 |
Glob Reg Health Technol Assess 2023; 10: 62-69 ISSN 2283-5733 | DOI: 10.33393/grhta.2023.2561 ORIGINAL RESEARCH ARTICLE |
 |
Cost-effectiveness analysis of abemaciclib with endocrine therapy (ET) versus ET alone for HR+, HER2−, node-positive, high-risk early breast cancer in Italy
ABSTRACT
Background: Abemaciclib was recently approved by the European Medicines Agency in combination with adjuvant endocrine therapy (ET) for adult patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−), node-positive early breast cancer (EBC) at high risk of recurrence.
Objective: To evaluate the cost-effectiveness of abemaciclib plus ET vs. ET alone in patients with HR+, HER2−, node-positive EBC at high risk of disease recurrence, from the Italian healthcare system perspective.
Methods: A cohort state transition model was developed with five states: invasive disease-free survival (IDFS), nonmetastatic recurrence, remission, metastatic recurrence, and death. The analysis had a time horizon of 30 years. Individual patient-level data from the monarchE trial (NCT03155997) were used to generate IDFS estimates. Resource use included drug acquisition/administration, best supportive care, terminal care, adverse events, hospitalization, post-progression therapy, and associated resource use in the metastatic disease health state. Health state utilities were derived from monarchE patient-level data and other sources, applying Italian tariffs where feasible.
Results: The estimated total discounted costs (€39,249 vs. €16,806; difference: €22,443) and quality-adjusted life years (QALYs) (11.49 vs. 10.50; difference: 0.99) were higher for abemaciclib plus ET compared with ET alone. The incremental cost-effectiveness ratio was €22,651 per QALY gained. The likelihood of abemaciclib plus ET being cost-effective vs. ET alone was 99% at a willingness-to-pay threshold of €30,000 per QALY gained.
Conclusion: Abemaciclib plus ET is a cost-effective treatment option vs. ET alone for those with HR+, HER2− node-positive EBC at high risk of recurrence in Italy.
Keywords: Abemaciclib, Cost-effectiveness, Early breast cancer, Endocrine therapy, HER2−, HR+
Received: January 12, 2023
Accepted: August 10, 2023
Published online: September 28, 2023
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Breast cancer has surpassed lung cancer as the most diagnosed cancer globally (1). Approximately 90% of patients with breast cancer have early-stage disease at the time of diagnosis (2). Among patients with early breast cancer (EBC), approximately 70% have tumors that are hormone receptor positive (HR+) and human epidermal growth factor receptor 2 negative (HER2−) (3).
Most patients with HR+, HER2− EBC will not experience disease recurrence with standard therapy (combinations of surgery, radiotherapy, [neo]adjuvant chemotherapy, and [neo]adjuvant endocrine therapy [ET]) (4,5). More than half of all breast cancer recurrences occur within the first 5 years, peaking at 2 years (6). For patients with HR+, HER2− EBC who have high-risk clinical pathologic features, such as a high number of involved regional lymph nodes, larger tumor size, and higher histologic grade, the risk of disease recurrence is higher (4,7). It is important to ensure patients at higher risk of disease recurrence receive optimal adjuvant treatment, with the aim of reducing the risk of metastatic disease. While significant advances have been made in breast cancer treatment, approximately 12,500 deaths were still estimated in 2021 in Italy (8-10).
Abemaciclib was recently approved by the European Medicines Agency (EMA) for use in combination with adjuvant ET in adult patients with HR+, HER2−, node-positive EBC at high risk of recurrence (11). It is currently the only cyclin-dependent kinase 4 and 6 (CDK4 and 6) inhibitor approved for this indication. EMA approval was based on data from the monarchE trial (NCT03155997), a large patient population with HR+, HER2−, node-positive EBC at high risk of recurrence, which was defined by clinical and pathologic features. The monarchE trial demonstrated that 2 years of abemaciclib added to physician’s choice of adjuvant ET, for a planned minimum duration of 5 years, resulted in a significant improvement in invasive disease-free survival (IDFS) compared with ET alone (12,13).
The aim of the current study was to assess the cost-effectiveness of abemaciclib combined with ET vs. ET alone in a patient population with EBC comparable to that of the EMA labeled indication (11), from the Italian healthcare system perspective.
Methods
Model overview
A five-state cohort Markov model was developed to evaluate the cost-effectiveness of abemaciclib plus ET vs. ET alone in patients with HR+, HER2−, node-positive EBC at high risk of recurrence. The model health states were IDFS, nonmetastatic recurrence, remission, metastatic disease recurrence, and death (Fig. 1). The cohort started in the IDFS health state and patients could move to nonmetastatic recurrence, metastatic disease, or death. Transitions could occur from the nonmetastatic recurrence health state to remission or death, and from the remission health state to metastatic disease or death. The model had a cycle length of 28 days. Half-cycle correction was applied to account for events not occurring at the start or end of every cycle. Costs and outcomes were calculated over a 30-year time horizon, considering both average age reported for patients and current life expectancy in Italy (14,15), and discounted at an annual rate of 3.0% as recommended by Agenzia Italiana del Farmaco (AIFA) (16). The analysis was conducted from the Italian healthcare system perspective. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS 2022) statement was used for reporting guidance in the preparation of this article (17,18).
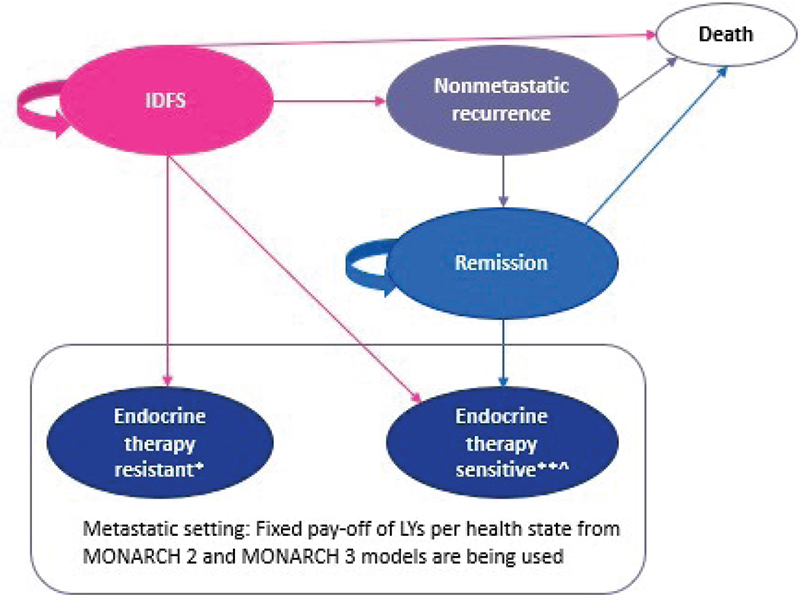
Fig. 1 - Schematic representation of the Markov model structure. *Disease recurrence while receiving or within 12 months of completing prior adjuvant endocrine therapy; **Disease recurrence at least 12 months after completion of prior adjuvant endocrine therapy; Ù Includes treatment with tamoxifen. IDFS = invasive disease-free survival; LYs = life years.
Target population
The patient population was comparable to that of the EMA labeled indication for abemaciclib (11). The population included patients with HR+, HER2−, node-positive EBC at high risk of recurrence, which was defined by clinical and pathologic features as either ≥4 positive axillary lymph nodes (pALN) or 1-3 pALN and at least one of the following criteria: tumor size ≥5 cm or histologic grade 3.
Treatment comparators and data sources
According to monarchE trial design, maximum time on abemaciclib was 2 years with a minimum planned duration of 5 years for adjuvant ET (12). Clinical guidelines were used to inform the treatments included in the nonmetastatic disease recurrence setting (4,19). As per the monarchE trial, the specific ET used was according to physician’s choice in both treatment arms (i.e., anti-estrogen tamoxifen and the aromatase inhibitors anastrozole, exemestane, and letrozole).
State transition and survival model parameters
The model was parametrized using data primarily from the monarchE trial extrapolated to the horizon of interest. The transition probabilities from IDFS health state were governed by the probability of remaining in IDFS health state, which was also the monarchE trial primary endpoint. Additional time-to-event outcomes included overall survival (OS) without distant recurrence and time-to-treatment discontinuation (TTD). Rates of nonmetastatic and metastatic disease recurrence combined with IDFS were required for transition probabilities from IDFS to nonmetastatic and metastatic health states.
To extrapolate beyond the monarchE observed period, the Kaplan-Meier estimate of survival curves of these three time-to-event endpoints were fit to a range of nine parametric and cubic spline models.
The best fitting distributions were selected using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) in addition to visual assessment of conformity to the original Kaplan-Meier survival curves. External data and thought leader judgment were also considered for long-term plausibility and external validity. For each of the time-to-event endpoints, the proportional hazards assumption was assessed to determine whether the two treatment arms could be modeled jointly or separately; this included visual inspection of both the Schoenfeld residuals and corresponding global test. The model selection and diagnostics were made according to National Institute for Health and Care Excellence (NICE) Decision Support Unit 14 recommendations (20).
Analyses were carried out using SAS Software 9.4 (SAS Institute Inc., Cary, NC, USA; traditional parametric models) and R 3.6.2 Software (cubic spline models).
In the absence of published literature for HER2− EBC, the nonmetastatic recurrence health state was informed by previous health technology assessments in HER2+ EBC (21-23), clinical guidelines (4,19), and clinical expert opinion. Patients were assumed to have a very low risk of experiencing disease metastases during the 12-month treatment period following diagnosis. After 12 months, patients transitioned to remission.
The latest monarchE data cut at the time of the analysis did not have sufficient longer term follow-up data to inform the metastatic setting. The health state was instead informed by data from a broader advanced breast cancer population, which included patients at high risk of disease recurrence, using the MONARCH 2 (24) and MONARCH 3 (25) trials. In this health state, patients were classified as ET-resistant if they experienced disease recurrence while receiving adjuvant ET or within 12 months of completing adjuvant ET. Patients were considered ET-sensitive if they experienced disease recurrence more than 12 months after completing their adjuvant ET.
Resource use
Resource use included drug acquisition and administration, best supportive care, terminal care, adverse events, hospitalizations, post-progression therapies, and associated resources in the metastatic health state. Unit costs were derived from national sources in Italy and previous health technology appraisals. Drugs were costed at the net realized price (effective price paid by the Italian healthcare system). Costs were sourced for the year 2021; when these costs were not available, latest available data were used.
Health state utilities
Health state utilities for IDFS were derived from the EuroQoL 5-dimension 5-level (EQ-5D-5L) monarchE patient-level data, and applying country-specific current index scores for Italy (26). For the metastatic recurrence health state, EQ-5D-5L utilities were derived from the MONARCH 2 and MONARCH 3 trials and cross-walked to EQ-5D-3L utilities using the algorithm by van Hout et al (27), applying UK utility weights (Tab. I), as per the available metastatic breast cancer models at the time of the cost-effectiveness analysis. This is not expected to affect results, particularly since there is no difference in summary statistics between the EQ-5D-3L and -5L utilities from the monarchE trial. For monarchE, Italian EQ-5D-3L valuation was based on Scalone et al (28) after cross-walk from EQ-5D-5L to EQ-5D-3L utilities using the algorithm by van Hout et al (27). As the data showed no statistically significant difference between treatment arms, overall utilities were applied to both treatment arms. The base-case analysis used age-adjusted utilities to consider the potential negative effect of age on health-related quality-of-life (HRQoL) (29,30).
For the nonmetastatic recurrence health state, published utility values were used (31), and post-metastatic recurrence utility estimates were applied from the MONARCH 2 and MONARCH 3 trials and literature-based assumptions (without cross-walking).
Assumptions
The key settings and assumptions used in the base-case analysis are provided in Table I. Results are reported in terms of the incremental cost per quality-adjusted life year (QALY) gained.
Sensitivity analyses
Two types of sensitivity analyses were conducted to address the underlying uncertainties in the model and inputs. A one-way (deterministic) sensitivity analysis was run, which modified one input parameter at a time. Low and high values were allocated based on the 95% confidence interval (CI) where applicable/available from the data source. Where no distributional information were available (e.g., standard error), input parameters were varied by ±20% of the mean estimate.
A probabilistic sensitivity analysis (PSA) was conducted by assigning distributions to input parameters and sampling from these distributions. The type of distribution was based on the upper and lower bounds that each parameter is constrained between. For example, a beta distribution was used for values that ranged between 0 and 1, and a gamma distribution was used for costs and resource use estimates, which is appropriate for variables that are always positive and have skewed distributions. In total, 1000 iterations were run as the model outcomes stabilized at approximately 500 iterations.
Results
In the base-case analysis, estimated total discounted costs (€39,249 vs. €16,806; difference: €22,443) and QALYs (11.49 vs. 10.50; difference: 0.99) were higher for abemaciclib plus ET compared with ET alone, respectively. The addition of up to 2 years of abemaciclib to adjuvant ET resulted in an incremental cost-effectiveness ratio (ICER) of €22,651 per QALY gained.
Drug acquisition was the main driver of costs in the abemaciclib plus ET arm, largely due to the higher cost of abemaciclib (Fig. 2A). For patients receiving ET alone, total cost in the metastatic setting was the key driver. This was specifically due to the use of CDK4 and 6 inhibitors (abemaciclib, palbociclib, or ribociclib). The CDK4 and 6 inhibitors plus ET are the mainstay of treatment for patients with metastatic breast cancer who have not received a prior CDK4 and 6 inhibitor plus ET. Most patients in the abemaciclib plus ET cohort who had metastatic recurrence received ET, with one-third of patients with ET-resistant disease receiving ET plus everolimus. Total discounted costs in the metastatic disease recurrence health state were €5,408 (abemaciclib plus ET) vs. €13,349 (ET alone).
| Setting | Option for base case |
|---|---|
| Population | HR+, HER2−, node-positive, high-risk EBC. High risk was defined as either ≥4 pALN, or 1-3 pALN and at least one of the following criteria: tumor size ≥5 cm or histologic grade 3 |
| Perspective | Italian healthcare system perspective |
| Time horizon | 30 years |
| Cycle length | 28 days |
| Discount rate QALYs | 3.0% |
| Discount rate costs | 3.0% |
| Intervention | Abemaciclib + physicians’ choice ET |
| Comparator | Physicians’ choice ET alone |
| Survival curve used for cost estimates |
• TTD for active treatment costs of abemaciclib + ET and ET • IDFS—disease management and background therapy • OS without distant recurrence—terminal care costs for IDFS, nonmetastatic recurrence, and remission health states |
| Endpoint for utility estimates |
• IDFS utility values were derived from the EQ-5D-5L monarchE patient-level data, and applying country-specific current index scores for Italy (26) • Published utility values for nonmetastatic disease health states (31) • EQ-5D-5L cross-walked 3L utilities using van Hout algorithm (27) from the global MONARCH 2 and MONARCH 3 cost-effectiveness models for the ET-resistant and ET-sensitive metastatic recurrence health state utilities, respectively • Post-metastatic recurrence utility estimates applied from the abemaciclib ABC trials (MONARCH 2 and MONARCH 3) and literature-based assumptions |
| Consideration of extrapolations |
• Yes, TTD from last data point of monarchE AFU1 data cut (April 1, 2021) until Year 5, when the clinical stopping rule is introduced • Yes, IDFS and OS with distant relapse, for the full-time horizon chosen by the user |
| Survival curve fitting | Dependent model fitting for IDFS and OS without distance recurrence |
| IDFS distribution | Log-logistic distribution following internal validity checks and assessment of external evidence |
| Long-term treatment effect | Waning of treatment effect assumed after 8 years until the crossing of the ET hazard rate with the general population mortality |
| NMR tunnel state duration | All patients who experience an NMR are assumed to receive additional adjuvant therapy for 12 months. After 12 months, patients are assumed to either transition into the remission health state or die due to all-cause mortality |
| OS without distant recurrence distribution | Exponential distribution following internal validity checks. Hazard of dying in IDFS health state assumed same as hazard of dying in the NMR and REM health states |
| TTD distribution | Extrapolations carried out using Hazard spline 2 knot. Clinical stopping rule at 2 years for abemaciclib and 5 years for ET was applied |
| Probability of recurrence |
• Transitions from IDFS health state: constant proportion over time between nonmetastatic recurrence and metastatic recurrence • Transitions from REM health state: constant monthly probability of transition from remission to the metastatic health state |
| Consideration of subsequent therapies |
• Yes, clinical guidelines inform the treatments included in the nonmetastatic disease recurrence setting • Yes, treatments prescribed for ET-resistant and ET-sensitive metastatic disease recurrence health state have been included based on the abemaciclib cost-effectiveness models for ABC (MONARCH 2 and MONARCH 3, respectively) |
| Consideration of second primary neoplasm cancer events |
• No, other than when a patient enters the nonmetastatic recurrence health state |
| Maximum or minimum time on treatment |
monarchE clinical trial, ET clinical guidelines • 2 years for abemaciclib (maximum) • 5 years for ET (minimum) |
| Wastage considered | No/NA all oral treatments modeled |
| Hospitalization costs |
• Dictated by the monarchE trial |
| Age-adjusted utilities | Yes |
ABC = advanced breast cancer; AFU1 = additional follow-up one; EBC = early breast cancer; EQ-5D-5L, 3L = EuroQoL 5-dimension 5-level, 3-level; ET = endocrine therapy; HER2− = human epidermal receptor 2 negative; HR+ = hormone receptor positive; IDFS = invasive disease-free survival; NA = not applicable; NMR = nonmetastatic recurrence; OS = overall survival; pALN = positive axillary lymph nodes; QALYs = quality-adjusted life years; REM = remission; TTD = time-to-treatment discontinuation.
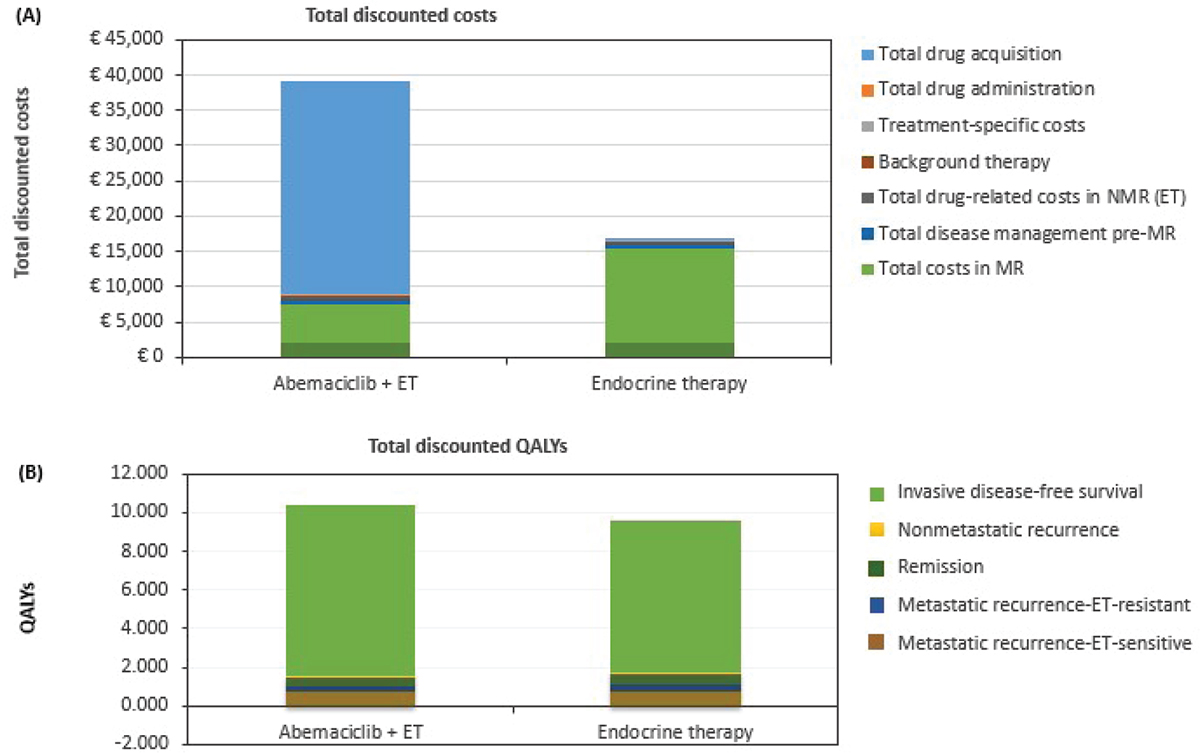
Fig. 2 - Breakdown of total discounted costs (A) and QALYs (B) by category for abemaciclib plus ET and for ET alone. ET = endocrine therapy; MR = metastatic recurrence; NMR = nonmetastatic recurrence; QALYs = quality-adjusted life years.
In terms of HRQoL (Fig. 2b), the IDFS health state was an important contributor of QALY gains in both treatment arms (86% and 83% of total QALYs for abemaciclib plus ET and ET alone, respectively). For this health state, the addition of abemaciclib resulted in incremental QALY gains of 1.14 relative to ET alone (9.86 vs. 8.72, respectively). Total QALYs in the metastatic recurrence health state were lower for abemaciclib plus ET relative to ET alone (total: 1.01 vs. 1.14; 0.27 vs. 0.39 [ET-resistant]; 0.74 vs. 0.75 [ET-sensitive]). The QALY gain in the ET arm was also driven by the use of CDK4 and 6 inhibitors.
The deterministic one-way sensitivity analysis showed the model was most sensitive to the proportion of patients moving to nonmetastatic recurrence, irrespective of treatment received (ICER range: €16,648 to €38,932; Fig. 3). Changes to other parameters resulted in small changes to the ICER.
The PSA showed a moderate degree of uncertainty in the QALY outcomes for both abemaciclib plus ET and ET alone (Fig. 4). All simulations were in the north-eastern quadrant of the cost-effectiveness plane, indicating that abemaciclib plus ET results in an improvement in QALYs but at a higher cost compared with ET alone. Cost-effectiveness acceptability curves indicated that the likelihood of abemaciclib plus ET being cost-effective compared with ET alone was 99% at a willingness-to-pay threshold of €30,000 per QALY gained (Fig. 5).
Discussion
Although several cost-effectiveness analyses have been conducted in HR+ EBC, most have focused on HR+, HER2+ EBC (19-21). We did not identify any other analyses that were representative of the monarchE patient population.
This cost-effectiveness analysis was informed by the first model for the monarchE specific population, which has been reviewed and recommended by NICE in the UK (32). The model showed that up to 2 years of abemaciclib administered with a minimum of 5 years of adjuvant ET is a cost-effective treatment option, from the Italian healthcare system perspective. The primary driver of costs for the abemaciclib plus ET arm was the abemaciclib drug acquisition costs in the IDFS health state. Due to delayed or avoided distant recurrence, substantial QALY gains were also estimated for abemaciclib plus ET vs. ET alone in the IDFS health state. The upfront cost of abemaciclib was offset in ET alone by the higher cost of treating more patients in the metastatic setting. The use of the CDK4 and 6 inhibitors in the metastatic recurrence health state showed higher costs and QALY gains for patients initially receiving adjuvant ET alone. Due to an absence of clinical data, based on expert opinion, it was assumed that patients receiving abemaciclib in the EBC setting would not be retreated (rechallenged) with a CDK4 and 6 inhibitor in the metastatic setting. It is acknowledged that a proportion of patients, likely among those in the ET-sensitive pathway, would be rechallenged with a CDK4 and 6 inhibitor and that payers may consider covering the additional cost of a second CDK4 and 6 inhibitor in certain circumstances. The size of the proportion that may be eligible for rechallenge is unknown.
The deterministic and probabilistic analyses showed that the ICER results were most sensitive to changes in the proportion of patients moving from IDFS to the nonmetastatic recurrence health state. ICER results were generally favorable when compared with commonly used/accepted cost-effectiveness or willingness-to-pay thresholds for health technologies in Europe and the USA (33-36), including Italy (37). Median ICER values for innovative oncologic therapies approved by AIFA in the period 2010-2013 ranged from €53,273 (for drugs with statistically significant OS gains) to €69,568 per life year gained (for drugs with nonsignificant OS differences) (38). In Italy, price and reimbursement for new medicines are simultaneously negotiated by AIFA and the relevant pharmaceutical company (39).
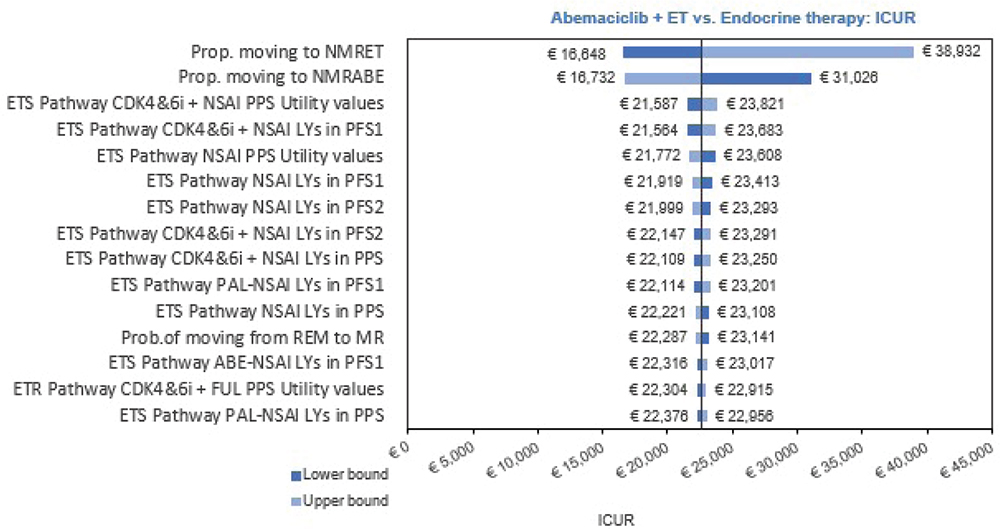
Fig. 3 - Results of one-way (deterministic) sensitivity analysis. ABE = abemaciclib; CDK4&6i = cyclin-dependent kinase 4 and 6 inhibitors; ET = endocrine therapy; ETR = endocrine therapy resistant; ETS = endocrine therapy sensitive; FUL = fulvestrant; ICER = incremental cost-effectiveness ratio; LY = life years; MR = metastatic recurrence; NMRABE = nonmetastatic recurrence—abemaciclib; NMRET = nonmetastatic recurrence—endocrine therapy; NSAI = nonsteroidal aromatase inhibitor; PFS = progression-free survival; PFS1 = progression-free survival first-line advanced breast cancer; PFS2 = progression-free survival second-line advanced breast cancer; PPS = post-progression survival; Prop = proportion; REM = remission.
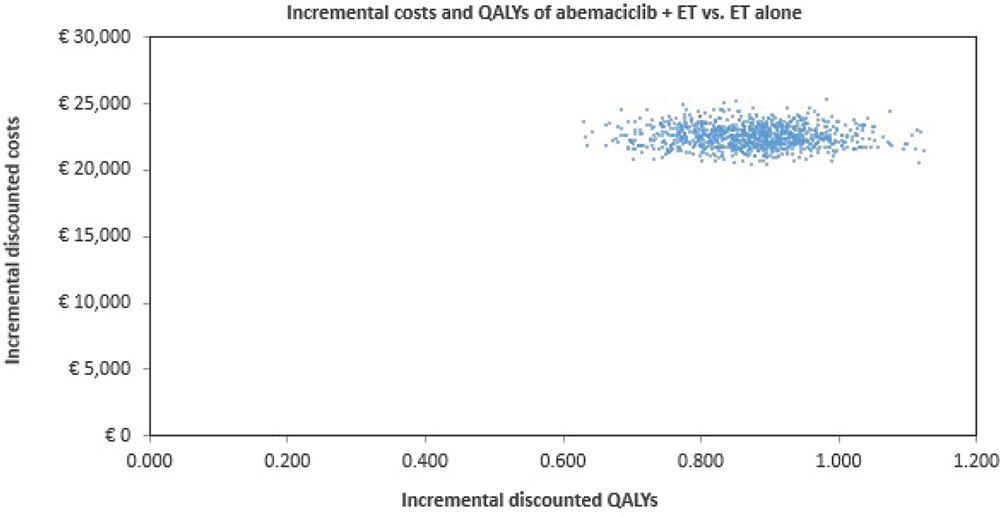
Fig. 4 - Results of probabilistic sensitivity analysis. ET = endocrine therapy; QALYs = quality-adjusted life years.
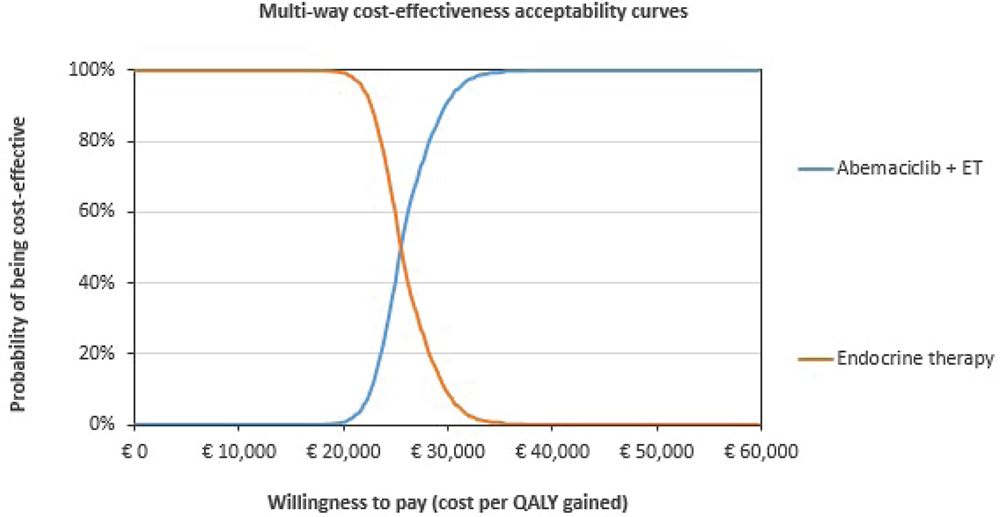
Fig. 5 - Cost-effectiveness acceptability curves for abemaciclib plus ET and for ET alone. ET = endocrine therapy; QALY = quality-adjusted life year.
There are several limitations related to the data and assumptions used to populate this model. Although monarchE met its primary endpoint, the follow-up time for the trial meant data were relatively immature for the purpose of extrapolating lifetime outcomes. In our analysis, we used clinical data from the latest data cut available at the time of the analysis. Paucity of clinical data outside of the monarchE trial prevented external validation of the OS without distant metastatic recurrence extrapolations. We relied on internal validation from the monarchE trial, which was the most recent and relevant data source for this population. This could introduce bias by over- or underestimating long-term survival outcomes. The assumption of a constant risk of disease recurrence or death is not reflective of a monarchE population, as the post-nonmetastatic recurrence pathway and the nonmetastatic recurrence death rate assumptions were informed by data from a HER2+ population. The model does not allow a second primary neoplasm cancer event to be captured in the EBC pathway, other than when a patient enters the nonmetastatic disease recurrence health state. Despite these limitations, the sensitivity analyses results support findings of the base-case analysis that abemaciclib plus ET is a cost-effective treatment, from the Italian healthcare system perspective.
Conclusion
The addition of up to 2 years of abemaciclib to a minimum 5 years of adjuvant ET was cost-effective compared with ET alone for patients with HR+, HER2−, node-positive EBC at high risk of disease recurrence in Italy.
Acknowledgments
The authors would like to acknowledge Greg Plosker and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript.
Disclosures
Conflict of interest: Alison Davie, Sory Traoré, and Massimo Giovannitti are employees of Eli Lilly and Company. Giuseppe Pompilio has no conflicts of interest to declare. Mark Lambton, Esra Cakar, and Anuja Chatterjee are employees of OPEN Health Evidence & Access, which received funding from Eli Lilly and Company for consulting.
Financial support: This study was funded by Eli Lilly and Company.
References
- 1. Cancer.Net. Breast cancer: statistics. Online. Accessed August 2023.
- 2. American Cancer Society. Breast cancer facts and figures 2019–2020. Atlanta: American Cancer Society, Inc. 2019. Online. Accessed October 2022.
- 3. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. CrossRef PubMed
- 4. Cardoso F, Kyriakides S, Ohno S, et al; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. CrossRef PubMed
- 5. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-1352. CrossRef PubMed
- 6. Cheng L, Swartz MD, Zhao H, et al. Hazard of recurrence among women after primary breast cancer treatment – a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev. 2012;21(5):800-809. CrossRef PubMed
- 7. Mamounas EP, Tang G, Paik S, et al. 21-Gene recurrence score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: results from NSABP B-28/NRG oncology. Breast Cancer Res Treat. 2018;168(1):69-77. CrossRef PubMed
- 8. AIOM. AIRTUM, SIAPEC-IAP. I numeri del cancro in Italia 2020. Online. Accessed September 2022.
- 9. AIOM. I numeri del cancro 2021. Online. Accessed September 2022.
- 10. AIOM. AIRO, A.N.I.S.C, SIAPEC-IAP, SICO, SIRM. Linee Guida Neoplasie della Mammella. Edizione 2021, aggiornata a 11.11.2021b. Online. Accessed September 2022.
- 11. European Medicines Agency (EMA). Abemaciclib (Verzenios®) summary of product characteristics. Online. Accessed May 2022.
- 12. Johnston SRD, Harbeck N, Hegg R, et al; monarchE Committee Members and Investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987-3998. CrossRef PubMed
- 13. Toi M, Boyle F, Im Y-H, et al. Adjuvant abemaciclib combined with endocrine therapy: efficacy results in monarchE cohort 1. Oncologist. 2023;28(1):e77-e81. CrossRef PubMed
- 14. ISTAT. 2021. Noiitalia2022. Online. Accessed January 2023.
- 15. Tagliabue G, Fabiano S, Contiero P, et al; AIRTUM Working Group. Molecular subtypes, metastatic pattern and patient age in breast cancer: an analysis of Italian network of cancer registries (AIRTUM) data. J Clin Med. 2021;10(24):5873. CrossRef PubMed
- 16. AIFA (Agenzia Italiana del Farmaco). Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale ai sensi del D.M. 2 agosto 2019. Online. Accessed January 2023.
- 17. Husereau D, Drummond M, Augustovski F, et al; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3-9. CrossRef PubMed
- 18. Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10-31. CrossRef PubMed
- 19. National Comprehensive Cancer Network (NCCN). Breast cancer (Version 2.2022). Online. Accessed May 2022.
- 20. Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data. Sheffield: Report by the Decision Support Unit. 2011 (updated 2013);(0). Online. Accessed November 2022.
- 21. National Institute for Health and Care Excellence (NICE). Trastuzumab emtansine for adjuvant treatment of HER2-positive early breast cancer [Technology Appraisal Guidance TA632] 2020. Online. Accessed January 2023.
- 22. National Institute for Health and Care Excellence (NICE). Neratinib for extended adjuvant treatment of hormone receptor-positive, HER2-positive early stage breast cancer after adjuvant trastuzumab [Technology Appraisal Guidance TA612] 2019a. Online. Accessed January 2023.
- 23. National Institute for Health and Care Excellence (NICE). Pertuzumab for adjuvant treatment of HER2-positive early stage breast cancer [Technology Appraisal Guidance TA569] 2019b. Online. Accessed January 2023.
- 24. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. CrossRef PubMed
- 25. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646. CrossRef PubMed
- 26. Finch AP, Meregaglia M, Ciani O, Roudijk B, Jommi C. An EQ-5D-5L value set for Italy using videoconferencing interviews and feasibility of a new mode of administration. Soc Sci Med. 2022;292:114519. CrossRef PubMed
- 27. van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708-715. CrossRef PubMed
- 28. Scalone L, Cortesi PA, Ciampichini R, et al. Italian population-based values of EQ-5D health states. Value Health. 2013;16(5):814-822. CrossRef PubMed
- 29. Ara R, Wailoo A. NICE DSU technical support document 12: the use of health state utility values in decision models. 2011a. Online. Accessed January 2023.
- 30. Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011b;14(4):539-545. CrossRef PubMed
- 31. Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073-1081. CrossRef PubMed
- 32. National Institute for Health and Care Excellence (NICE). Abemaciclib with endocrine therapy for adjuvant treatment of hormone receptor-positive, HER2-negative, node-positive early breast cancer at high risk of recurrence [Technology Appraisal Guidance TA810]. 2022. Online. Accessed January 2023.
- 33. Brouwer W, van Baal P, van Exel J, Versteegh M. When is it too expensive? Cost-effectiveness thresholds and health care decision-making. Eur J Health Econ. 2019;20(2):175-180. CrossRef PubMed
- 34. Cherla A, Renwick M, Jha A, Mossialos E. Cost-effectiveness of cancer drugs: comparative analysis of the United States and England. EClinicalMedicine. 2020;29-30:100625. CrossRef PubMed
- 35. Ryen L, Svensson M. The willingness to pay for a quality adjusted life year: a review of the empirical literature. Health Econ. 2015;24(10):1289-1301. CrossRef PubMed
- 36. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness – the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. CrossRef PubMed
- 37. Fattore G. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. PharmacoEcon Ital Res Artic. 2009;11(2):83-93. CrossRef
- 38. Martone N, Lucioni C, Mazzi S, et al. Valutazione di costo-efficacia dei nuovi farmaci oncologici immessi sul mercato italiano. Glob Reg Health Technol Assess. 2014;1(2):31-43.
- 39. Villa F, Tutone M, Altamura G, et al. Determinants of price negotiations for new drugs. The experience of the Italian Medicines Agency. Health Policy. 2019;123(6):595-600. CrossRef PubMed