 |
Glob Reg Health Technol Assess 2024; 11: 154-160 ISSN 2283-5733 | DOI: 10.33393/grhta.2024.2892 ORIGINAL RESEARCH ARTICLE |
 |
Cabozantinib use in second or subsequent line of treatment in renal cell carcinoma: an analysis of Italian administrative databases
ABSTRACT
Background: Cabozantinib use in everyday clinical practice for advanced or metastatic renal cell carcinoma (RCC) is relatively recent, and real-world data on treatment persistence, adherence and sequencing are still limited.
Methods: We conducted an analysis based on an integrated administrative database, covering around 6.9 million health-assisted Italian individuals, to explore the use of cabozantinib for RCC. Patients with at least one prescription for cabozantinib during 2017-2020 were searched. These were characterized during all available period (i.e. from 2010 onwards) before the index date and were observed after inclusion.
Results: A total of 113 patients treated with cabozantinib in second or subsequent line were included, and their demographic, clinical and treatment characteristics were described. About half of these RCC patients were aged >65 years (47.8%). Sixty patients (53.1%) were highly adherent to cabozantinib therapy, and the median cabozantinib treatment duration of use was 8.7 months (95% confidence interval: 5.8-11.1). During the first year of follow-up, the average total cost per patient was €32,508.
Conclusions: We described second or subsequent line cabozantinib treatment for RCC in a real-world setting and the economic burden of disease in Italy, taking advantage of large, integrated administrative databases.
Keywords: Administrative databases, Cabozantinib, Real-world studies, Renal cell carcinoma, Treatment
Received: December 13, 2023
Accepted: June 5, 2024
Published online: July 3, 2024
This article includes supplementary material
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Kidney cancer affected more than 430,000 new patients and caused about 180,000 deaths in the year 2020 worldwide, ranking 14th in cancer incidence and 15th in mortality (1). In Italy, a total of 13,500 new diagnoses of kidney cancer were estimated during the same year (2). Renal cell carcinoma (RCC) is the most frequent histological type of kidney cancer, and its incidence – although relatively low as compared to other cancers – is rising in both sexes in most world areas (3). Survival in patients with metastatic disease – that is, about one-third of cases at diagnosis – is poor (4).
Rapid developments in the treatment options for advanced/metastatic RCC have led, during the last few years, to major improvements in the management of the disease and consequently in clinical outcomes. Immune checkpoint inhibitors and anti-angiogenesis tyrosine kinase inhibitors (TKIs) are now available, and the ESMO guidelines have synthesized through an algorithm and a list of recommendations the first- and second-line systemic treatment of advanced RCC (5). Cabozantinib is an oral small-molecule inhibitor of multiple tyrosine kinase receptors, including MET, vascular endothelial growth factor (VEGF) and AXL. Cabozantinib monotherapy was shown to improve survival and objective response in front-line patients with both intermediate or poor risk RCC and in those with advanced RCC following prior VEGF-targeted therapy (6-8). Subsequently, in the CheckMate 9ER phase III trial, the efficacy and safety of cabozantinib was demonstrated also as a front-line treatment for advanced RCC patients of all risk groups, in combination with nivolumab (9).
In Italy, cabozantinib was first approved as a second-line treatment for advanced RCC in November 2017. Its indication was extended in August 2019 to first-line treatment of adults with intermediate or poor risk disease and in October 2022, in combination with nivolumab, to first-line treatment of advanced RCC patients (favourable, intermediate, poor risk). Therefore, given the recent initiation of use of cabozantinib in clinical practice (particularly as a front-line therapy), real-world data on treatment persistence, adherence and sequencing are still limited.
Healthcare databases are increasingly being recognized and used in clinical research to provide new evidence – complementary to those from clinical trials – on the adoption and impact of different patient management and treatment pathways, in a real-world setting (10). Studies based on administrative data and focused specifically on RCC are still limited and rarely related to Italian experience in metastatic RCC (11-13). Thus, with the aim to provide real-world information describing – without comparative purposes – the use of cabozantinib in second or subsequent line of treatment for RCC in clinical practice, we conducted an analysis by integrating data from administrative database, covering more than 10% of the Italian population.
Methods
Our study is a retrospective analysis of RCC based on> Italian Healthcare Departments’ administrative databases, covering around 6.9 million health-assisted individuals in six regions of northern, central and southern Italy (i.e. Veneto, Toscana, Umbria, Lazio, Campania and Puglia). Patients were included in the cohort if they satisfied both the following criteria: (i) at least one hospitalization discharge diagnosis of RCC (according to the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 189.0), including both main and secondary diagnosis, identified during December 2017-December 2020; and (ii) at least one dispensation for cabozantinib (Anatomical Therapeutic Chemical [ATC] code L01XE26 [L01EX07 from 2021]) during December 2017-December 2019 (inclusion period) and at least 12 months of potential observation. Supplementary Figure 1 shows in detail the time periods of the study. Since cabozantinib is also indicated for hepatocellular carcinoma (HCC), patients with a hospitalization discharge diagnosis for HCC (ICD-9-CM code 155) during the whole period 2010-2020 were excluded from the cohort. Therefore, the cohort included a total of 144 patients. Given the small number of RCC patients treated with cabozantinib as front-line therapy (n = 31) and their short follow-up period available so far (i.e. cabozantinib was approved for first-line treatment in August 2019, and our observation period ends in December 2020), the latter were excluded, and the cohort was focused on second and subsequent line of treatment only (n = 113). The index date was defined as the date of first cabozantinib dispensation (in the second or subsequent line setting) within the inclusion period. The line of cabozantinib treatment was identified according to the number of previous systemic therapy lines, including TKI, immunotherapy, everolimus and bevacizumab (more details are given below). Patients were characterized during all available period before the index date (characterization period, that is, from 2010 onwards, independently of their presence in the database during the whole period) and were observed during all the available period after the inclusion (follow-up, i.e. until December 2020).
Patients’ characteristics were derived from the> administrative databases, including age at index date and gender.
Data sources
Data were extracted from the following databases: (i) demographic database, which consists of all patient demographic data, such as gender, age, death; (ii) pharmaceutical database, which supplies information on medicinal products reimbursed by the National Health System (NHS) as the ATC code, number of packages, number of units per package, unit cost per package and dispensation date; (iii) hospitalization database, which comprise all hospitalization data for patients in analysis, such as the discharge diagnosis codes classified according to ICD-9-CM, Diagnosis-Related Group (DRG) and DRG-related charge (provided by the NHS); (iv) outpatient specialist services database, which incorporates all information about visits and diagnostic tests for patients under analysis (date and type of dispensation, description activity and laboratory test or specialist visit charge); and (v) payment exemption database, which contains data of the exemption codes that allow to avoid the contribution charge for services/treatments when specific diseases are diagnosed.
An anonymous univocal numeric code was assigned to each study individual to guarantee patients’ privacy, in full conformity with the European General Data Protection Regulation (GDPR) (2016/679). The patient code in each database permitted electronic linkage among all databases. The results were produced as aggregated summaries and were never attributable to a single institution, department, doctor, individual or individual prescribing behaviours. The project from which these analyses were drawn was approved by the Ethics Committee involved in the analysis (the full list, with protocol codes and dates of approval, is reported in Supplementary Table 1).
Pharmaco-utilization analysis in the cabozantinib cohort
During the whole period of follow-up, the therapeutic pathway was evaluated considering the following therapies/procedures: systemic therapy (TKI: sunitinib, ATC code: L01XE04, current ATC code: L01EX01; axitinib, ATC code: L01XE17, current ATC code: L01EK01; cabozantinib, ATC code: L01XE26, current ATC code: L01EX07; sorafenib, ATC code: L01XE05, current ATC code: L01EX02; pazopanib, ATC code: L01XE11, current ATC code: L01EX03); immunotherapy (nivolumab, ATC code: L01XC17, current ATC code: L01FF01); other therapies (everolimus, ATC code: L01XE10, current ATC code: L01EG02; bevacizumab, ATC code: L01XC07, current ATC code: L01FG01); and surgery (nephrectomy, partial or total, identified by ICD-9-CM codes: 55.4, 55.5). Unspecific chemotherapies were not considered. Cabozantinib drug utilization was evaluated for treatment interruption (defined as no dispensation during a 3-month period); treatment adherence (calculated as number of cabozantinib tablets dispensed from first to penultimate dispensation, divided by the expected number of tablets according to the number of days between first and last dispensation [medical possession ratio, MPR]; a patient was defined adherent with an MPR ≥80%); line of treatment (identified by number of previous systemic therapy lines) and duration.
Healthcare cost analysis in the cabozantinib cohort
In alive RCC patients, during the first year of follow-up, the healthcare costs were estimated considering the expenses for reimbursable drugs (both RCC-related and overall), referring to the NHS purchase price (applied to outpatient or inpatient medications, as appropriate), for hospitalizations (determined by using the DRG tariffs, for RCC-related hospitalizations and all-cause), and for outpatient specialist services (according to regional tariffs, for tests and visits, prescriptions). Outliers, defined as values that exceed more than three times the standard deviation (SD), were excluded from cost analysis. Data were reported as the mean annual healthcare cost per patient.
Statistical analysis
Continuous variables were reported as mean ± SD or median and 95% confidence interval (CI), and categorical variables were expressed as numbers and percentages. Comparisons in the adherence to cabozantinib treatment between subgroups according to age and line of therapy were performed using the chi-square test. To take into account time-to-event outcome, without any comparative purpose but only with a descriptive intent, cabozantinib treatment persistence was analysed using Kaplan-Meier product-limit survival curve estimates (14), starting from the date of first dispensation until treatment interruption (defined as no dispensation during a 3-month period) plus last dispensation coverage or until death (events), or until end of follow-up period with the patient being still alive and on treatment (censored). Sensitivity analyses were conducted for treatment adherence and treatment persistence. These were performed by adopting different criteria as compared to the main analysis: for treatment adherence, MPR cutoffs in sensitivity analyses were set at (i) ≥70% and (ii) ≥90% (as compared to ≥80% in the main analysis); for treatment interruption, the grace periods in sensitivity analyses were set at (i) 2 months and (ii) 4 months (as compared to 3 months in the main analysis). All analyses were performed using Stata SE version 12.0 (StataCorp, College Station, TX, USA). According to “Opinion 05/2014 on Anonymisation Techniques” drafted by the “European Commission Article 29 Working Party”, analyses involving fewer than three patients were not reported, as they were potentially traceable to single individuals. Therefore, results referred to three or lesser patients were indicated as “not reported” (NR).
Results
Table 1 reports the characteristics at baseline of RCC patients treated with cabozantinib. A total of 84 (74.3%) patients were male and 29 (25.7%) females. Almost half of RCC patients were older than 65 (47.8%), with a mean of 62.7 years (SD: 10.8).
| N (%) | |
|---|---|
| Sex | |
| Females | 29 (25.7) |
| Males | 84 (74.3) |
| Age (mean, SD) | 62.7 (10.8) |
| ≤65 years | 59 (52.2) |
| >65 years | 54 (47.8) |
SD = standard deviation.
Table 2 gives the results of second or subsequent line cabozantinib adherence, based on the MPR, in RCC patients. In the main analysis (i.e. adherence defined with MPR ≥80%), 60 out of 113 patients (53.1%) were highly adherent to cabozantinib therapy. Two sensitivity analyses were performed: the first one fixed a cutoff for MPR ≥70%, reporting adherence in 68.1% of patients; the second one had a cutoff ≥90%, and found a 38.1% treatment adherence. In stratified analyses, the proportion of highly adherent patients was similar in the subgroups of second (52.3%) and third or subsequent line (54.2%) of cabozantinib treatment and was slightly higher in patients aged ≤65 (57.6%) than in those aged >65 years (48.1%).
| N (%) | p-Value | |
|---|---|---|
| Main scenario (MPR ≥80%) | 60/113 (53.1) | |
| Sensitivity analyses | ||
| Scenario 2 (MPR ≥70%) | 77/113 (68.1) | |
| Scenario 3 (MPR ≥90%) | 43/113 (38.1) | |
| Subgroup analyses† | ||
| According to agen> | 0.31 | |
| ≤65 years | 34/59 (57.6) | |
| >65 years | 26/54 (48.1) | |
| According to line of cabozantinib treatmentn> | 0.84 | |
| 2nd line | 34/65 (52.3) | |
| 3rd or higher line | 26/48 (54.2) | |
p-Values for comparison between subgroups were derived using the chi-square test.
MPR = medical possession ratio; RCC = renal cell carcinoma.
†Subgroup analyses were conducted according to the main scenario (i.e. MPR ≥80%).
Figure 1 shows drug persistence of cabozantinib in RCC patients treated in second or subsequent line of therapy. A total of 86 events (76.1%) were observed, with a median treatment duration of 8.7 months (95% CI: 5.8-11.1). Sensitivity analyses based on different grace periods to define treatment interruption (i.e. absence of dispensations during last 2 or 4 months) did not materially change the results, as the median drug persistence remained equal to 8.7 months (data not shown).
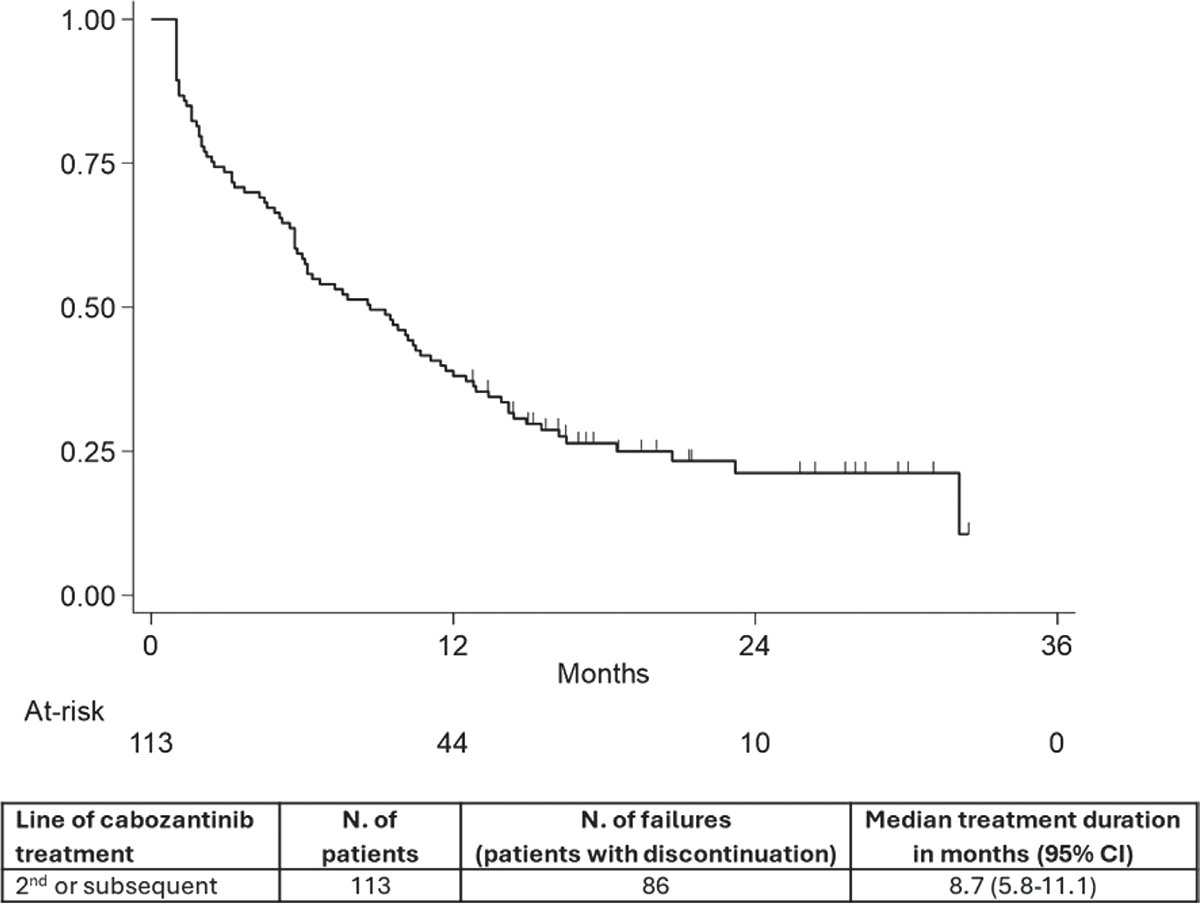
FIGURE 1 - Kaplan-Meier curve of second or subsequent line cabozantinib treatment persistence.
Table 3 describes the sequence of RCC treatments in patients with second or subsequent line cabozantinib dispensation. About 57% of patients (n = 65) were dispensed with cabozantinib in the second line: sunitinib was the most frequently used drug before cabozantinib, with 27 out of 65 (41.5%) second-line patients reporting the sunitinib-cabozantinib sequence, 7 patients (10.8%) the sunitinib-cabozantinib-everolimus sequence and 7 patients (10.8%) the sunitinib-cabozantinib-nivolumab sequence. Pazopanib followed by cabozantinib was used in 16 out of 65 patients (24.6%). Forty-eight patients (42.5%) were dispensed with cabozantinib in third> or higher lines.
Cost analysis was performed on the 113 RCC patients on second and subsequent line of cabozantinib treatment and with at least 1 year of follow-up, excluding outliers (Fig. 2). During the first year of follow-up, the average total cost per patient was €32,508, largely accounted for by the cost of RCC drugs (€27,495, i.e. 85% of total costs). Other major costs were related to laboratory tests (€1,999 per patient, on average), other drugs (€1,629) and hospitalizations (€563 for RCC-related plus €694 for other hospitalizations).
| 1st line | 2nd line | 3rd line | N (%) |
|---|---|---|---|
| Cabozantinib total 2nd line | 65 (57.5) | ||
| Sunitinib | Cabozantinib | 27 (23.9) | |
| Pazopanib | Cabozantinib | 16 (14.2) | |
| Sunitinib | Cabozantinib | Everolimus | 7 (6.2) |
| Sunitinib | Cabozantinib | Nivolumab | 7 (6.2) |
| Other combinations | 8 (7.1) | ||
| Cabozantinib total ≥3rd line | 48 (42.5) | ||
| Sunitinib | Nivolumab | Cabozantinib | 11 (9.7) |
| Sunitinib | Axitinib | Cabozantinib | 6 (5.3) |
| Pazopanib | Nivolumab | Cabozantinib | 5 (4.4) |
| Other combinations | 26 (23.0) | ||
Results referred to ≤3 patients were not reported for data privacy.
RCC = renal cell carcinoma.
Discussion
This study based on administrative data provided a detailed description of the use of cabozantinib in treatment-experienced subjects with RCC in Italy. In this real-world setting, about half of these patients were older than 65 years. Treatment adherence, defined as an MPR ≥80%, was also achieved by about half of patients. The median treatment duration with second or subsequent line cabozantinib was 8.7 months (95% CI: 5.8-11.1), in line with findings from other analyses focused on clinical practice data (15,16). During the study period examined, cabozantinib was often used after sunitinib or – less frequently – pazopanib as a second- or third-line treatment for RCC in Italy.
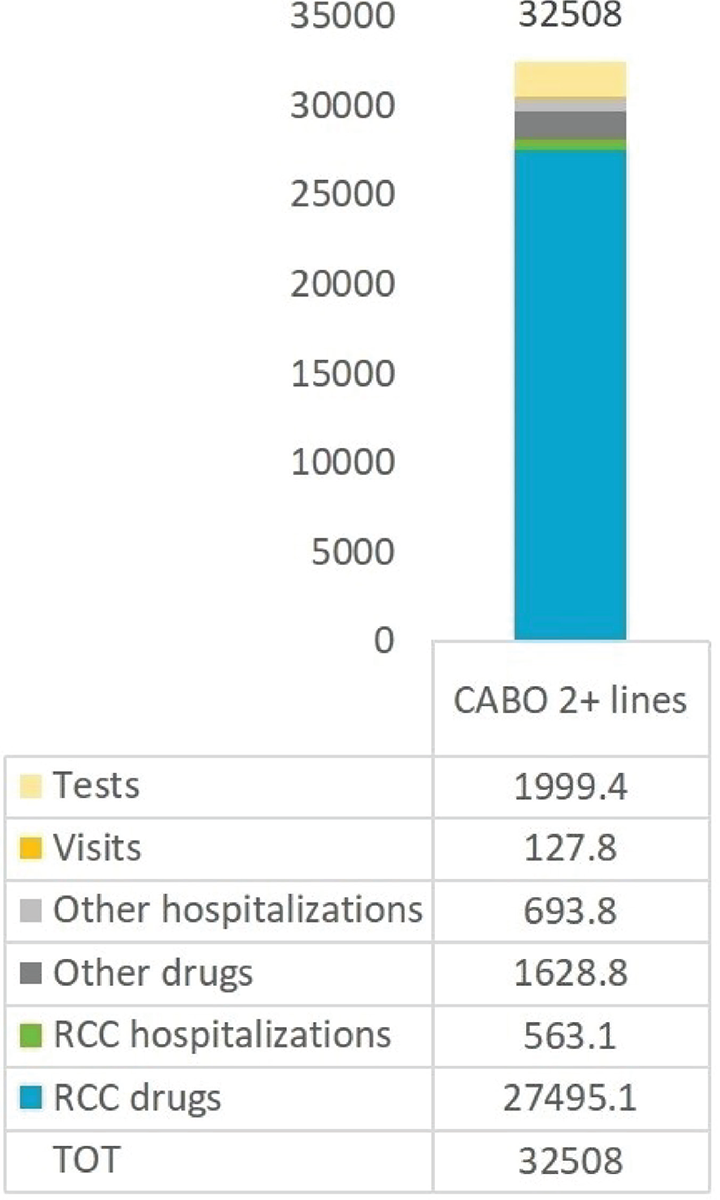
FIGURE 2 - Mean costs (€) of renal cell carcinoma patients receiving cabozantinib therapy during the first year of follow-up (alive patients).
Real-world studies reporting results on cabozantinib treatment persistence in patients with advanced RCC are still limited. In the French CABOREAL Early Access Program, including 410 patients with metastatic RCC starting cabozantinib therapy between 2016 and early 2018, the median duration of treatment was 7.6 months (15). Similar results emerged in the International Metastatic RCC Database Consortium, involving 413 patients from various countries, where the median times to cabozantinib treatment failure were 8.3 months for first-line, 7.3 months for second-line, 7.0 months for third-line and 8.0 months for fourth-line treatment (16). A recent, smaller study conducted in northeastern Italy reported a median duration of treatment of 6.6 months for second-line and 7.5 months for third-line cabozantinib use, thus confirming the activity of cabozantinib regardless of its line of use (17). Our finding of a median treatment persistence of cabozantinib of 8.7 months is, therefore, consistent with previous results from other unselected European and worldwide RCC patient populations. When we conducted sensitivity analyses by using different treatment interruption criteria in our assessment, results were robust and the estimates were further confirmed. Besides relevant clinical considerations, these findings support a reliable use of healthcare administrative databases in this oncological setting.
This analysis provides new data from Italy about the most frequently used treatment sequences involving cabozantinib in advanced RCC. Given the availability of a number of new therapies in the last decade, information on common clinical practice patterns is increasingly needed. Cabozantinib was frequently used as second-line treatment, mostly after front-line sunitinib or pazopanib therapy, or as a third-line treatment, often after nivolumab. This finding is in line with another Italian analysis (17). When, however, cabozantinib was given as third-line treatment or beyond, a large number of different sequencies were reported and no pattern of therapies use could clearly be identified. Numbers were too sparse to examine treatment persistence according to different sequences. Other studies – including an Italian analysis of 84 patients with metastatic RCC – showed however that the effectiveness and time to treatment failure of second-line cabozantinib were generally independent of type of first-line treatment (18-20).
Earlier studies evaluated the role of compliance to other treatments for RCC (12,21), but data on cabozantinib adherence are still scanty. In agreement with previous analyses, we considered highly compliant those patients with an MPR ≥80%, and we conducted both sensitivity and stratified analyses to evaluate the variation in treatment adherence according to the MPR cutoff as well as in specific subgroups of patients. Compliance was relatively low in this population, being achieved by just about half of patients. When, however, a lower threshold was used for MPR (i.e. ≥70%), more than two-thirds of RCC patients resulted adherent to cabozantinib treatment. No relevant differences emerged in cabozantinib adherence across different age groups nor according to the line of treatment. Non-adherence to second-line therapies for RCC is particularly relevant, as it was shown to affect progression-free survival (PFS) significantly in a previous study (21). Aside from the limitations of our analyses based on administrative data, which are discussed below, efforts to increase treatment adherence in this patient setting are therefore warranted.
A comprehensive analysis of administrative Italian data for year 2015, that is, a year before the approval of cabozantinib, reported an average expenditure of €22,067 for the NHS, for each patient with metastatic RCC (13). In our study, the mean cost of RCC patients during the first year of follow-up after cabozantinib treatment was about 50% higher, that is, about €32,500, largely accounted for by the high costs of innovative RCC drugs associated with increased patient survival. This confirms and further quantifies the elevated economic burden of RCC in Italy. According to the National Report “The Use of Medicines in Italy (Year 2022)”, per capita costs of vascular endothelial growth factor receptor (VEGFR)-targeted TKI (i.e. the therapeutic class of cabozantinib) more than doubled in Italy between 2014 and 2022 (22). A number of studies have examined the cost-effectiveness of RCC drugs, including cabozantinib, both at an international level and in the Italian context (17,23,24). These studies are useful instruments to evaluate the impact of new treatments, in consideration of the rapidly evolving scenario and of the increasing number of therapeutic options for advanced RCC. Further studies on this topic are therefore needed.
Limitations of this study are those typical to real-world observational analyses and to administrative data use applied to the clinical research setting (25-27), including potential information and selection bias, and lack of relevant individual patient information that cannot be retrieved in administrative databases. For example, information bias may derive from the availability of databases restricted to a specific time period only. In fact, one or more earlier lines of treatment may have been missed for some patients. Furthermore, treatment adherence calculation may suffer from information bias, as it was based on tablet dispensations rather than on a direct clinical measurement method (28). With reference to the lack of clinical and other covariates, reasons for treatment discontinuation and known lifestyle risk factors for RCC (e.g. smoking habits, body mass index, etc.) were not available. Also, data on patient comorbidities were not derived from the administrative databases. Cabozantinib was approved to treat advanced/metastatic medullary thyroid cancer in Italy in June 2019, but a hospitalization discharge diagnosis for medullary thyroid cancer was not among our patient exclusion criteria. This notwithstanding, in an a posteriori data check, no prescriptions specific to thyroid cancer were detected and the RCC patient population was thus confirmed. Despite these limits, real-world investigations are important to integrate data from clinical studies, by providing information from everyday clinical practice. Drug persistence is a useful measure, which can be computed in the absence of specific clinical information, and that integrates other clinical measures. Among the strengths of this study, the analyses were based on large data coverage from several regions throughout Italy. The use of healthcare administrative data can contribute to better understand the real-world utilization of drugs in the oncological setting, and their results have potentially relevant clinical and regulatory implications. Previous studies of RCC based on healthcare administrative databases, considering both clinical and economic aspects, are however lacking in Italy (13).
Conclusions
This study provided relevant information to improve the knowledge of RCC treatment in the real-world setting, by describing the use of second or higher line cabozantinib in the clinical practice in Italy, as well as the economic burden of disease, taking advantage of large, integrated administrative databases.
Acknowledgements
The authors thank Statinfo (Italy) for providing medical writing and editorial support, which was industry sponsored in accordance with Good Publication Practice guidelines (GPP 2022).
Disclosures
Conflict of interest: CL: received honoraria for consulting (advisory board) or as a speaker, from IPSEN, Bristol Myers Squibb and MSD. VA, AB, EP: employees of IPSEN. SS: received honoraria from IPSEN, MSD, Bristol Myers Squibb and Janssen. The other authors reported no conflict of interests.
Financial support: The study report was developed by CliCon S.r.l. Società Benefit and supported by IPSEN. The agreement signed by CliCon S.r.l. and IPSEN does not create any joint venture or any similar relationship between parties. CliCon S.r.l. is an independent company. Neither CliCon S.r.l. nor any of their representatives are employees of IPSEN for any purpose.
Author contributions: Conceptualization, Data curation and Formal Analysis: LDE; Funding acquisition, Project administration: VA, AB and EP; Supervision: SS; Validation: CL, AV, SS; Writing – review & editing: CL, AV, LDE, VA, AB, EP, SS.
Data availability statement: All data used for the current study are available upon reasonable request next to CliCon s.r.l. which is the body entitled of data treatment and analysis by local health units.
References
- 1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France 2020: Online Accessed December 2023.
- 2. AIOM-AIRTUM. I numeri del cancro in Italia. Il Pensiero Scientifico Editore; 2021.
- 3. Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519-530. CrossRef PubMed
- 4. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79-87. CrossRef PubMed
- 5. Powles T, Albiges L, Bex A, et al; ESMO Guidelines Committee. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol. 2021;32(12):1511-1519. CrossRef PubMed
- 6. Choueiri TK, Escudier B, Powles T, et al; METEOR Investigators. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814-1823. CrossRef PubMed
- 7. Choueiri TK, Escudier B, Powles T, et al; METEOR investigators. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917-927. CrossRef PubMed
- 8. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591-597. CrossRef PubMed
- 9. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. CrossRef PubMed
- 10. Corrao G, Cantarutti A. Building reliable evidence from real-world data: needs, methods, cautiousness and recommendations. Pulm Pharmacol Ther. 2018;53:61-67. CrossRef PubMed
- 11. Escudier B, de Zélicourt M, Bourouina R, Nevoret C, Thiery-Vuillemin A. Management and health resource use of patients with metastatic renal cell carcinoma treated with systemic therapy over 2014-2017 in France: a national real-world study. Clin Genitourin Cancer. 2022;20(6):533-542. CrossRef PubMed
- 12. Hackshaw MD, Nagar SP, Parks DC, Miller LA. Persistence and compliance with pazopanib in patients with advanced renal cell carcinoma within a U.S. administrative claims database. J Manag Care Spec Pharm. 2014;20(6):603-610. PubMed
- 13. Ronconi G, Dondi L, Piccinni C, et al. Metastatic renal cancer: real-world evidence from a large Italian claims database. Glob Reg Health Technol Assess. 2021;8(1):1-7. CrossRef PubMed
- 14. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. CrossRef
- 15. Albiges L, Fléchon A, Chevreau C, et al. Real-world evidence of cabozantinib in patients with metastatic renal cell carcinoma: Results from the CABOREAL Early Access Program. Eur J Cancer. 2021;142:102-111. CrossRef PubMed
- 16. Gan CL, Dudani S, Wells JC, et al. Cabozantinib real-world effectiveness in the first-through fourth-line settings for the treatment of metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer Med. 2021;10(4):1212-1221. CrossRef PubMed
- 17. Maruzzo M, Pierantoni F, Bortolami A, et al. Real-world treatment with nivolumab or cabozantinib for metastatic renal cell carcinoma (mRCC) in the Veneto region of Italy: results of AMOUR study. Target Oncol. 2022;17(4):467-474. CrossRef PubMed
- 18. Iacovelli R, Ciccarese C, Facchini G, et al. Cabozantinib after a previous immune checkpoint inhibitor in metastatic renal cell carcinoma: a retrospective multi-institutional analysis. Target Oncol. 2020;15(4):495-501. CrossRef PubMed
- 19. Navani V, Wells JC, Boyne DJ, et al. CABOSEQ: the effectiveness of cabozantinib in patients with treatment refractory advanced renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Clin Genitourin Cancer. 2023 Feb;21(1):106.e1-106.e8. CrossRef PubMed
- 20. Santoni M, Heng DY, Bracarda S, et al. Real-world data on cabozantinib in previously treated patients with metastatic renal cell carcinoma: focus on sequences and prognostic factors. Cancers (Basel). 2019;12(1):84. CrossRef PubMed
- 21. Shafrin J, Sullivan J, Chou JW, Neely MN, Doan JF, Maclean JR. The effect of medication nonadherence on progression-free survival among patients with renal cell carcinoma. Cancer Manag Res. 2017;9:731-739. CrossRef PubMed
- 22. Italian Medicine Agency (AIFA). National report on medicines use in Italy – Year 2022 Rome, Italy. 2023. Online Accessed June 2024.
- 23. 23. Edwards SJ, Wakefield V, Cain P, et al. Axitinib, cabozantinib, everolimus, nivolumab, sunitinib and best supportive care in previously treated renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess. 2018;22(6):1-278. CrossRef PubMed
- 24. Stanisic S, Cicchetti A, Porta C, Procopio G, Berto P. Costo-Efficacia di cabozantinib nel trattamento di seconda linea del tumore a cellule renali metastatico (mRCC) in Italia. Glob Reg Health Technol Assess. 2018;5:1-9. CrossRef
- 25. Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012;307(13):1433-1435. CrossRef PubMed
- 26. Gini R, Schuemie MJ, Pasqua A, et al. Monitoring compliance with standards of care for chronic diseases using healthcare administrative databases in Italy: strengths and limitations. PLoS One. 2017;12(12):e0188377. CrossRef PubMed
- 27. Trifirò G, Gini R, Barone-Adesi F, et al. The role of European healthcare databases for post-marketing drug effectiveness, safety and value evaluation: where does Italy stand? Drug Saf. 2019;42(3):347-363. CrossRef PubMed
- 28. Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155-159. CrossRef PubMed