSEN, Janssen. V.A., P.M., G.M.: employees of IPSEN stock ownership in IPSEN. G.Fo.: Advisory board and travel from IPSEN. The other authors reported no conflict of interests.
Financial support: The study report was developed by CliCon S.r.l. Società Benefit and supported by IPSEN. The agreement signed by CliCon S.r.l. and IPSEN does not create any joint venture or any similar relationship between parties. CliCon S.r.l. is an independent company. Neither CliCon S.r.l. nor any of their representatives are employees of IPSEN for any purpose.
Author contributions: Conceptualization: LDE; Data curation: LDE; Formal analysis: LDE; Funding acquisition: VA, GM and PM; Project administration: VA, GM and PM; Supervision: OC, GF, GF; Validation: OC, GF, GF; Writing – review and editing: OC, GF, LDE, VA, GM, PM, GF.
Data Availability Statement: All data used for the current study are available upon reasonable request next to CliCon S.r.l., which is the body entitled of data treatment and analysis by local health units.
References
- 1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France. 2020. Online. (Accessed February 2024)
- 2. ISTAT. 2022. Online. (Accessed February 2024)
- 3. AIOM-AIRTUM. I numeri del cancro in Italia. Intermedia Editore; 2021. Online (Accessed February 2024)
- 4. AIRTUM. Italian cancer figures, report 2016: survival of cancer patients in Italy. Epidemiol Prev. 2017;41(2):1-248. Online (Accessed February 2024)
- 5. Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity – United States, 2001-2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473-1480. CrossRef PubMed
- 6. Rebello RJ, Oing C, Knudsen KE, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7(1):9. CrossRef PubMed
- 7. Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet. 2021;398(10305):1075-1090. CrossRef PubMed
- 8. Jenster G. The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol. 1999;26(4):407-421. PubMed
- 9. Merseburger AS, Hupe MC. An update on triptorelin: current thinking on androgen deprivation therapy for prostate cancer. Adv Ther. 2016;33(7):1072-1093. CrossRef PubMed
- 10. Heyns CF, Simonin MP, Grosgurin P, Schall R, Porchet HC; South African Triptorelin Study Group. Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int. 2003;92(3):226-231. CrossRef PubMed
- 11. Martínez-Piñeiro L, Schalken JA, Cabri P, Maisonobe P, de la Taille A; Triptocare Study Group. Evaluation of urinary prostate cancer antigen-3 (PCA3) and TMPRSS2-ERG score changes when starting androgen-deprivation therapy with triptorelin 6-month formulation in patients with locally advanced and metastatic prostate cancer. BJU Int. 2014;114(4):608-616. CrossRef PubMed
- 12. Gil T, Aoun F, Cabri P, Maisonobe P, van Velthoven R. A prospective, observational grouped analysis to evaluate the effect of triptorelin on lower urinary tract symptoms in patients with advanced prostate cancer. Ther Adv Urol. 2015;7(3):116-124. CrossRef PubMed
- 13. Lebret T, Culine S, Davin JL, et al. Quality of life of 1276 elderly patients with prostate cancer, starting treatment with a gonadotropin-releasing hormone agonist: results of a French observational study. Aging Male. 2014;17(2):87-93. CrossRef PubMed
- 14. Thiery-Vuillemin A, Rigaud J, Crehange G, Pello Leprince Ringuet N, Grandoulier AS, Lebret T. Baseline parameters of patients with prostate cancer (PCa) initiating a triptorelin treatment for at least 12 months in real life, TALISMAN study interim analysis. Am Soc Clin Oncol. 2022;40(6):Abstract. CrossRef
- 15. Corrao G, Cantarutti A. Building reliable evidence from real-world data: needs, methods, cautiousness and recommendations. Pulm Pharmacol Ther. 2018;53:61-67. CrossRef PubMed
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. CrossRef PubMed
- 17. Hupe MC, Hammerer P, Ketz M, Kossack N, Colling C, Merseburger AS. Retrospective analysis of patients with prostate cancer initiating GnRH agonists/antagonists therapy using a German claims database: epidemiological and patient outcomes. Front Oncol. 2018;8:543. CrossRef PubMed
- 18. Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263-282. CrossRef PubMed
- 19. Heidenreich A, Bastian PJ, Bellmunt J, et al; European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467-479. CrossRef PubMed
- 20. Heidenreich A, Bastian PJ, Bellmunt J, et al; European Association of Urology. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent – update 2013. Eur Urol. 2014;65(1):124-137. CrossRef PubMed
- 21. Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer – 2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243-262. CrossRef PubMed
- 22. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. CrossRef
- 23. Swanson LJ, Seely JH, Garnick MB. Gonadotropin-releasing hormone analogs and prostatic cancer. Crit Rev Oncol Hematol. 1988;8(1):1-26. CrossRef PubMed
- 24. Ploussard G, Mongiat-Artus P. Triptorelin in the management of prostate cancer. Future Oncol. 2013;9(1):93-102. CrossRef PubMed
- 25. van den Driessche H, Mattelaer P, van Oyen P, et al. Changes in body image in patients with prostate cancer over 2 years of treatment with a gonadotropin-releasing hormone analogue (triptorelin): results from a Belgian non-interventional study. Drugs Real World Outcomes. 2016;3(2):183-190. CrossRef PubMed
- 26. Morgia G, Russo GI, Tubaro A, et al; Members of the LUNA Foundation, Società Italiana d’Urologia. Patterns of prescription and adherence to European Association of Urology guidelines on androgen deprivation therapy in prostate cancer: an Italian multicentre cross-sectional analysis from the Choosing Treatment for Prostate Cancer (CHOICE) study. BJU Int. 2016;117(6):867-873. CrossRef PubMed
- 27. Fourcade RO, Benedict A, Black LK, Stokes ME, Alcaraz A, Castro R. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy, Spain and the UK. BJU Int. 2010;105(1):49-56. CrossRef PubMed
- 28. Lazzaro C. Gruppo Informale di Studio sugli aspetti Economico-Sanitari del Carcinoma Prostatico in I. [Managing patients with prostate cancer in Italy during the first year after diagnosis. A cost description based on a sample of 8 urological wards]. Arch Ital Urol Androl. 2003;75(3):138-149. PubMed
- 29. Borsoi L, Ciani O, Fornarini G, et al. Direct healthcare costs of non-metastatic castration-resistant prostate cancer in Italy. Int J Technol Assess Health Care. 2023;39(1):e2. CrossRef PubMed
- 30. Restelli U, Ceresoli GL, Croce D, et al. Economic burden of the management of metastatic castrate-resistant prostate cancer in Italy: a cost of illness study. Cancer Manag Res. 2017;9:789-800. CrossRef PubMed
- 31. Svensson J, Lissbrant IF, Gauffin O, et al. Time spent in hormone-sensitive and castration-resistant disease states in men with advanced prostate cancer, and its health economic impact: registry-based study in Sweden. Scand J Urol. 2021;55(1):1-8. CrossRef PubMed
- 32. Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012;307(13):1433-1435. CrossRef PubMed