 |
Glob Reg Health Technol Assess 2024; 11: 22-30 ISSN 2283-5733 | DOI: 10.33393/grhta.2024.2696 ORIGINAL RESEARCH ARTICLE |
 |
Italian healthcare resource consumption for patients on hemodialysis treated for chronic kidney disease-associated pruritus (CKD-aP)
ABSTRACT
Background: Chronic kidney disease-associated pruritus (CKD-aP) affects patients on hemodialysis. This study identified hemodialysis patients presumably affected or not affected by CKD-aP and integrated healthcare costs, from the perspective of the Italian administrative healthcare data.
Methods: Through cross-linkage of Italian administrative healthcare data collected between 2015 and 2017 (accrual period) in the database of Fondazione ReS (Ricerca e Salute), patients undergoing in-hospital/outpatient hemodialysis were selected. Cohorts with and without CKD-aP were created based on the presence/absence of CKD-aP-related treatment (according to common clinical practice and guidelines) supplies and assessed in terms of CKD-aP-related treatments and mean healthcare costs per capita paid by the Italian National Health Service (INHS).
Results: Of 1,239 people on hemodialysis for ≥2 years, CKD-aP affected 218 patients. Patients with CKD-aP were older and with more comorbidities. During the follow-up year, on average, the INHS spent €37,065 per case, €31,286 per control and € 35,988 per non-CKD-aP subject. High-efficiency dialytic therapies performed to people on hemodialysis with CKD-aP largely weighed on the overall mean annual cost.
Conclusions: This real-world study identified patients on chronic hemodialysis potentially treated for CKD-aP. Interestingly, high-efficiency dialysis seems the most frequent and expensive choice for the treatment of CKD-aP. The discovery of appropriate and effective treatments for this condition might offer cost offsets.
Keywords: Costs, Dialysis, End-stage kidney disease, Italy, Retrospective analysis, RWE, Treatment, Uremic pruritus
Received: October 24, 2023
Accepted: November 29, 2023
Published online: January 15, 2024
This article includes Supplementary Material
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Chronic kidney disease-associated pruritus (CKD-aP) is a generalized persistent and refractory itching, more frequently affecting patients on hemodialysis; it is also known as uremic pruritus, despite etiology being independent from uremia (1,2). Considering the Dialysis Outcome and Practice Patterns Study (DOPPS) phase 4 to 6 and published epidemiological data, the proportion of subjects affected by moderate pruritus was highest in the United Kingdom (47%), lowest in Germany (27%) and in average around 40% when considering moderate to severe CKD-aP (2). Pruritus augments with increasing CKD severity (3) and the time spent on hemodialysis (4). CKD-aP generally occurs with dry skin and frequent complications (5), which evolve to a high negative symptom burden consisting in discomfort, fatigue, poor sleep quality and depression (3). Overall, the mental and physical health status of patients with CKD-aP worsen with increasing pruritus severity (3).
The pathophysiology of CKD-aP is probably multifactorial but still unknown, therefore no causative or etiology-specific treatments are established (6,7). European guidelines recommend the use of topical therapy (capsaicin) and systemic treatments (7) that are first- and second-generation antihistamines, corticosteroids, the κ-opioid receptor agonist nalfurafine, gabapentin, pregabalin and, sporadically, thalidomide. Among non-pharmacological options, ultraviolet (UV) phototherapy is effective for pruritus, especially the narrowband UVB (7). Whereas according to the British Association of Dermatologists, the only definitive treatment for CKD-aP is renal transplantation (6). Besides the unmet medical needs, there is scarce literature about pathophysiology, long-term outcomes (8) and healthcare resource consumption of CKD-aP that contributes to the lack of knowledge, and the consequent underestimation of its burden, among clinicians and CKD patients (1,2,9).
This Fondazione ReS (Ricerca e Salute)’s retrospective observational study of Italian administrative healthcare data aimed to raise the interest in the healthcare burden of CKD-aP hemodialysis patients within the Italian nephrologists’ current therapeutic approaches, given the absence of an effective treatment. Therefore, the purposes of this study were to describe clinical and socio-demographic features of CKD-aP hemodialysis patients, and to evaluate the proper use of the recommended therapies and direct healthcare burden on the Italian National Health Service (INHS).
Materials and methods
Data source
This study originates from the cross-linkage of the administrative healthcare data routinely collected in the ReS database, under specific agreements with several Italian local and regional health authorities (HAs), and periodically conveyed to the Italian Ministry of Health for reimbursement purposes. The INHS is a universal coverage single-payer healthcare system, thus the healthcare data collected by HAs represent the healthcare of all the INHS beneficiaries. Given the reliable representativeness of the Italian population (large catchment community and superimposable age distributions with those reported for the entire country by the Italian Institute of Statistics—ISTAT 1 (10)), Fondazione ReS has been conducting several observational studies on a range of clinical questions (11-13) since 2018. Its aims are to integrate findings from clinical trials and registries, which are still the most common bases of guidelines and recommendations, and to contribute to the evidence-based and patient-centered medicine. The following administrative databases establish the ReS database. The demographic database considers age, sex, HA of residency and disease waiver claim for co-payment. The pharmaceutical dataset consists of all drugs reimbursed by the INHS and supplied by local and hospital pharmacies: active substances can be analyzed by marketing code, Anatomical Therapeutic Chemical code (World Health Organization’s ATC classification) (14), dose, package number and dispensing date. The hospitalization database is analyzable through in-hospital diagnoses and procedures recorded in the hospital discharge forms related to overnight and daily hospitalizations (Italian version of the 9th International Classification of Disease—Clinical Modification [ICD-9-CM]) (15). The outpatient specialist care dataset is composed of examinations, diagnostics and invasive/non-invasive procedures performed in local facilities affiliated with the INHS and are analyzed based on the related current national classification system (2017 version of the “Nomenclatore tariffario”). Italian administrative healthcare databases also include all costs directly paid by the INHS, because of reimbursement purposes. The ReS database is physically placed into Cineca’s servers (16), whose collaboration guarantees compliance with international standard certifications of data quality and security. This retrospective observational study has analyzed Italian administrative healthcare data that are anonymized at the source and in aggregated form, according to the specific agreements with the HAs, owners of the data and European privacy laws. For these reasons and the institutional purposes of this study, neither informed consent nor ethical approval were applicable.
Cohort selection
From the ReS database, during the 3-year accrual period (2015-2017), patients with at least one in-hospital or outpatient hemodialysis procedure (ICD-9-CM code 39.95) were identified. About 5 million inhabitants captured annually by the ReS database correspond to about 15% of the Italian residents (10). The most recent date of hemodialysis represented the index date (supplementary figure 1). After having selected only patients with at least 2 years of ongoing hemodialysis before the index date, those potentially affected since 2013 by other diseases that generally cause pruritus (chronic liver disease/cirrhosis, systemic lupus erythematosus, arterial vasculitis and disease/inflammatory conditions of the skin and subcutaneous tissue) were excluded (supplementary table 1). Given the lack of a specific administrative code for CKD-aP and of most clinical information (e.g., dialysis registries, general practitioner [GP]’s database, laboratory values), the reimbursed supplies of gabapentin, pregabalin, thalidomide and antihistamines, recommended by the current guidelines (6,7), and the performance of UV phototherapy were used as proxies of CKD-aP. The final sample was categorized according to the presence/absence of the aforementioned CKD-aP-related treatments 180 days before and/or after the index date: gabapentin, pregabalin, thalidomide and antihistamines and the UV phototherapy (supplementary table 2). The time span of 180 days before and/or after the index date was chosen for the highest possible accuracy when associating CKD-aP and specific treatment supplies. CKD-aP-related therapies and the proxy that associates them with people on hemodialysis were suggested by the common clinical practice, the international guidelines (6,7) and the nephrologists who supported the researchers of Fondazione ReS for this study.
Epidemiological and clinical characterization
Age and sex at baseline were given. Moreover, within 2 previous years (until 2013), potential CKD-aP-related comorbidities (anemia, xerosis cutis, hyperparathyroidism, hypercalcemia, hyperuricemia and hyperphosphatemia) and other comorbidities of interest (hypothyroidism, diabetes mellitus, depression, arterial hypertension, viral hepatopathies and coronary artery disease [CAD]) were assessed (supplementary table 3).
CKD-aP-related treatments
The CKD-aP-related treatments reimbursed by the INHS were searched among pharmaceuticals (gabapentin, pregabalin, thalidomide and antihistamines), hospital discharges and outpatient specialist care (UV phototherapy) databases. Data 1 year before and 1 year after the index date were available for each patient (supplementary figure 1). The use of CKD-aP-related therapies was described within 1 year before and after the index date (to be consistent with the further follow-up analyses). DDD (defined daily dose—the assumed average maintenance daily dose of a drug used in its main indication (14)), drug packages’ and phototherapy procedures’ number, and patients supplied with a CKD-aP-related treatment at least once during one previous or subsequent year assessed the specific treatment consumption.
Healthcare integrated costs
One-year healthcare costs were assessed for CKD-aP and non-CKD-aP patients. Moreover, an individual matched pair case-control analysis was performed to assess the average annual cost by healthcare administrative database and overall, through an even more realistic perspective. Matched variables were sex, age and local HA of residency. Cases were people on hemodialysis potentially affected by CKD-aP, while controls were those without CKD-aP, according to the categorization by the presence or absence of CKD-aP-related treatments. Mean costs for cases and controls were compared through a z-test and a p-value <0.05 was considered statistically significant. Only INHS direct costs due to reimbursed pharmaceuticals, hospitalizations and outpatient specialist services are recorded in the Italian administrative databases. Specifically, pharmaceutical costs, by pharmaceutical group/active substance of interest, were calculated through sum and provided as mean per capita, starting from prices of community and hospital pharmacies (inclusive of value-added tax). The in-hospital expenditure was derived by the DRG (diagnosis-related group) system fee, which is used to calculate the reimbursed in-hospital stay costs per patient. Each DRG code corresponds to all in-hospital cares (from admission to discharge) in their entirety and complexity, without distinguishing single performed services. Local outpatient diagnostics and invasive/non-invasive procedures were assessed through the current national fees, listed in the 2017 version of the “Nomenclatore tariffario.” Pharmaceutical expenses were split into CKD-aP-related treatments and “other drugs” (i.e. all drugs different from the CKD-aP-related ones).
Statistical analyses
To compare patients’ demographics and comorbidities, a chi-square test between frequencies was performed, except for differences between patient distributions by age group, for which a chi-square test between distributions was performed. A p-value <0.05 was considered statistically significant. An individual matched pair case-control analysis was performed to assess the average annual cost by healthcare administrative database and overall, through an even more realistic perspective. Matched variables were sex, age and local HA of residency. The categorization of cases and controls was based by the presence and absence of CKD-aP-related treatments, respectively. Mean total costs per case and per control were compared through a Mann-Whitney non-parametric U-test, and a p-value <0.05 was considered statistically significant. All analyses were performed by means of Oracle SQL Developer Italian version 18.1.0.095 (California, United States).
Results
Epidemiological and clinical characterization
From the 2015-2017 ReS population (on average about 5 million inhabitants per year), 6,147 patients (about 1.2 × 1,000/year) were treated at least once with in-hospital or local outpatient hemodialysis (Fig. 1), of which, 1,589 patients (1,589/6,147; 25.9%; 0.3 × 1,000/year) were treated for at least 2 years.
After having applied the exclusion criteria, the final sample (n = 1,239) was split into people with CKD-aP (n = 218) and without CKD-aP (n = 1,021). Both cohorts were mostly males and elderly (patients with CKD-aP were slightly older). Age distributions showed prevalence increasing with age, with a peak at 70-79 years (Tab. I).
The analysis of potentially CKD-aP-related comorbidities showed that hyperphosphatemia, hyperparathyroidism, anemia and hyperuricemia affected both cohorts in the same descending order of frequency (Tab. I). People on hemodialysis with CKD-aP were slightly more affected by all of them, except hyperuricemia. Among the other comorbidities, hypertension, diabetes mellitus, hypothyroidism, depression, CAD and viral hepatopathies were in the same descending order of frequency in both cohorts (Tab. I).
CKD-aP-related treatments
Table II shows that 58.3% (127/218) of people on hemodialysis with CKD-aP in the previous year and 65.1% (142/218) in the subsequent year received at least one CKD-aP-related drug. In both observation periods, about 10% of the cohort (23/218; 20/218) was treated with gabapentin and around 50% (108/218; 122/218) with antihistamines (53/218; 24.3% cetirizine), whose mean consumption (4.6 packs/patient) was in line with an annual chronic use in Italy. For all CKD-aP-related drugs, the annual mean consumption (package number and DDD) was very similar at one preceding and follow-up year. At least one phototherapy treatment/cycle was performed on 1.4% (3/218) of people on hemodialysis with CKD-aP in both periods and, on average, two times and four times per patient within one previous and subsequent year, respectively.
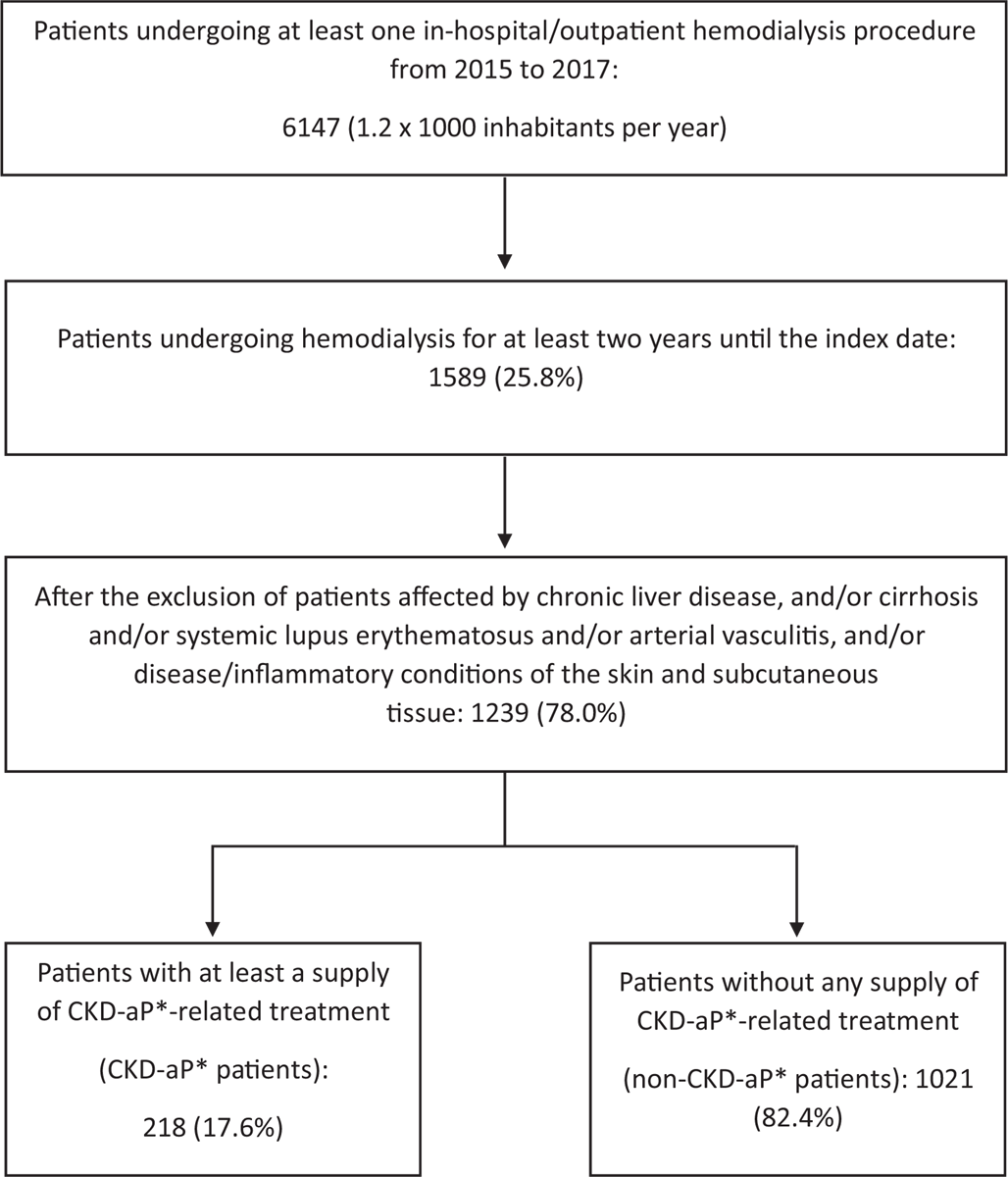
Fig. 1 - Selection of subjects undergoing hemodialysis and potentially affected or not by CKD-aP.
*CKD-aP: chronic kidney disease-associated pruritus
Healthcare integrated costs
During the follow-up year, on average, the INHS spent €37,065 per case, €31,286 per control and €35,988 per non-CKD-aP subject (Tab. III).
For all cohorts, the mean costs related to each administrative healthcare database similarly weighed on the overall expenditure: about 80% (€29,809/€37,065; €25,437/€31,286; €28,917/€35,988; respectively) was due to outpatient specialist services, about 10% to pharmaceuticals (€3,667/€37,065; €2,721/€31,286; €3,430/€35,988) and to hospitalizations (€3,590/€37,065; €3,129/€31,286; €3,640/€35,988). Most of the pharmaceutical expenditure was due to concomitant drugs. The hemodialysis performed out-of-hospital accounted for around 80% of the overall local outpatient care spending (€23,239/€29,809; €19,926/€25,437; €22,697/€28,917), while the in-hospital hemodialysis contributed more than 60% to the total expense for hospitalizations (€2,238/€3,590; €1,980/€3,129; €2,413/€3,640). High-efficiency dialytic therapies performed to people on hemodialysis with CKD-aP largely weighed on the overall mean annual cost. For each cost item and total healthcare expenditure, numerical but non-significant differences (p>0.05) between cases and controls were found.
| CKD-aP patients
(n = 218) |
Non-CKD-aP patients
(n = 1,021) |
p-Value | |
|---|---|---|---|
| Males (n; %) | 121; 55.5 | 583; 57.1 | 0.72 |
| Median age (Q1; Q3) | 71 (61; 80) | 68 (53; 79) | 0.07 |
| Mean age (±standard deviation) | 69 (±13) | 66 (±15) | |
| Distribution by age group (n; %) | 0.03* | ||
| <18 | 0; 0.0 | 2; 0.2 | |
| 18-29 | 2; 0.9 | 8; 0.8 | |
| 30-39 | 3; 1.4 | 39; 3.8 | |
| 40-49 | 10; 4.6 | 118; 11.6 | |
| 50-59 | 30; 13.8 | 156; 15.3 | |
| 60-69 | 59; 27.1 | 235; 23.0 | |
| 70-79 | 61; 28.0 | 263; 25.8 | |
| ≥80 | 53; 24.3 | 200; 19.6 | |
| Total | 218; 100.0 | 1,021; 100.0 | |
| Potential CKD-aP-related comorbidities (n; %) | |||
| Hyperphosphatemia | 150; 68.8 | 623; 61.0 | 0.03* |
| Hyperparathyroidism | 102; 46.8 | 437; 42.8 | 0.3 |
| Anemia | 81; 37.2 | 364; 35.7 | 0.7 |
| Hyperuricemia | 36; 16.5 | 178; 17.4 | 0.7 |
| Other comorbidities of interest (n; %) | |||
| Arterial hypertension | 162; 74.3 | 732; 71.7 | 0.4 |
| Diabetes mellitus | 57; 26.1 | 195; 19.1 | 0.01* |
| Hypothyroidism | 41; 18.8 | 138; 13.5 | 0.04* |
| Depression | 31; 14.2 | 85; 8.3 | 0.007* |
| Coronary artery disease | 21; 9.6 | 86; 8.4 | 0.6 |
| Viral hepatopathies | 2; 0.9 | 5; 0.5 | 0.4 |
CKD-aP = chronic kidney disease-associated pruritus.
*Differences statistically significant (p-value <0.05) through a chi-square test between distributions for distributions by age, and through a chi-square test between frequencies for demographics and comorbidities.
| CKD-aP-related treatments | –365 days | +365 days | ||||
|---|---|---|---|---|---|---|
| Patients; % on CKD-aP cohort (n = 218) | Mean no of packages per patient treated | Mean DDD per patient treated | Patients; % on CKD-aP cohort (n = 218) | Mean no of packages per patient treated | Mean DDD per patient treated | |
| Gabapentin | 23; 10.6 | 4.7 | 21.0 | 20; 9.2 | 5.4 | 25.5 |
| Antihistamines | 108; 49.5 | 4.6 | 93.6 | 122; 56.0 | 4.2 | 88.2 |
| Ultraviolet light therapy | 3; 1.4 | 2.0 | NA | 3; 1.4 | 4.0 | NA |
| At least one CKD-aP-related treatment | 127; 58.3 | 142; 65.1 | ||||
Differences between drug dispensations within 365 days before and after the index date were not significant (p-value >0.05) through a chi-square test.
CKD-aP = chronic kidney disease-associated pruritus; DDD = defined daily dose; NA = not available.
| Administrative healthcare database Specific cost item | CKD-aP patients n = 218)—cases | Non-CKD-aP patients (n = 218)—controls | Non-CKD-aP patients (n = 1,021) |
|---|---|---|---|
| Mean cost (€) per capita; % on total/single healthcare database* | Mean cost (€) per capita; % on total/single healthcare database* | Mean cost (€) per capita; % on total/single healthcare database* | |
| Pharmaceuticals | 3,667; 9.9 | 2,721; 8.7 | 3,430; 9.5 |
| CKD-aP-related drugs | 20; 0.5 | 1; 0.1 | <1; 0.0 |
| Other drugs | 3,647; 99.5 | 2,720; 99.9 | 3,430; 100.0 |
| Hospitalizations | 3,590; 9.7 | 3,129; 10.0 | 3,640; 10.1 |
| Hemodialysis | 2,238; 62.3 | 1,980; 63.3 | 2,413; 66.3 |
| Local outpatient specialist services | 29,809; 80.4 | 25,437; 81.3 | 28,917; 80.4 |
| Ultraviolet light therapy | <1; 0.0 | <1; 0.0 | 0; 0.0 |
| Hemodialysis | 23,239; 77.9 | 19,926; 78.3 | 22,697; 78.4 |
| Bicarbonate hemodialysis with biocompatible membrane | 13,763; 46.2 | 12,187; 47.9 | 13,565; 46.9 |
| Other hemodiafiltration | 5,465; 18.3 | 4,890; 19.2 | 5,296; 18.3 |
| Hemodiafiltration | 1,680; 5.6 | 1,364; 5.4 | 2,083; 7.2 |
| Hemodiafiltration, limited assistance | 1,286; 5.6 | 745; 2.9 | 619; 2.1 |
| Acetate and bicarbonate hemodialysis | 783; 2.6 | 548; 2.1 | 775; 2.7 |
| Hemofiltration | 91; 0.3 | 1; 0.0 | 126; 0.4 |
| Hemodialysis – hemofiltration | 89; 0.3 | 88; 0.3 | 160; 0.5 |
| Acetate and bicarbonate hemodialysis, limited assistance | 81; 0.3 | 104; 0.4 | 72; 0.2 |
| Home acetate and bicarbonate hemodialysis | 0; 0.0 | 0; 0.0 | <1; 0.0 |
| Total cost | 37,065; 100.0 | 31,286; 100.0 | 35,988; 100.0 |
Differences between mean total costs per case and control were considered significant (p = 0.007) through a Mann-Whitney non-parametric U-test.
CKD-aP = chronic kidney disease-associated pruritus.
*The italic font identifies only the % of the sub-total of a specific item related to each specific administrative healthcare database (i.e., pharmaceuticals, local outpatient specialist care and hospitalizations).
Discussion
The Italian ReS database of about 5 million inhabitants per year, from 2015 to 2017, included about 1.2 × 1,000/year people on acute or chronic hemodialysis. Of those, for the purposes of our study, only patients on chronic hemodialysis (i.e., those treated for ≥2 years) were further selected. This reduced the annual prevalence to 0.3 × 1000 inhabitants. People on chronic hemodialysis with CKD-aP were mostly males and elderly (mean age 69 ± 13 years).
Within one previous and follow-up year, antihistamines were dispensed to about 50% of patients on hemodialysis with CKD-aP. This study found around 10% (23/218) of the cohort treated with gabapentin, but no patient received pregabalin or thalidomide reimbursed by the INHS. Overall, each patient on hemodialysis with and without CKD-aP has a similar economic burden for the INHS. Particularly, concomitant drugs corresponded with the entire pharmaceutical expenditure for each subject without CKD-aP, and with 99.5% (€3,590/€3,667) for each one with CKD-aP. Interestingly, the cost analysis showed that high-efficiency dialyses were the most frequent and expensive choice for the treatment of CKD-aP, with hemodiafiltration accounting for the highest cost.
The prevalence is underestimated compared to that reported by the Italian Registry of Dialysis and Transplantation (17) (i.e., 0.7 × 1000 inhabitants) for the same period, probably because, to select patients on chronic hemodialysis (≥2 years), we excluded people with a negative outcome, who were instead included by the Registry. Moreover, evidence exists on the critical tendency of underreporting pruritus by patients and overlooking it by healthcare professionals (1,2,9). The DOPPS phase 5 (2012-2015) (2) found from 5% to 20% of hemodialysis patients at least moderately bothered by pruritus. Nevertheless, the 17.6% (218/1,239) of patients on chronic hemodialysis for ≥2 years and potentially affected by CKD-aP are hard to compare with a scarce and inconsistent literature originating from different data sources (2,18). Despite other limitations in the identification criteria (see “Strengths and limitations” section), demographics were in line with literature (1,2,9), as well as the prevalence of some concomitant metabolic disorders (e.g., related to phosphorus, ferritin and parathormone) (1-4). This evidence suggests that the identified cohort can be representative of the real-world CKD-aP patients, for the purposes of this analysis.
Although this analysis could assess only the recommended pharmacotherapies reimbursed by the INHS and evaluable through administrative databases, rates of patients with at least one CKD-aP-recommended therapy (127/218, 58.3% before; 142/218, 65.1% after index date), including rare UV phototherapy performances, were close to the findings of the DOPPS phase 5, as shown in a recent review (2). In people on hemodialysis with pruritus: oral and topical antihistamines were prescribed as first choice, while gabapentin or pregabalin were prescribed to <10% of patients. In the ReS database, since patients with dermatitis or autoimmune disease potentially treated with antihistamines were excluded, antihistamine supplies appear to be the greater marker of CKD-aP in Italy. Whereas although gabapentin is, nowadays, the only drug worldwide marketed with the highest evidence against CKD-aP (19), our findings confirm the Italian clinical practice (2). It is worth explaining that patients treated at least once during the preceding and following years were not 100% of the cohort, because the therapeutic approach was analyzed in the previous period separately from the follow-up. Whereas for the selection, therapies could also be supplied/performed within both 180 days before and after the index date. The substantial use of high-efficiency dialysis is consistent with the common stepwise management found in the DOPPS phase 5, as shown in a recent review (2) namely, in case of serious pruritus, the dialysis dose is increased before prescribing medications. Indeed, high-flux hemodialysis, hemodiafiltration with hemoperfusion and high-permeability hemodialysis have shown significant relief of CKD-aP compared to the conventional hemodialysis (19). At the same time, the heterogeneous therapeutic approach appears to be a continuous research of an effective dialysis treatment, despite the absence of any real improvement evidence (18).
Strengths and limitations
Administrative data represent a large and unselected community reliably reflecting the real population, with high accuracy in identifying patients with CKD (20). Nevertheless, limitations on the exclusive use of administrative data are several. First of all, given that a specific therapy for CKD-aP does not exist and the CKD-aP diagnosis is not available, a proxy had to be used for the identification of the cohort (i.e., supply of recommended therapies during 180 days before and/or after the index date + exclusion of some conditions frequently causing pruritus and treated by the same therapies (6,7)). Also, the inability to properly identify all the recommended therapies (e.g., therapies for mineral and bone disorders and topical treatments are not evaluable through administrative data (7)) and the INHS reimbursement conditions of antihistamines and gabapentin limit the findings. Indeed, the prolonged dispensation of antihistamines is reimbursed whether a chronic severe disease (e.g., the excluded ones) exists or in case of long-term treatment of seasonal allergic rhino-conjunctivitis (21), which is a condition hard to identify, but likewise uncommon; whereas gabapentin reimbursement is limited to specific neuropathies not including CKD-aP (22) and to epilepsy, leading to a misclassification.
The absence of out-of-pocket purchase, clinical information (e.g., dialysis vintage or dose, renal function) and other relevant patient characteristics could have contributed to slightly underestimate the cohort selection, also making it impossible to carry out some specific analyses. The ReS database still does not link to other databases, such as dialysis registries, GP’s operating system or those collecting laboratory outcomes, and consequently, some patient on hemodialysis with CKD-aP that is assisted by GPs, together with those whose healthcare is not reimbursed by the INHS, remain undetected by this study. Moreover, given the absence of many clinical variables and diagnoses mandatory for the prescription and for reimbursement purposes, only in-hospital diagnoses can be used for selection and analyses. This probably led to the inclusion of patients with an excluded disease on the one hand, and to the underestimation of people with comorbidities on the other hand. Moreover, the absence of a full panel of clinical variables has as limitation that a complete propensity score matching could not been performed, therefore we choose an exact/direct matching method 1:1 based only on the available and most reliable variables, such as sex, age and local HA of residency.
Finally, costs are slightly underestimated, mostly because they do not register the out-of-pocket purchase of healthcare services (e.g., over-the-counter drugs for CKD-aP seem very frequent (3)) and all indirect costs (e.g., those due to productivity loss or caregiver support).
The CKD-aP symptom burden is high and needs to be reduced early. The most updated guidelines (6,7) do not recommend a clear management, but recent studies and reviews (2,18,23,24) reported the tendency of a stepwise approach. Given the lack of an established and effective cure, the identification of comorbidities is essential to recognize the potential underlying mechanism and establish an appropriate therapeutic strategy.
The cost analysis is crucial for understanding the care pathway of people on chronic hemodialysis with CKD-aP and comparing it to that of patients on hemodialysis without pruritus. Our findings prove that the wide use of high-efficiency hemodialytic strategies was too expensive and should be delayed in favor of approaches based on strongest evidence, also following the discovery of an effective treatment (promising novel therapies, e.g., difelikefalin, are currently approved by the Food and Drug Administration [FDA] and European Medicines Agency [EMA] (24)). People on hemodialysis with CKD-aP still have significant unmet needs. Healthcare policy makers and payers should take action soliciting researches to improve treatments, but this effort accomplishes above all through the awareness of the healthcare professionals and patients themselves. Indeed, given the poor understanding of the pathophysiology of CKD-aP and lack of knowledge of its long-term outcomes (9), updated guidelines and education initiatives for clinicians and patients are deserved. Larger and higher-quality trials are needed (19), as well.
Conclusions
Even if the identified cohort results are underestimated, this study highlights some current critical therapeutic strategies in the Italian nephrology settings. The observed widespread antihistamine use in Italy for treating CKD-aP still lacks enough evidence, whereas the very low use of gabapentin due to INHS reimbursement criteria was not in line with the strongest evidence for the CKD-aP treatment at the time of the analysis. Despite only being descriptive, this direct cost analysis integrates the very reduced knowledge in the CKD-aP economic and clinical panorama, and suggests that, probably, in Italy, high-efficiency hemodialytic therapies are among the first therapeutic choices leading to a sensitive cost increase for treating CKD-aP. In conclusion, we suggest that healthcare policy makers and clinicians would adopt a stronger evidence-based approach to treat CKD-aP, and this might be a guide to cost offsets, an optimization of the healthcare budget and better outcomes for patients.
Disclosures
Conflict of interest: L.M. declares his participation in the advisory boards of Vifor Pharma, GlaxoSmithKline and Galderma. F.A. declares his participation in the advisory board of Vifor Pharma. The other authors declare that they have no competing interests.
Financial support: Funding for this study was provided by an unconditional grant from Vifor Pharma, Glattbrugg, Switzerland. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing and publishing the report.
Author Contributions: Conceptualization: S.C., G.R., L.D., A.P., L.M. and F.A.; data curation: L.D.; formal analysis: L.D.; funding acquisition: I.E., A.A. and N.M.; investigation: G.R., S.C. and L.D.; methodology: S.C., G.R., L.D., L.M. and F.A.; project administration: G.R.; software: L.D.; supervision: A.P. and N.M.; validation: S.C., G.R., L.D., A.P., L.M. and F.A.; writing—original draft: S.C., L.M. and F. A.; writing—review and editing: S.C., C.P., G.R., L.D., L.D., L.M. and F.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement: This was a retrospective observational study of Italian administrative data, which have been analyzed in an aggregated form after their anonymization at the source, according to the specific agreements with the regional/local health authorities, owners of the data and European privacy laws. For these reasons and because of the institutional purposes of this study, ethical approval was not applicable.
Informed Consent Statement: This is a retrospective observational study of Italian administrative data, which have been analyzed in an aggregated form after their anonymization at the source, according to the specific agreements with the regional/local health authorities, owners of the data and European privacy laws. For these reasons and because of the institutional purposes of this study, informed consent was not applicable.
Data Availability Statement: The datasets analyzed during the current study are not publicly available and are not available from the corresponding author on reasonable request, because they are owned by the Italian regional/local health authorities who have not authorized Fondazione ReS to make them available.
References
- 1. Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2020;3(1):42-53.e1. CrossRef PubMed
- 2. Kim D, Pollock C. Epidemiology and burden of chronic kidney disease-associated pruritus. Clin Kidney J. 2021 Oct;14(suppl 3):i1-i7. CrossRef
- 3. van der Willik EM, Lengton R, Hemmelder MH, et al. Itching in dialysis patients: impact on health-related quality of life and interactions with sleep problems and psychological symptoms—results from the RENINE/PROMs registry. Nephrol Dial Transplant. 2022;37(9):1731-1741. CrossRef PubMed
- 4. Kimata N, Fuller DS, Saito A, et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int. 2014;18(3):657-667. CrossRef PubMed
- 5. Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87(4):685-691. CrossRef PubMed
- 6. Millington GWM, Collins A, Lovell CR, et al. British Association of Dermatologists’ guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol. 2018;178(1):34-60. CrossRef PubMed
- 7. Weisshaar E, Szepietowski JC, Dalgard FJ, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99(5):469-506. CrossRef PubMed
- 8. Ting SW, Fan PC, Lin YS, et al. Association between uremic pruritus and long-term outcomes in patients undergoing dialysis. J Am Acad Dermatol. 2020;83(3):924-925. CrossRef PubMed
- 9. Aresi G, Rayner HC, Hassan L, et al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manage. 2019;58(4):578-586.e2. CrossRef PubMed
- 10. Istituto Nazionale di Statistica and ISTAT. Resident population by age, sex and marital status. 2015. Online. Accessed December 2023.
- 11. Piccinni C, Cevoli S, Ronconi G, et al. Insights into real-world treatment of cluster headache through a large Italian database: prevalence, prescription patterns, and costs. Expert Rev Clin Pharmacol. 2021;14(9):1165-1171. CrossRef PubMed
- 12. Maggioni AP, Dondi L, Andreotti F, et al. Prevalence, clinical impact and costs of hyperkalaemia: special focus on heart failure. Eur J Clin Invest. 2021;51(8):e13551. CrossRef PubMed
- 13. Ronconi G, Dondi L, Calabria S, et al. Real-world prescription pattern, discontinuation and costs of ibrutinib-naïve patients with chronic lymphocytic leukemia: an Italian healthcare administrative database analysis. Clin Drug Investig. 2021;41(7):595-604. CrossRef PubMed
- 14. WHO. Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2023. Online. Accessed May 2023.
- 15. Ministero del Lavoro, della salute e delle politiche sociali. Classificazione delle malattie, dei traumatismi, degli interventi chirurgici e delle procedure dagnostiche e terapeutiche. Versione italiana della ICD9-CM. 2007. Online. Accessed December 2023.
- 16. CINECA—Interuniversity Consortium. Online. Accessed May 2023.
- 17. Società Italiana di Nefrologia. Registro italiano di dialisi e trapianto [Italian Registry of Dialysis and Transplantation]. Online. Accessed May 2023.
- 18. Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387-1402. CrossRef PubMed
- 19. Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017 Nov;70(5):638-655. CrossRef PubMed
- 20. van Oosten MJM, Logtenberg SJJ, Edens MA, et al. Health claims databases used for kidney research around the world. Clin Kidney J. 2020;14(1):84-97. CrossRef PubMed
- 21. Agenzia Italiana del Farmaco. Nota 89. GU Serie Generale n.259 del 04-11-2004 2004. Online. Accessed December 2023.
- 22. Agenzia Italiana del Farmaco. Nota 4. Farmaco in nota: duloxetina, gabapentin, pregabalin. Online. Accessed December 2023.
- 23. Arzhan S, Roumelioti ME, Unruh ML. Itch and ache on dialysis: new approaches to manage uremic pruritus and restless legs. Blood Purif. 2020;49(1-2):222-227. CrossRef PubMed
- 24. Trachtenberg AJ, Collister D, Rigatto C. Recent advances in the treatment of uremic pruritus. Curr Opin Nephrol Hypertens. 2020;29(5):465-470. CrossRef PubMed