 |
Glob Reg Health Technol Assess 2023; 10: 46-52 ISSN 2283-5733 | DOI: 10.33393/grhta.2023.2560 ORIGINAL RESEARCH ARTICLE |
 |
COVID-19 impact on the decision process of the Italian Medicine Agency: a quantitative assessment
ABSTRACT
Background: Since the COVID-19 pandemic has placed more attention on drugs’ approval process and the importance of rapid decision-making in the healthcare sector, it is crucial to assess how time to market (TTM) of drugs varied.
Objective: To estimate the impact of the COVID-19 pandemic on TTM of drugs in Italy.
Methods: An IQVIA database was used to retrieve information on drugs that obtained positive opinion from the Committee for Medicinal Products for Human Use between January 2015 and December 2021. The available observations were divided into three groups (Pre COVID, Partially COVID, and Fully COVID) according to the timing of their negotiation process. Differences in average TTM among the three groups were analyzed in three steps: (1) descriptive statistics; (2) univariate analysis; (3) multivariate analysis, using a matching estimator.
Results: A total of 363 unique combinations of molecule and indication met the inclusion criteria: 174 in the Pre COVID group, 69 in the Partially COVID group, and 123 in the Fully COVID group. Descriptive statistics and univariate analysis found a statistically significant difference in TTM among the three periods, with average TTM increasing during the pandemic (+136 days, p = 0.00) and then decreasing afterward (−23 days, p = 0.09). In the matching analysis, results for the Partially COVID period were confirmed (+108 days, p = 0.00) while results for the Fully COVID period lost significance but maintained a negative sign.
Conclusions: The results suggest that after an adjustment phase in the Partially COVID period, a return to the status quo was reached.
Keywords: COVID-19, Drugs, Italy, Price and reimbursement, Time to market
Received: January 5, 2023
Accepted: April 4, 2023
Published online: May 31, 2023
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
In Italy, for drugs approved at the European level through centralized procedure by the European Medicine Agency (EMA), reimbursement eligibility and/or prices are defined at the national level. In particular, prices and coverage of new pharmaceutical technologies are decided through negotiations between the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) and the Market Authorization Holder (MAH) that must submit a price and reimbursement (P&R) dossier (1).
The request can be submitted after the publication of the European Commission (EC) decision in the Union Register of medicinal products for human use, following the Committee for Medicinal Products for Human Use (CHMP) positive opinion. For orphan drugs, drugs with exceptional therapeutic relevance, and drugs that can be used only in hospital settings, the request can be submitted to AIFA immediately after the CHMP positive opinion. After an administrative check and a preliminary assessment, the P&R dossiers are evaluated by the Technical Scientific Commission (Commissione Tecnico Scientifica, CTS) and thereafter by the Prices and Reimbursement Committee (Comitato Prezzi e Rimborso, CPR) (2).
The CTS evaluates medicinal products from a clinical point of view: it establishes the treatment place in therapy, identifies the comparators and evaluates, when the MAH submits a request, if a product is innovative according to AIFA criteria (unmet therapeutic need, therapeutic added value, and quality of evidence) (3). The same commission establishes whether a medicinal product will be reimbursed by the Italian National Healthcare Service (NHS) and the class of reimbursement (class A, H, or C when not reimbursed). Afterward, if the CTS gives a positive opinion on the reimbursement of the product, the CPR carries out the activity of negotiation with pharmaceutical companies for setting the price and other conditions (managed entry agreements [MEAs], confidential discounts) for medicinal products reimbursed by the NHS (class A or H). In case of unreimbursed drugs (class C), the MAH will communicate their price to AIFA. The CPR examines the submissions considering the CTS’ assessments, compares the treatment cost with other therapeutic alternatives, and assesses the drugs’ potential market share uptake in the upcoming years, as well as its market value in other European countries (2). The national process ends with the validation of the agreement between AIFA and the MAH by the Board of Directors and the publication of the P&R resolution in the Italian Official Journal (4). Except for drugs recognized as innovative (5), additional approval steps are required to gain access at the regional level.
EMA authorized drugs, awaiting AIFA P&R assessment, are temporarily classified in “class C non-negotiated” (Cnn), meaning they can be commercialized without being reimbursed by the Italian NHS (6). Since this process is fully independent from the P&R procedure and the commercialization in Cnn class is a company’s choice, these drugs are usually not included in any consideration about time to availability or time to market (TTM), which considers the end of the P&R process.
In the period 2016-2019, Italian time to availability, defined as the number of days from European market authorization to reimbursement in the member state, was below the European average (418 days vs. 506 days) and the rate of availability, defined as the number of medicines available to patients with respect to the number of medicines approved by EMA, was among the highest (75%) of all member states (7). Still, time to availability varies significantly, reaching a maximum over 3 years, and overall increased in the last years (7).
Since 2020, with the outbreak of the COVID-19 pandemic, more attention was placed on the drugs’ approval process and the awareness on the relevance of rapid decision-making within the healthcare framework increased.
This study builds on existing evidence published by the same coauthors in abstract form (8) and aims at assessing the impact that COVID may have had on the reimbursement and negotiation process of drugs in Italy in terms of TTM, defined as the number of days between the beginning of the drug’s P&R dossier evaluation by the CTS and the day of the publication of the P&R resolution in the Italian Official Journal.
Methods
Data
To evaluate patient access during the COVID-19 period, data were collected from an IQVIA proprietary database on Italian negotiation dynamics. The database was updated with respect to the previous study (8) and includes all the new active substances receiving a CHMP positive opinion from January 2015 to December 2021, comprehensive of all further extensions of therapeutic indications, new pack sizes, and reimbursement conditions’ renegotiation.
For each drug pack the database collects information on over 100 variables that can be clustered in three groups, namely drug and disease, process, and outcome:
• Drug and disease variables refer to drug brand, molecule and company, therapeutic area, number of indications in Italy and Europe, drug use in terms of line of treatment and combinations, orphan drug designation, population size and median progression-free survival (PFS) (only available for oncological and onco-hematological drugs in the database);
• Process variables include dates of CHMP opinion, EMA authorization, CTS and CPR start and end, P&R resolution publication on the Italian Official Journal; moreover, they include details on innovativeness, inclusion in the list ex law 648/1996, activation of compassionate use program, and 5% AIFA fund;
• Negotiation outcome variables collect information on number of milligrams and units per pack, administration mode, official price, reimbursement class, lawful and confidential discount, presence of AIFA monitoring registry and of MEAs, prescription rules, and first regional sell-out date.
Data were gathered from the EMA website, the reports of the CTS and CPR meetings, the analysis of the administrative acts of marketing authorization and reimbursement published in the Italian Official Journals, relevant literature, and IQVIA proprietary sales data.
For this analysis we were interested in the TTM for each indication of a drug that completed the Italian negotiation pathway. Duplicates in terms of pack or formulation changes, price renegotiations and drugs not negotiated (class Cnn) were excluded from the analysis; therefore, each observation of the analyzed database corresponds to a unique combination of molecule and indication. Given the exceptional circumstances of the pandemic, vaccines were also excluded from the analysis.
To assess the impact of COVID-19 on TTM of a drug, we defined three different periods, based on the beginning of the drug’s negotiation pathway (identified with the first CTS evaluation), end of the negotiation pathway (identified with the P&R resolution publication on the Italian Official Journal), and the beginning of the COVID-19 pandemic (identified with March 2020).
Each observation was assigned to:
• the “Pre COVID” period, when the negotiation pathway finished before the beginning of the pandemic;
• the “Partially COVID” period, when the negotiation pathway started before the beginning of the pandemic and finished during the COVID period;
• the “Fully COVID” period, when the negotiation pathway started after the beginning of the pandemic.
In Figure 1 an illustrative example of the identified periods is reported.
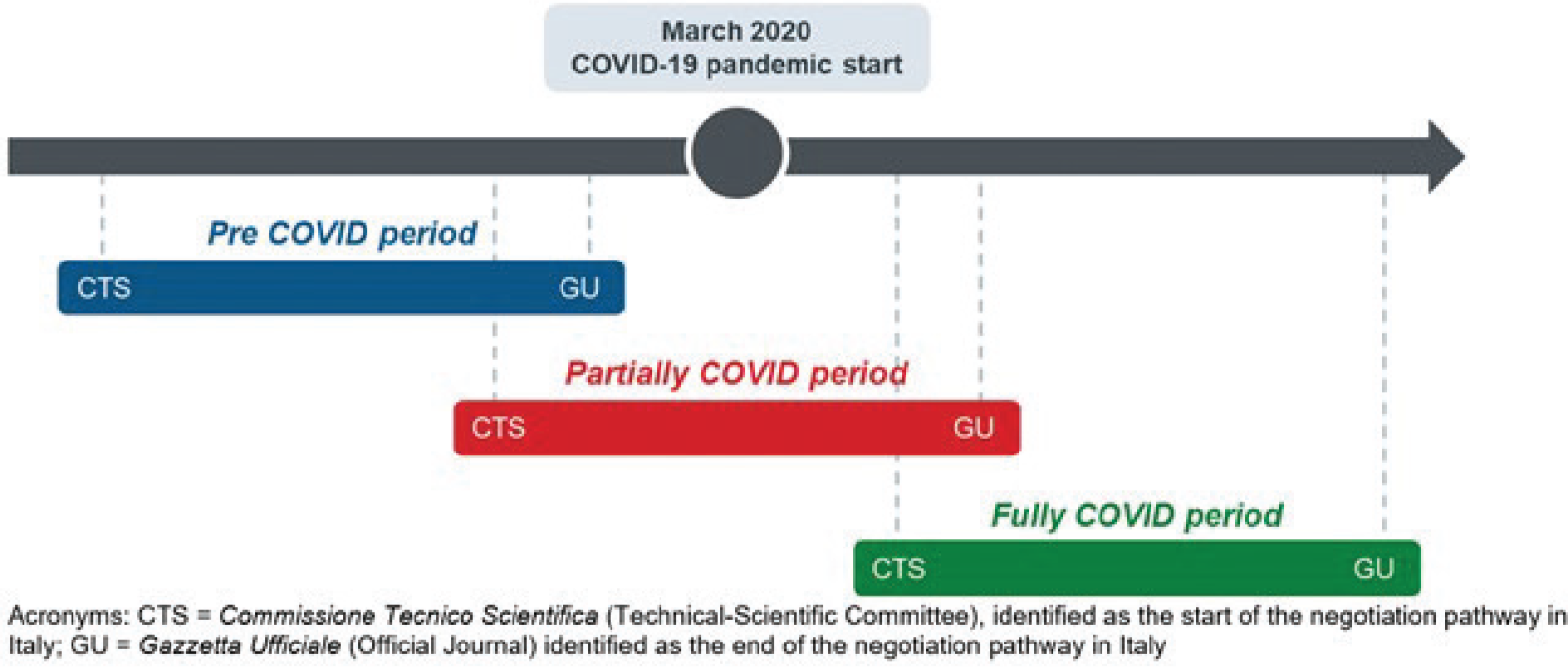
Fig. 1 - Identified periods according to drugs’ negotiation pathway and COVID-19 pandemic start.
Statistical analysis
Statistical analysis is divided into three phases: a preliminary phase to validate the meaningfulness of the research question, a descriptive phase, and an inferential phase. All analyses were conducted using Stata/MP 14 software.
In the preliminary phase differences of TTM among the three periods (Pre COVID, Partially COVID, and Fully COVID) were assessed to visually inspect differences in TTM. Kernel density graphs were plotted using the width of the density window that minimizes the mean integrated squared error if the data were Gaussian and a Gaussian kernel were used (9).
In a second phase, descriptive statistics of possible associated factors were calculated for the overall sample and by COVID period. Furthermore, for each factor a two-sample test was performed to test the null hypothesis that mean TTM is equal between drugs identified by the factors, against the alternative hypothesis that it differs. Factors for which the test revealed that mean TTM differs in a statistically significant way (p-value ≤ 0.10) were identified as relevant for the analysis.
Finally, in the inferential phase, observations across the three periods were matched with each other based on the covariates identified as relevant through the two-sample test in the previous phase.
A nearest-neighbor matching estimator was implemented to impute the missing potential outcome (i.e., TTM) for each observation by using an average of the outcomes of similar drugs that were negotiated in a different COVID period, based on a weighted function of the covariates for each observation. This method allows to reduce the heterogeneity bias due to differences in associated factors across the population and thus to estimate the average treatment effect (ATE) of the pandemic on drugs’ TTM, that is, the change in average TTM attributable solely to the drug negotiation period, independently of other associated factors. In particular, the ATE is computed by taking the average of the difference between the observed and imputed potential outcomes for each subject (10).
To test the solidity of results, various sensitivity analyses were conducted. First, different specifications of the matching estimator model were explored, by setting the number of minimum matches equal to 5 and to 10, and by adding the bias-adjustment option, which removes part of the heterogeneity bias that remains after matching (11). Second, to reduce the selection bias derived from the truncation of the post-COVID period, the analysis was performed only on data from May 2018, that is, setting the Pre COVID period equal to the post-COVID period. Lastly, since currently published literature studying average TTM (12,13,14,15) only refers to reimbursed drugs (class A and H drugs), we performed a sensitivity analysis excluding approved but not reimbursed medical products (class C drugs).
Results
Between January 2015 and December 2021, 380 drugs1 received a positive CHMP opinion, for a total of 561 indications. Of these, 363 passed the inclusion criteria for our analysis, being a unique combination of molecule and indication, excluding vaccines (N = 18) and non-negotiated indications at the time the analysis was conducted (N = 180), either because they had not started the negotiation process or because the negotiation process was ongoing at the time the analysis was conducted.
Indications negotiated before the COVID-19 pandemic represented the most numerous group (N = 174), followed by indications that started their negotiation after the COVID-19 pandemic onset (N = 123), and by indications starting the negotiating process before the COVID-19 pandemic onset but finishing it after (N = 69). In the Fully COVID period, the proportion of indications negotiated as first indication was lower than in the overall sample (60.2% vs. 66.4%), as well as proportion of indications receiving AIFA’s monitoring registry, negotiating a MEA or a confidential discount (37.6% vs. 42.1%, 13.7% vs. 19.5%, and 65.3% vs. 70.6%, respectively), and the proportion of indications assessed with AIFA’s accelerated procedure (5.3% vs. 10.1%).
Details on analyzed drugs according to the start of their negotiation process with respect to COVID-19 pandemic onset are reported in Table I.
| No. of observations | All | Pre COVID | Partially COVID | Fully COVID |
|---|---|---|---|---|
| 366 | 174 | 69 | 123 | |
| First indication | 66.4% | 77.2% | 50.7% | 60.2% |
| Early access program* | 27.8% | 25.7% | 29.0% | 30.1% |
| Innovativeness status | 74.7% | 78.9% | 68.1% | 72.4% |
| AIFA Monitoring Registry | 42.1% | 45.0% | 42.6% | 37.6% |
| Presence of MEA | 19.5% | 21.9% | 23.5% | 13.7% |
| Negotiation of confidential discount | 70.6% | 72.4% | 75.4% | 65.3% |
| Orphan medicine | 24.1% | 23.5% | 23.1% | 25.4% |
| EMA conditional approval | 4.6% | 3.6% | 4.6% | 6.1% |
| EMA exceptional circumstances | 2.0% | 1.2% | 6.2% | 0.9% |
| AIFA accelerated assessment | 10.1% | 12.0% | 13.8% | 5.3% |
| Advanced therapy | 2.0% | 3.0% | 1.5% | 0.9% |
| Monotherapy | 29.3% | 29.5% | 33.8% | 26.3% |
| Orphan disease | 33.1% | 33.3% | 37.7% | 30.1% |
| Therapeutic area | ||||
| Blood and immune system | 9.6% | 11.7% | 8.7% | 7.3% |
| Digestive system | 2.5% | 2.9% | 2.9% | 1.6% |
| Endocrine and metabolic | 13.2% | 12.9% | 17.4% | 11.3% |
| Infectious and parasites | 10.5% | 12.3% | 5.8% | 10.6% |
| Musculoskeletal | 2.8% | 2.3% | 0.0% | 4.9% |
| Oncology | 37.2% | 38.0% | 40.6% | 34.1% |
| Other | 24.2% | 19.9% | 24.6% | 30.1% |
AIFA = Agenzia Italiana del Farmaco, Italian Medicines Agency; EMA = European Medicines Agency; MEA = managed entry agreements.
*Early access programs considered are Law 648, 5% fund, and compassionate use.
The main objective of our study was to assess the impact of the COVID-19 pandemic on TTM. From the graphical representation of TTM by period (Fig. 2), some degree of heterogeneity emerges especially when visually inspecting the Partially COVID period. Moreover, descriptive statistics confirmed these results: indeed, average TTM for drugs receiving a positive CHMP opinion from January 2015 to December 2021 was 309.1 days (standard deviation [SD] = 179.0), with the lowest TTM for Fully COVID drugs (266.6 days, SD = 141.3) and the highest TTM for Partially COVID (425.8 days, SD = 250.6). Pre COVID average TTM was 289.5 days (SD = 146.4).

Fig. 2 - Kernel density function of drugs’ time to market, by COVID-19 period.
After inspecting differences in TTM visually, univariate analyses were run on the variables that were identified as associated factors of TTM: variables signaling the innovativeness of the drug (accelerated assessment, advanced therapy, innovative status), unmet needs (captured by the index variable early access, orphan medicine, orphan disease), drug characteristics (monotherapy, first indication, therapeutic area), and complexity of negotiation outcome (presence of an AIFA’s monitoring registry, negotiation with confidential discount, MEA). For these variables, a two-sample test analysis on mean TTM was performed (Tab. II).
Finally, considering the significant variables in Table II, the nearest matching estimator was implemented (Tab. III). For the first analysis (Partially COVID vs. Fully COVID period) 4 observations were excluded as they were found to have no exact matches, and the matching was performed on 224 observations, using a minimum of 1 match per observation to a maximum of 27; for the second analysis (Pre COVID vs. Fully COVID period), the matching was performed on 272 observations, using a minimum of 1 match per observation to a maximum of 21.
| Mean TTM (days) | p-Value | ||
|---|---|---|---|
| NO | YES | ||
| Fully COVID* | 289.49 | 266.64 | 0.09 |
| Partially COVID* | 289.49 | 425.84 | 0.00 |
| EMA conditional approval | 309.58 | 322.50 | 0.78 |
| EMA exceptional circumstances | 305.37 | 542.57 | 0.00 |
| AIFA accelerated assessment | 311.24 | 300.83 | 0.75 |
| Advanced therapy | 309.91 | 323.43 | 0.84 |
| Monotherapy | 313.39 | 302.43 | 0.61 |
| First indication | 300.04 | 311.52 | 0.56 |
| Orphan medicine | 299.01 | 345.46 | 0.04 |
| Early Access Program** | 298.45 | 331.55 | 0.11 |
| Innovative status | 320.13 | 303.43 | 0.44 |
| AIFA monitoring registry | 288.54 | 336.49 | 0.01 |
| Presence of MEA | 294.81 | 366.16 | 0.00 |
| Negotiation of confidential discount | 273.98 | 321.67 | 0.02 |
| Orphan disease | 297.78 | 327.68 | 0.13 |
| Therapeutic area*** | – | – | 0.00 |
Statistically significant variables (p ≤ 0.1) in bold.
AIFA = Agenzia Italiana del Farmaco, Italian Medicines Agency; EMA = European Medicines Agency; MEA = Managed Entry Agreements; TTM = time to market.
*Tested against Pre COVID; **Early access programs considered are Law 648, 5% fund, and compassionate use; ***Categorical variable tested with analysis of variance (ANOVA).
| ATE (days) | SE | z | p-value | Lower bound
(95% CI) |
Upper bound
(95% CI) |
No. of observations | Matches requested (min-max) | |
|---|---|---|---|---|---|---|---|---|
| Partially COVID vs. Pre COVID | 108.04 | 38.02 | 2.84 | 0.00 | 33.51 | 182.56 | 224 | 1 (1–27) |
| Fully COVID vs. Pre COVID | −16.00 | 19.52 | −0.82 | 0.41 | −54.27 | 22.26 | 272 | 1 (1–21) |
ATE = average treatment effect; CI = confidence interval; SE = standard error.
From the matching analyses, the impact of COVID-19 emerges as statistically significant only for the Partially COVID period, for which the ATE vs. the Pre COVID period was estimated at 108.04 days (p = 0.00). On the other hand, while the impact of the Fully COVID period tested against the Pre COVID period is still negative as in the univariate analysis (Tab. II), it loses significance when confounding factors are considered (ATE = −16.00, p = 0.41).
The increase in duration of the negotiation processes that started before COVID-19 and were concluded during the COVID-19 period and its subsequent reduction for negotiations that both started and ended after the beginning of the COVID-19 pandemic may be explained by a temporary shock that involved both the regulator and pharmaceutical companies. On the one hand, AIFA had to face extraordinary epidemiological circumstances: according to the law decree 18/2020, article 17, the CTS was asked to evaluate all data from experimental studies and compassionate use programs of medicinal products indicated for COVID-19 (16), which resulted in additional burden for the commission. On the other hand, the pharmaceutical sector was affected, along with all other sectors, by the restrictive measures that Italy faced in 2020, which had an impact on the business processes of pharmaceutical companies.
In all sensitivity analyses, a significant ATE can still be associated with the Partially COVID period. TTM of drugs approved in the Pre COVID period did not show significance compared to drugs approved in the Post COVID period in the bias-adjusted analysis or the analyses performed on drugs reimbursed from May 2018 and on class A and H drugs only, while its coefficient gained significance when increasing the minimum number of matches requested per observation (Tab. IV).
| ATE (days) | p-value | No. of observations | Matches requested (min-max) | |
|---|---|---|---|---|
| Partially COVID vs. Pre COVID | ||||
| Baseline | 108.04 | 0.00 | 224 | 1 (1–27) |
| Matches requested = 5 | 123.36 | 0.00 | 193 | 5 (5–31) |
| Matches requested = 10 | 139.29 | 0.00 | 171 | 10 (10–32) |
| Bias-adjustment option | 101.38 | 0.00 | 224 | 1 (1–27) |
| Pre COVID period = Fully COVID period | 96.35 | 0.02 | 70 | 1 (1–13) |
| Class A and H | 102.98 | 0.00 | 197 | 1 (1–27) |
| Fully COVID vs. Pre COVID | ||||
| Baseline | −16.00 | 0.41 | 272 | 1 (1–21) |
| Matches requested = 5 | −30.37 | 0.12 | 255 | 5 (5–25) |
| Matches requested = 10 | −34.96 | 0.10 | 231 | 10 (10–28) |
| Bias-adjustment option | −18.85 | 0.34 | 272 | 1 (1–21) |
| Pre COVID period = Fully COVID period | 0.82 | 0.98 | 106 | 1 (1–16) |
| Class A and H | −5.03 | 0.80 | 229 | 1 (1–21) |
ATE = average treatment effect.
Conclusions
This study estimated the impact of COVID-19 on TTM, defined as the number of days between the drugs’ first assessment by the CTS and the P&R resolution published in the Italian Official Journal.
It was found that TTM first increased (+108 days, p = 0.00) with the COVID-19 pandemic outbreak and then settled on levels not significantly different from the Pre COVID period, suggesting that a return to the status quo was reached after an adjustment period. The initial increase in TTM might be attributable to a temporary shock that affected both the regulator and the pharmaceutical sector.
Results were confirmed by a set of sensitivity analyses, varying both the model specifications and the frame of the analysis.
This study builds on evidence published by the same coauthors (8), by applying the same methodology to an updated version of the same database and expanding the timeframe of analysis to indications that received a CHMP positive opinion up until December 2021. In the study, we assessed TTM according to different time waves, implementing an inferential statistical method that allowed the estimation of the impact of the COVID-19 outbreak while controlling for other covariates. This analysis was made possible by the richness of the IQVIA proprietary database on negotiation dynamics, where variables on the drug and disease, process, and negotiation outcomes from January 2015 are systematically collected and updated.
Moreover, it contributes to the existing literature providing an update about the average TTM of drugs in Italy and of the main determinants of AIFA decisions (12,13,14,15).
The result of the present study in terms of mean TTM (309.1 ± 179.0 days) is aligned with previously published literature. Lidonnici et al (13) reported 258 days as average TTM, considering a shorter period than our study (2015-2018) and only including reimbursed drugs (class A and H). In a later publication based on the same data, Raimondo et al (15), found that TTM increased to 287 days in the 2018-2020 three-year period. Similarly, Prada et al (12,14), only including reimbursed new active substances approved from 2014 to 2019, estimated an average of 228 days and, when focusing on the reimbursed oncological drugs, estimated a time to reimbursement of 248 days.
On the other hand, there is uncertainty about the association of some explanatory variables with negotiation outputs. Indeed, as in our study, Lidonnici et al (13) confirmed the significant impact of the therapeutic area and the significant negative impact of the registry presence on TTM, while Prada et al (14) recognized drug innovativeness as the only significant element in the negotiation.
In addition to the uncertainty about the association of some explanatory variables with negotiation outputs, this study presents some limitations. First, some variables that might influence TTM, such as the initial drug price proposed by the company in the negotiation, were not publicly available and could not be assessed. While the impact of high initial proposed pricing should at least still be partially captured by the dichotomous variable of confidential discount, which is a negotiation tool to decrease drug acquisition costs, other aspects that could have an impact on negotiation outcomes remain unexplored. For example, Russo et al (17) find that incremental cost-effectiveness ratio (ICER) resulting from the economic evaluation of a drug is a predictor of its final price outcome, and it might play a role in the drugs’ TTM as well.
Secondly, there could be a selection bias in the considered drugs. In particular, we included all the drugs for which the P&R resolution was published on the Italian Official Journal: indeed, this might bring to underestimate the TTM of those drugs that received positive CHMP opinion recently and still hadn’t had their P&R resolution published on the Italian Official Journal. The implementation of a matching estimator aimed at reducing the selection bias led to a more reliable estimate of the impact of COVID-19 on the time of reimbursement. Nevertheless, future research on the topic would benefit from a longer timeframe of analysis, which would allow to estimate variations in TTM with a smaller confidence interval.
Finally, it should be considered that there is additional time elapsing between submission of the P&R dossier and initiation of the assessment process, which was not factored into this study. Lidonnici et al (13) estimated that this procedural step between 2015 and 2017 lasted on average 93 days with a great variability (between 23 and 237 days).
To conclude, despite some limitations, this study provides insights on the COVID-19 pandemic’s impact on AIFA negotiation outcomes and on TTM. Moreover, it updates and reinforces the current literature about drug time to reimbursement in Italy.
Acknowledgments
Original research
Part of the results of this research were presented at the ISPOR 2022 conference in Vienna and published in abstract form in the journal Value in Health by the same authors of this manuscript.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Guidelines for pricing and reimbursement of medicines (determina DG/1372/2020) [Internet]. Agenzia Italiana del Farmaco; Online (Accessed January 2023)
- 2. Commissioni consultive e tecnico-scientifiche [Internet]. Agenzia Italiana del Farmaco; Online (Accessed January 2023)
- 3. Criteri di valutazione dell’innovatività. Agenzia Italiana del Farmaco; Online (Accessed January 2023)
- 4. GU Serie Generale n.185 del 24-07-2020. Criteri e modalità con cui l’Agenzia italiana del farmaco determina, mediante negoziazione, i prezzi dei farmaci rimborsati dal Servizio sanitario nazionale Online (Accessed January 2023)
- 5. GU Serie Generale n.263 del 10-11-2012 - Suppl. Ordinario n. 201. Disposizioni urgenti per promuovere lo sviluppo del Paese mediante un più alto livello di tutela della salute. Online (Accessed January 2023)
- 6. GU Serie Generale n.263 del 10-11-2012 – Suppl. Ordinario n. 201. Legge 189/2012, Art. 5. Sect. 2012;5:189. Online (Accessed January 2023)
- 7. Newton M, Scott K, Troein P. EFPIA patients W.A.I.T. indicator 2020 survey. Online (Accessed January 2023)
- 8. Fiorentino F, Canali B, Candelora L, et al. HPR108 impact of COVID-19 pandemic on the decision process of the Italian Medicine Agency: a quantitative assessment. Value Health. 2022;25(12):S251-S252. Online
- 9. Silverman BW. Density estimation for statistics and data analysis. 1st ed. London: Chapman & Hall; 1986. Online (Accessed January 2023)
- 10. Abadie A, Imbens G. Simple and bias-corrected matching estimators for average treatment effects. NBER. 2002; Technical Working Paper No. 283:57. Online
- 11. Abadie A, Drukker D, Herr JL, Imbens GW. Implementing matching estimators for average treatment effects in Stata. Stata J. 2004;4(3):290-311. CrossRef
- 12. Prada M, Ruggeri M, Sansone C, De Fazio D, Tettamanti A, Mantovani M. Timeline of authorization and reimbursement for oncology drugs in Italy in the last 3 years. Med Access Point Care. 2017;1(1):e29-e36. CrossRef
- 13. Lidonnici D, Ronco V, Isernia M, et al. Tempi di accesso ai farmaci in Italia nel periodo 2015-2017: Analisi delle tempistiche di valutazione dell’Agenzia Italiana del Farmaco. Glob Reg Health Technol Assess. 2018;1-9. CrossRef
- 14. Prada M, Rossi L, Mantovani M. Time to reimbursement and negotiation condition in Italy for drugs approved by the European Medicines Agency during the period 2014-2019. AboutOpen. 2020;7(1):89-94. CrossRef
- 15. Raimondo P, Casilli G, Isernia M, et al. AIFA time-to-reimbursement: a comparison between the last two committees from 2015 to 2020. Glob Reg Health Technol Assess. 2020;7(1):109-114. CrossRef PubMed
- 16. Gazzetta Ufficiale. Decreto legge 18/2020, Art. 17. Sect. 2020;17:18. Online (Accessed January 2023)
- 17. Russo P, Zanuzzi M, Carletto A, Sammarco A, Romano F, Manca A. Role of economic evaluations on pricing of medicines reimbursed by the Italian National Health Service. Pharmacoeconomics. 2023;41(1):107-117. CrossRef PubMed
Fotnoter
- 1 Excluding biosimilars and generic, hybrid, and informed consent drugs.