 |
Glob Reg Health Technol Assess 2022; 9: 82-90 ISSN 2283-5733 | DOI: 10.33393/grhta.2022.2449 ORIGINAL RESEARCH ARTICLE |
 |
Cost-benefit analysis of ALK diagnosis vs. non-diagnosis in patients with advanced non–small cell lung cancer in Spain
ABSTRACT
Introduction: In recent years, target therapies to specific molecular alterations in advanced non–small cell lung cancer (NSCLC) have been identified and have shown superior efficacy compared to non-targeted treatments. Anaplastic lymphoma kinase (ALK) is one of the therapeutic targets; nevertheless, ALK diagnosis is not performed in all NSCLC patients in Spain. The objective of this study is to estimate in monetary terms the benefit for the Spanish society of ALK diagnosis in advanced NSCLC patients.
Methods: A cost-benefit analysis of ALK diagnosis vs. non-diagnosis in advanced NSCLC patients was carried out from the Spanish social perspective, with a time horizon of 5 years. Costs, benefits and the cost-benefit ratio were measured. The analysis has considered the overall survival in advanced NSCLC patients treated with the ALK-tyrosine kinase inhibitor (TKI) alectinib. The natural history of NSCLC was simulated using a Markov model. A 3% discount rate was applied to both costs and benefits. The result was tested using a deterministic sensitivity analysis.
Results: The cost of ALK diagnosis vs. non-diagnosis in the base case would be €10.19 million, generating benefits of €11.71 million. The cost-benefit ratio would be €1.15. In the sensitivity analysis, the cost-benefit ratio could range from €0.89 to €2.10.
Conclusions: The results justify the universal application of ALK diagnosis in advanced NSCLC, which generates a benefit for Spanish society that outweighs its costs and allows optimal treatment with targeted therapies for these patients.
Keywords: ALK, Cost-benefit, Cost-effectiveness, Economic evaluation, Lung cancer, Non–small cell lung cancer
Received: July 4, 2022
Accepted: August 4, 2022
Published online: September 12, 2022
This article includes supplementary material
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2021 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Lung cancer (LC) is the most diagnosed cancer worldwide, with more than 2 million new cases in 2018, being the leading cause of death from malignancy (1). In Spain, LC is the fourth most diagnosed cancer, with an estimated incidence of 29,549 new cases in 2021. Due to its high mortality, its 5-year prevalence is low (35,815 patients in 2020) (2). Despite latest therapeutic advances, more than half of LC diagnosed patients die within 1 year of diagnosis and the 5-year survival is approximately 18% (3). Non–small cell lung cancer (NSCLC) accounts for approximately 85% of all LC (4).
Several molecular alterations have been identified in NSCLC, including rearrangements in the anaplastic lymphoma kinase (ALK) gene, which are present in about 5% of NSCLC (5). More than 40% of ALK-positive (ALK+) patients have brain metastasis (BM) at diagnosis, presenting a worse prognosis (6).
In patients with advanced NSCLC, targeted therapies based on driver genes improve survival (7). ALK-tyrosine kinase inhibitors (TKIs) such as alectinib, crizotinib, ceritinib, brigatinib and lorlatinib have been developed and authorized for the treatment of ALK+ NSCLC patients (8).
Treatment with ALK-TKIs relies on needs to perform a previous diagnostic test on NSCLC patients. From biopsy or cytological samples, the diagnostic test can identify various genetic alterations, including ALK rearrangements (9).
Therefore, in order to evaluate the efficiency of ALK diagnosis, the aim of this study was to estimate, in monetary terms, the benefit for the Spanish society of the ALK diagnosis in NSCLC patients vs. non-diagnosis.
Methods
A cost-benefit analysis (CBA) was carried out from the Spanish social perspective, with a 5-year time horizon and a 3% discount rate (10). To calculate the cost-benefit ratio, the benefit of ALK diagnosis in NSCLC patients was compared with non-diagnosis of ALK, regarding the additional cost of performing it.
According to the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) clinical practice guidelines published at the time of this analysis, alectinib is the preferred first-line treatment option for advanced ALK+ NSCLC patients. Therefore, it was assumed that these patients would be treated with alectinib. For non-diagnosed patients, it was assumed that they would be treated with chemotherapy and/or immunotherapy, which is the standard treatment in patients with unknown driver mutations (11,12).
The natural history of the disease was simulated using a Markov model based on three health states (stable, progression and death), with 6-month cycles (Fig. 1). The model starts with patients in stable state, every 6 months the patient state was reviewed to allocate the corresponding costs and benefits. States were modeled according to the alectinib survival curves of progression-free survival (PFS) and overall survival (OS), the non-diagnosed patient’s survival curves and the mortality risk in the general population by sex and age.
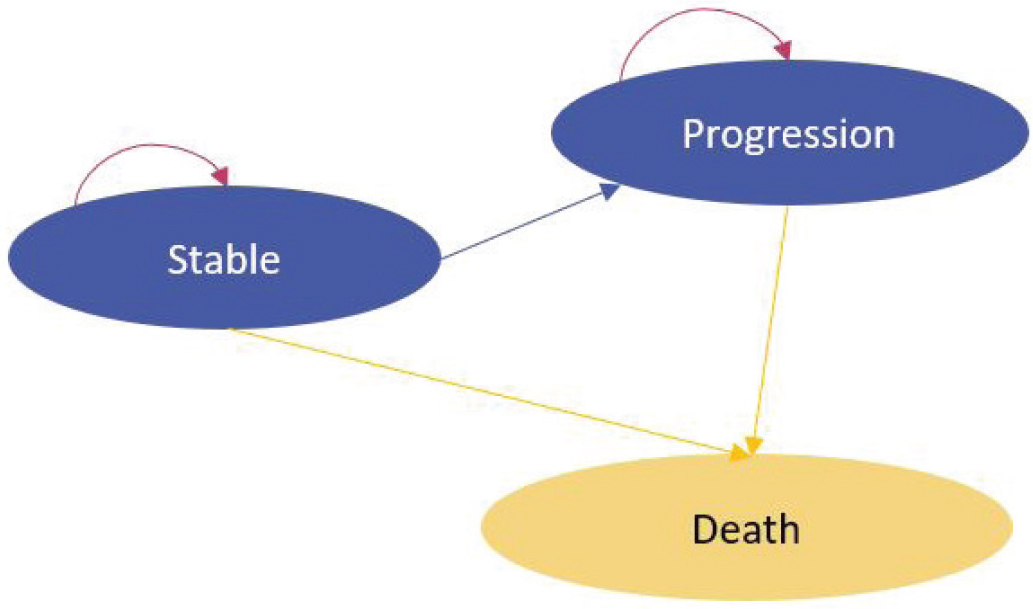
Fig. 1 - Markov model health states.
Data sources
Epidemiology data, survival rates, healthcare resource use, utilities, productivity and formal and informal care were obtained from a literature review, including national and international references (the latter were used whenever national data were not available). Databases consulted are included in Supplementary material (Supplementary Table S1). All extracted data were contrasted and validated by an expert group (a pathologist and three oncologists). To complete the necessary information about follow-up visits, medication, adverse events (AEs), disease progression and impact of ALK diagnosis on the different areas of patients’ lives, three telephone interviews were conducted with patients.
Furthermore, to quantify the tangible and intangible benefits, patients’ experience was considered, performing a focus group with seven patients and one caregiver in 2020.
Population
The candidate population (Fig. 2) for ALK diagnosis (7,724 patients) includes 28,833 patients diagnosed with LC in Spain in 2020 (13), with NSCLC (85%) (4) in stage IV (54.5%) (14), non-squamous (66.9%) and squamous (33.1%) (15) who have never smoked (16%) (16), and were tested for ALK (80.1%) (17). The target population (263 patients) was the subset of the candidate population for ALK diagnosis who obtained an ALK+ result (3.4%) in a molecular diagnostic test (17). The identification of ALK rearrangement allows the administration of personalized therapies that encompasses the strategy of matching this molecular subtype with effective targeted therapies, such as alectinib. The average age of ALK+ patients considered at diagnosis was 61 years, 40.6% were men, and 42.11% presented BM at diagnosis (Tab. I) (14).
The last updated median PFS in advanced ALK+ NSCLC patients treated with alectinib with and without BM in the ALEX trial was 25.4 and 38.6 months, respectively (6), and the last reported OS analysis of patients treated with alectinib is shown in Supplementary Table S2 (6). The median PFS and OS data of patients without molecular diagnosis have been estimated based on the median survival of patients treated with alectinib (6) and the hazard ratio (HR) of alectinib vs. chemotherapy estimated in a meta-analysis of ALK+ NSCLC patients (PFS HR: 0.23 [0.17; 0.030]; OS HR: 0.57 [0.39; 0.83]) (Tab. I and Supplementary Table S2) (18). The survival probability by age in the general population was estimated from all-cause mortality rates in the general population (Supplementary Table S3).
Cost and resource use
Costs were expressed in €2020, including direct healthcare costs (DHCs), direct non-healthcare costs (DNHCs) and indirect costs (ICs). Unit healthcare costs were the median value of the unit costs for each autonomous community in Spain (Median of the costs of the Official Gazettes of the Autonomous Communities, 2020). All prices corresponding to previous years were updated, using the general consumer price index (CPI) or medicines CPI (19).
DHCs included molecular diagnosis, imaging tests, drugs and their administration, palliative care, management of AEs and resources use. The diagnosis care process is shown in Supplementary Figure S1. It has been considered that ALK diagnosis was performed in parallel with LC diagnosis. The difference between groups was the re-biopsy when there was not enough tissue (77.1% of the invalid tests) (20), and also a first visit to the medical oncologist in the ALK diagnosis arm (21). The average cost of the ALK diagnosis test (€137.30), the cost of a consultation with the oncologist (€159.28), as well as the re-biopsy cost (€186.79) (Supplementary Tables S4 and S5) were also included in the ALK diagnosed arm.

Fig. 2 - Diagram of the target population for cost-benefit analysis (4,13-17). ALK = anaplastic lymphoma kinase; CBA = cost-benefit analysis; NSCLC = non–small cell lung cancer.
| Data inputs | References | |
|---|---|---|
| Epidemiology of ALK-positive NSCLC | ||
| ALK+ NSCLC incidence (n) | 263 | (4,13-17) |
| Progression-free survival | ||
| Median PFS in ALK diagnosed patients with BM (months) | 25.4 | (6) |
| Median PFS in ALK diagnosed patients without BM (months) | 38.6 | (6) |
| Median PFS in ALK non-diagnosed patients with BM (months) | 5.8 | (6,18) |
| Median PFS in ALK non-diagnosed patients without BM (months) | 8.9 | (6,18) |
| Patient characteristics | ||
| Male (%) | 40.6 | (14) |
| Mean age at diagnosis (years) | 61 | (14) |
| Mean weight (kg) | 71.08 | (14,51) |
| Body surface (m2) | 1.79 | (14,51) |
| Creatinine level (mg/dL) | 0.7 | (52) |
| BM at diagnosis (%) | 42.11 | (6) |
ALK = anaplastic lymphoma kinase; BM = brain metastasis; NSCLC = non–small cell lung cancer; PFS = progression-free survival.
The cost of ALK diagnosis test is applied to all the candidate population (7,724 patients), not just to the target population (263 patients). However, for the target population, resource use is different between ALK diagnosed and non-diagnosed patients, and depending on whether or not patients have BM (Supplementary Table S6). The unit DHCs are shown in Supplementary Table S5. The average annual cost for the ALK diagnosis arm was €4,907.72 per patient without BM and €15,054.61 per patient with BM. In the non-diagnosed ALK arm, costs were €4,625.11 and €14,605.79, respectively. In patients with BM, the costs related to neurosurgery (a neurosurgery consultation and hospitalization due to neurosurgery) were considered (€279.08). These costs are assumed only when performed (first 6 months).
The recommended drugs for the treatment of advanced NSCLC by ESMO guidelines and their market shares have been considered (Supplementary Table S7) (12). The respective dosages were obtained from the summary of product characteristics, the clinical trials submitted for the marketing authorization of the drug indication of interest, the ESMO Clinical Practice Guide (12) or the Therapeutic Positioning Guidelines of the Spanish Society of Hospital Pharmacy (SEFH) (Supplementary Table S8) (21). Drug costs were calculated using the list price (22), including Royal Decree Law 8/2010 deduction rate (23) and a 4% of the value-added tax (VAT) entitled for Spain (Supplementary Table S9). For intravenous drugs, the analysis also considered the cost of administration time for each drug in the day hospital (Supplementary Table S5). The administration time required is shown in Supplementary Table S10. The costs of treating AEs were also included, taking into account the different frequency and number of grade 1-2 and 3-4 AEs per year between the ALK diagnosed patients treated with alectinib and the non-diagnosed patients treated with chemotherapy and/or immunotherapy (Supplementary Table S11). The average unit cost of treating each AEs is shown in Supplementary Table S12.
The model also included the cost of palliative care. About 93.3% of patients received follow-up from primary care (PC) and 6.7% were assisted by a palliative home care team (24). The mean number of consultations required per week was 0.88, 0.38, 0.38 and 0.93 in home palliative care, PC physicians, PC nurses and nurse phone consultations, respectively (25). Supplementary Table S5 shows their unit costs. The drugs used in the palliative care phase were also considered (Supplementary Tables S13 and S14).
DNHCs included formal care (professional care financed by private or public funds), informal care (non-remunerated care from relatives/friends) and travel costs. About 0.50% of ALK diagnosed patients and 9.4% of non-diagnosed patients had a formal caregiver (26). Patients distinguished between informal care needs in the emotional sphere (support to face feelings such as fear, uncertainty, sadness, etc.) and in the functional sphere (help with limitations that affect functional activity and autonomy) (27). About 96.6% of patients, regardless of whether they were diagnosed or not, indicated that they had an informal caregiver related to the emotional sphere. However, no cost was assumed for that sphere. About 20% of diagnosed patients and 48.5% of non-diagnosed patients indicated that they had an informal caregiver in relation to the functional sphere (27). Based on interviews with patients, 2 hours of informal care was estimated in diagnosed patients. Based on published literature, 19.2 hours of informal care was used in non-diagnosed patients (28). A cost of €7.43/hour was assumed (minimum hourly wage for domestic workers) (29).
Traveling for follow-up visits, tests’ performance and day hospital for intravenous drugs administration implied a cost for patients, which amounts to €17.25 per trip (assuming an average 25 km/trip) (19,30-34). The number of day hospital visits for intravenous treatment administration was estimated depending on the type of treatment.
ICs included productivity loss (PL) due to attending consultations, performing tests and intravenous drug administration. The activity rate and the average salary of the general population were considered (Supplementary Table S15). The percentage of patients on sick leave at the time of diagnosis was 96.90% (35), assuming that 19% of the diagnosed patients would return to work after 4.5 months (36) and 11.20% of the non-diagnosed patients would return to work after 9 months (35). Based on this, the percentage of patients with NSCLC who work throughout the time horizon was estimated (Supplementary Table S16). It was assumed that, as the disease progresses, the percentage of patients on sick leave will increase. Based on the information obtained from the focus group and the patients’ interviews, a 1.5-hour PL was assumed for each test performed or visit to the hospital, including the traveling time and the time that they remained in the hospital. A PL was also assumed for visits to the day hospital to receive an intravenous treatment (Supplementary Table S10).
Benefits
The benefits in a CBA can be positive, negative, tangible, and intangible. To quantify intangible benefits, financial proxies (approximate value of something that does not have a specific market value) were used. Throughout the analysis, different benefits were detected related to: stigmatization and guilt of patients after diagnosis, quality of life (QoL) of patients and caregivers, impact of AEs in patients’ lives, time spent with the family and PL due to sick leave or premature death.
Patients with LC experience feelings of guilt and shame at the time of diagnosis (34.20%) (27,37). Based on the focus group and patients’ interviews, it was assumed that ALK diagnosis reduced this feeling by 75%. To quantify this benefit, it was assumed that patients suffering from these problems would need four visits to the psycho-oncologist after the diagnosis (in the first 6 months of the analysis).
The QoL loss associated with disease progression or death was estimated by utility loss as the disease progresses, which occurs earlier in the non-diagnosed patients (Supplementary Table S17). The economic proxy used was the cost-effectiveness threshold (cost per quality-adjusted life year [QALY] gained) recommended in Spain (€25,000) (38,39). After disease progression, patients who continued with active treatment (90% of diagnosed patients and 86.67% of non-diagnosed patients) lost 0.089 annual utilities, while patients who attended palliative care (10% of diagnosed and 13.33% of non-diagnosed patients) lost 0.344 annual utilities (40,41). Due to NSCLC mortality, both diagnosed and non-diagnosed patients lost an annual utility of 0.814 (40).
AEs, apart from generating a DHCs for their treatment, had an impact on different areas of patients’ lives: physical condition, self-care, autonomy, daily activities, leisure area, family and couple relationships, work environment, emotional sphere and care needs. These areas, as well as the percentage of patients affected, were identified through the focus group and patient interviews (Supplementary Table S18). According to the expert group, the impact of nausea and vomiting was considered only during the time of occurrence (3.5 days). Financial proxies used to quantify these impacts are shown in Supplementary Table S19.
Spending time with family has been recorded as a very important factor for cancer patients. Therefore, the time that patients who die are no longer with their family was quantified. In order to quantify this benefit, the analysis takes into account the minutes per day that couples spend doing activities together (42), which were quantified with a financial proxy (Supplementary Table S20).
Informal caregivers suffer an additional burden. After progression of the patients’ disease, there are changes in their treatment and worsening in their general condition. Therefore, informal caregivers have to spend more hours caring for these patients. Their mental and physical health worsens (43-45), as well as their social life (45). The benefits shown in Supplementary Table S21 were assumed per patient in disease progression with informal caregivers (96% had a caregiver in the emotional sphere in both groups and 20% and 48.5% of diagnosed and non-diagnosed patients had a caregiver in the functional sphere, respectively) (27). For the emotional sphere, the impact on mental and social health was included, and for the functional sphere the impact on health was included.
Finally, for those patients who were working at baseline, the PL due to disease progression or death was quantified as the lost wages (Supplementary Table S15).
Deterministic sensitivity analysis
A sensitivity analysis was carried out on the most relevant variables: 1) discount rate (0%; 5%); 2) price of ALK diagnosis tests (±20%); 3) HR of alectinib OS vs. chemotherapy OS (0.39; 0.83); 4) HR of alectinib PFS vs. chemotherapy PFS (0.17; 0.30); 5) cost per QALY gained (€22,000; €60,000); and 6) ±20% of the official rates of the autonomous communities in Spain.
Results
The cost results and the monetized benefits analyzed for diagnosed and non-diagnosed patients are detailed in Table II. The administration cost, the DHCs of treating AEs and the cost of formal and informal care were lower in the diagnosed patients’ arm, generating savings of €161,145, €625,958 and €1,111,823, respectively. Of the remaining additional costs, those that most affected the total cost were the anticancer drugs (67.74%), the follow-up (22.57%) and the ALK diagnosis (9.20%). The cost of traveling (visits, test and drug administration) and ICs had almost no effect in the total cost (Fig. 3A).
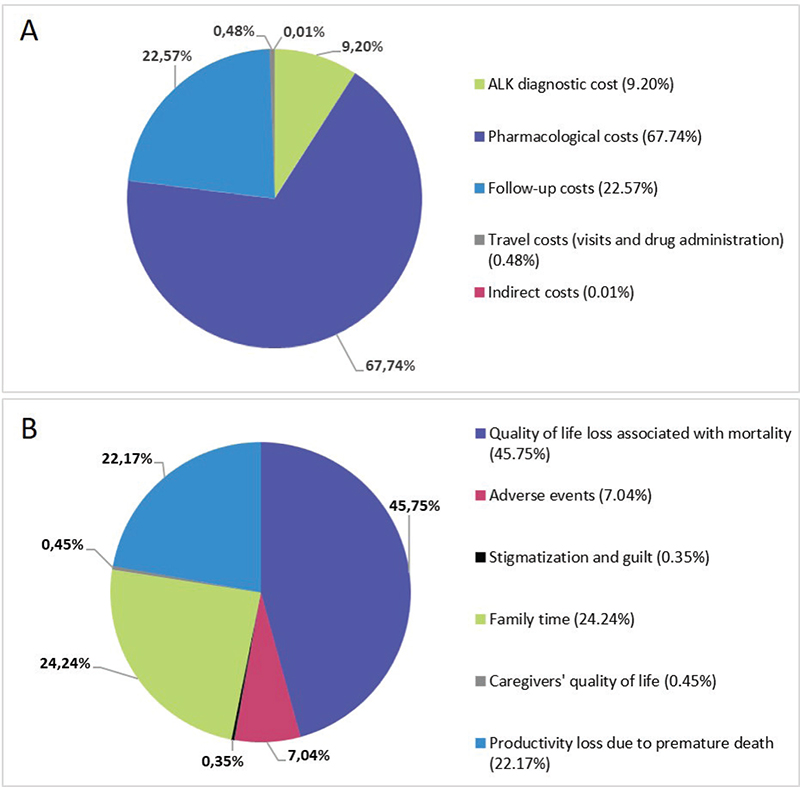
Fig. 3 - Distribution of additional costs (A) and benefits (B). ALK = anaplastic lymphoma kinase.
| Diagnosed patients | Non-diagnosed patients | Difference | |
|---|---|---|---|
| Costs | |||
| ALK diagnosis | €1,111,783 | €0 | €1,111,783 |
| Drugs | €50,792,934 | €42,602,890 | €8,190,044 |
| Drug administration | €104,629 | €265,774 | −€161,145 |
| Follow-up resources | €8,344,028 | €5,615,785 | €2,728,243 |
| Treating adverse events | €374,265 | €1,000,223 | −€625,958 |
| Formal care | €18,004 | €42,931 | −€24,927 |
| Informal care | €745,014 | €1,831,910 | −€1,086,896 |
| Traveling (visits and administration) | €422,944 | €364,589 | €58,355 |
| Productivity loss due to attendance visits and follow-up tests | €7,184 | €6,419 | €766 |
| Total costs | €61,920,785 | €51,730,521 | €10,190,265 |
| Benefits | |||
| Stigmatization and guilt | −€17,686 | −€63,540 | €45,854 |
| QoL loss associated with progression | −€513,869 | −€469,177 | −€44,692 |
| QoL loss associated with mortality | −€8,174,452 | −€14,183,585 | €6,009,133 |
| Adverse events | −€456,325 | −€1,381,624 | €925,299 |
| Time to spend with the family | −€4,214,901 | −€7,399,309 | €3,184,408 |
| QoL of caregivers | −€345,122 | −€404,806 | €59,684 |
| Productivity loss due to sick leave | −€6,880,171 | −€5,500,661 | −€1,379,510 |
| Productivity loss due to premature death | −€3,115,468 | −€6,027,573 | €2,912,105 |
| Total benefits | −€2,371,799 | −€35,430,276 | €11,712,282 |
| Cost-benefit ratio | €1.15 |
ALK = anaplastic lymphoma kinase; NSCLC = non–small cell lung cancer; QoL = quality of life.
The benefits related to utility loss associated with disease progression and the PL due to sick leaves were lower in the diagnosed patients’ arm than in the non-diagnosed arm (€44,692 and €1,379,510, respectively). Regarding the other benefits observed, the one that had the biggest impact was related to utility losses associated with mortality (45.75%) because the ALK diagnosed patients had a higher OS than the non-diagnosed patients (Fig. 3B). The next most important benefit was related to the PL due to premature death. Time spent with the family also had an important contribution (24.24%).
A cost increase of €10.19 million was obtained in the ALK diagnosed arm compared to the ALK non-diagnostic arm, which would generate benefits of €11.71 million. The cost-benefit ratio was €1.15.
Sensitivity analysis results
The results of the sensitivity analysis are shown in Figure 4. The most relevant parameters were the variations in OS (−13.58%; 82.78%) and PFS (−22.37%; 71.07%), as well as the cost per QALY gained (−6.11%; 71.29%). A total of 83% of the sensitivity analysis performed showed that the implementation of ALK diagnosis in NSCLC patients is cost-beneficial for society, demonstrating the robustness of the result obtained in the base case. This result could increase by 82.78% and reach a cost-benefit of €2.10.
Discussion
To our knowledge, this is the first study that has determined the CBA of ALK diagnosis in NSCLC patients. Until now, the economic evaluation of biomarker diagnosis in NSCLC has been studied only in terms of cost-effectiveness without showing a complete overview of their impact on patients’ lives and their informal caregivers (46). Furthermore, this is the first economic evaluation that includes intangible patient outcomes such as the stigmatization, the time that they lost with their families or the consequences that AEs have in all aspects of their daily life (self-care, autonomy, etc.).
Cost-effectiveness is a very common criterion by which to evaluate healthcare interventions. It compares costs with outcomes (effectiveness) in a long-term analysis. On the other hand, CBA, where both costs and outcomes are measured in monetary units is hardly used in the field of healthcare (47). The CBA can provide shorter-term results since it allows us to observe the social value of the healthcare intervention. For example, our analysis with a 5-year time horizon shows how the diagnosis of ALK rearrangements can affect relevant social aspects for the patient, such as leisure (travel, going to restaurants, theaters, etc.).
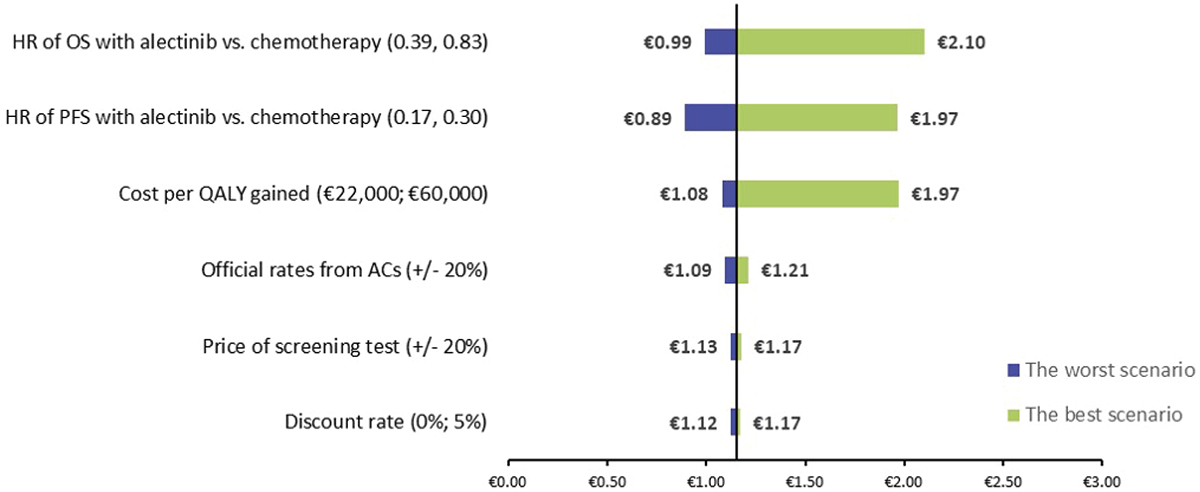
Fig. 4 - Tornado diagram of total cost-benefit ratio. ACs = autonomous communities; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; QALY = quality-adjusted life year.
The cost-benefit ratio of €1.15 obtained in this analysis means that for each €1 invested in ALK diagnosis in NSCLC patients, a social benefit of €1.15 would be obtained. The relevant benefits (QoL loss associated with mortality, time to spend with the family and PL due to premature death) are associated with an increased OS in patients treated with targeted therapies, reaching almost double. These results demonstrate that financing the ALK diagnosis by the Spanish National Health System would generate a benefit for Spanish society. Patients with NSCLC harboring ALK fusions can be successfully treated with ALK-TKIs that could substantially improve their QoL. Therefore, treatment with ALK-TKIs is recognized as the standard-of-care for these patients, with alectinib indicated by multiple national treatment guidelines as the preferred option until 2020 (6,11,48).
Several limitations must be acknowledged. The monetary value of some items in the study was scattered. Consequently, many data sources had to be considered in order to estimate a median. It was also necessary to make approximations or even assumptions for some items in which no accurate data were found. Secondly, the data obtained from the focus group of patients and caregivers may not be representative of the total number of patients and caregivers, given the small number of participants included. Another limitation of the study is the small number of interviews conducted with patients. However, it was not possible to include more patients or caregivers in the focus group, or interview more patients, due to the small number of patients with NSCLC ALK+ in Spain (n = 263). In addition, the real prices (financed prices) of the drugs have not been used since these are not public, so it has been decided to use the accessible prices (list prices). Finally, the absence of a probabilistic sensitivity analysis is also considered a limitation of the study.
Conclusions
The results suggest that ALK diagnosis in NSCLC is cost-beneficial, as it generates a benefit for the Spanish society that outweighs its costs, justifying the universal application of this diagnosis, which allows patients to be treated with effective and tailored options like targeted therapies.
Declarations
Ethics approval and consent to participate: The present study conforms with the ethical principles of the Declaration of Helsinki 1975/83. This study did not require approval by any ethics committee. According to the Spanish law (“Ley 14/2007, de 3 de julio, de Investigación biomédica” updated on June 2, 2011), research projects carried out on human beings or their biological material have to be approved by a Research Ethics Committee, excluding observational studies where any patient treatment or intervention is not modified (49). Moreover, according to the Spanish law (“Ley 41/2002, de 14 de noviembre, básica reguladora de la autonomía del paciente y de derechos y obligaciones en materia de información y documentación clínica el consentimiento informado” updated on December 6, 2018), the informed consent has to be signed only when the activity of the study can affect patients’ health status (50). As the present study did not affect the patients’ health status, it was not necessary for patients to sign an informed consent form.
Disclosures
Conflict of interest: MM reports speaker’s bureau and advisory board fees from Sanofi, Pfizer, Janssen, Bristol-Myers Squibb, MSD, Boehringer-Ingelheim, AstraZeneca, Roche, Kyowa Kyrin, Pierre Fabre, Takeda Pharmaceutical, and Bayer AG; and has received a research grant from Bristol-Myers Squib, outside the submitted work. FR has received consulting or advisory role fees from Roche, Pfizer, Novartis, BMS, Pierre Fabre, Incyte, Abbvie, Amgen, MSD and Lilly; and travel and accommodation support from Roche. RA has received consulting or advisory role fees from Pharmamar, Roche, Novartis, BMS, Bristol and Lilly; and travel and accommodation support from Roche and Pharmamar. LR, JFG and RG were working at Roche during the study. AGD, YIM and RSSC work at Weber, a company that received honoraria from Roche for the study. ALO declares that she has no competing interests.
Financial support: This study was funded by Roche Farma S.A., Spain.
Author’s contributions: All authors participated in the conception and design of the study. MM, RA, ALO, YIM, AGD, RSSC and FR participated in the acquisition of data. YIM, AGD and RSSC wrote the draft of the manuscript. All authors participated in the analysis and interpretation of data. All authors critically revised the manuscript for important intellectual content and approved the final version to be published.
Availability of data and materials: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. CrossRef PubMed
- 2. Sociedad Española de Oncología Médica (SEOM). Las cifras del cáncer en España. 2021. Accessed April 2021. Online
- 3. SEER. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Statistics Review, 1975-2016. Accessed May 2021. Online
- 4. Majem M, Juan O, Insa A, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol. 2019 Jan;21(1):3-17. CrossRef PubMed
- 5. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010 Oct 28;363(18):1693-1703. CrossRef PubMed
- 6. Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056-1064 CrossRef PubMed
- 7. Zhu QG, Zhang SM, Ding XX, He B, Zhang HQ. Driver genes in non-small cell lung cancer: characteristics, detection methods, and targeted therapies. Oncotarget. 2017;8(34):57680-57692. CrossRef PubMed
- 8. European Medicines Agency (EMA). Medicines [Internet]. European Medicines Agency. Accessed March 2020. Online
- 9. Update to the “Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors” [Internet]. IASLC. Accessed May 2021. Online
- 10. López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias [A proposed guideline for economic evaluation of health technologies]. Gac Sanit. 2010;24(2):154-170. CrossRef PubMed
- 11. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 3. 2020. Accessed May 2022. Online
- 12. Planchard D, Popat S, Kerr K, et al. Metastatic non-small-cell lung cancer. ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Updated version published 18 September 2019 by ESMO Guidelines Committee. European Society for Medical Oncology; 2019:71.
- 13. Sociedad Española de Onlología Médica (SEOM). Las cifras del cáncer en España 2020. Accessed April 2021. Online
- 14. Vidal J, Clavé S, de Muga S, et al. Assessment of ALK status by FISH on 1000 Spanish non-small cell lung cancer patients. J Thorac Oncol. 2014;9(12):1816-1820. CrossRef PubMed
- 15. Provencio M, Carcereny E, Rodríguez-Abreu D, et al. Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study). Transl Lung Cancer Res. 2019;8(4):461-475. CrossRef PubMed
- 16. de Castro J, Tagliaferri P, de Lima VCC, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl). 2017;26(6):e12734. CrossRef PubMed
- 17. Sociedad Española de Anatomía Patológica (SEAP). LungPath. Accessed April 2021. Online
- 18. Elliott J, Bai Z, Hsieh SC, et al. ALK inhibitors for non-small cell lung cancer: a systematic review and network meta-analysis. PLoS One. 2020;15(2):e0229179. CrossRef PubMed
- 19. Instituto Nacional de Estadística (INE). Índice de Precios de Consumo. Base 2016. Medias anuales. Índices nacionales: general y de grupos ECOICOP. INE. Accessed April 2021. Online
- 20. Chouaid C, Dujon C, Do P, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer. 2014;86(2):170-173. CrossRef PubMed
- 21. Sociedad Española de Farmacia Hospitalaria (SEFH). Grupos de trabajo. Docetaxel Monoterapia. 2005 [Internet]. Accessed May 2021. Online
- 22. Consejo General de Colegios Farmacéuticos. Botplusweb.portalfarma.com. BOT Plus 2. Base de Datos de Medicamentos [Internet]. Accessed May 2021. Online
- 23. Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Agencia Estatal Boletín Oficial del Estado. Accessed March 2020; Online
- 24. Alonso-Babarro A, Astray-Mochales J, Domínguez-Berjón F, et al. The association between in-patient death, utilization of hospital resources and availability of palliative home care for cancer patients. Palliat Med. 2013;27(1):68-75. CrossRef PubMed
- 25. Alonso-Babarro A, Bruera E, Varela-Cerdeira M, et al. Can this patient be discharged home? Factors associated with at-home death among patients with cancer. J Clin Oncol. 2011;29(9):1159-1167. CrossRef PubMed
- 26. Wood R, Taylor-Stokes G, Smith F, Chaib C. The humanistic burden of advanced non-small cell lung cancer (NSCLC) in Europe: a real-world survey linking patient clinical factors to patient and caregiver burden. Qual Life Res. 2019;28(7):1849-1861. CrossRef PubMed
- 27. Fundación más que ideas, Asociación Española de Cáncer de Pulmón. La esfera emocional y social del cáncer de pulmón. 2019. Accessed March 2020. Online
- 28. Nightingale CL, Steffen LE, Tooze JA, et al. Lung cancer patient and caregiver health vulnerabilities and interest in health promotion interventions: an exploratory study. Glob Adv Health Med. 2019;8:2164956119865160. CrossRef PubMed
- 29. Real Decreto BOE. 231/2020, de 4 de febrero, por el que se fija el salario mínimo interprofesional para 2020. [Internet]. Accessed May 2021. Online
- 30. DOG núm. 96, del 21 de mayo de 2014. DECRETO 56/2014, de 30 de abril, por el que se establecen las tarifas de los servicios sanitarios prestados en los centros dependientes del Servicio Gallego de Salud y en las fundaciones públicas sanitarias. 2014. Accessed March 2020. Online
- 31. DOCM núm. 226, del 21 de noviembre de 2014. Orden de 17/11/2014, de la Consejería de Sanidad y Asuntos Sociales, por la que se establecen los precios públicos de la asistencia sanitaria y de los servicios prestados en la red de centros sanitarios dependientes del Servicio de Salud de Castilla-La Mancha. [2014/15022] [Internet]. Accessed May 2021. Online
- 32. BORM núm. 133, del 11 de junio de 2020. Orden de 29 de mayo de 2020 de la Consejería de Presidencia y Hacienda, por la que se publican las tarifas de las tasas y precios públicos aplicables en el año 2020. Accessed March 2020. Online
- 33. BOPA núm. 77, del 4 de abril de 2013. Resolución de 25 de febrero de 2013, de la Consejería de Hacienda y Sector Público, por la que hace pública la relación de las cuantías exigibles por tasas y precios públicos en el ejercicio 2013. Accessed March 2020. Online
- 34. BOA núm. 156, del 10 de agosto de 2012. RESOLUCIÓN de 30 de julio de 2012, de la Dirección Gerencia del Servicio Aragonés de Salud, sobre revisión de las tarifas a aplicar por la prestación de servicios sanitarios a terceros obligados al pago o a usuarios sin derecho a asistencia sanitaria en la Comunidad Autónoma de Aragón. 2012. Accessed May 2021. Online
- 35. Molina Villaverde R, Feliu Batlle J, Jiménez Gordo AM, San José Valiente B. Actividad laboral en una cohorte de pacientes con carcinoma de pulmón. Med segur trab. 2012;58(226):6-12. https://dx.doi.org/10.4321/S0465-546X2012000100002.
- 36. Lister J, Stanisic S, Kaier K, Hagist C, Gultyaev D, Walzer S. Societal savings in patients with advanced non-squamous non-small-cell lung cancer receiving bevacizumab-based versus non-bevacizumab-based treatments in France, Germany, Italy, and Spain. Clinicoecon Outcomes Res. 2012;4:299-305. PubMed
- 37. Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ. 2004;328(7454):1470. CrossRef PubMed
- 38. Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? [What is an efficient health intervention in Spain in 2020?]. Gac Sanit. 2020;34(2):189-193. CrossRef PubMed
- 39. Ortega Eslava A, Marín Gil R, Fraga Fuentes MD, López-Briz E, Puigventós Latorre F. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos. GENESIS, Grupo de Evaluación de Novedades, Estandarización e Investigación en Selección de Medicamentos. SEFH, Sociedad Española de Farmacia Hospitalaria; 2017. Accessed March 2020. Online
- 40. Carlson JJ, Suh K, Orfanos P, Wong W. Cost effectiveness of alectinib vs. crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Pharmacoeconomics. 2018;36(4):495-504. CrossRef PubMed
- 41. Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6(1):84. CrossRef PubMed
- 42. Roman JG, Flood SM, Genadek KR. Parents’ time with a partner in a cross-national context: a comparison of the United States, Spain, and France. Demogr Res. 2017;36:111-144. CrossRef PubMed
- 43. Masanet E, La Parra D. [Relationship between the number of hours of informal care and the mental health status of caregivers]. Rev Esp Salud Pública. 2011;85(3):257-266. CrossRef PubMed
- 44. Grant M, Sun V, Fujinami R, et al. Family caregiver burden, skills preparedness, and quality of life in non-small cell lung cancer. Oncol Nurs Forum. 2013;40(4):337-346. CrossRef PubMed
- 45. Jassem J, Penrod JR, Goren A, Gilloteau I. Caring for relatives with lung cancer in Europe: an evaluation of caregivers’ experience. Qual Life Res. 2015;24(12):2843-2852. CrossRef PubMed
- 46. Gallacher D, Auguste P, Royle P, Mistry H, Armoiry X. A systematic review of economic evaluations assessing the cost-effectiveness of licensed drugs used for previously treated epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) negative advanced/metastatic non-small cell lung cancer. Clin Drug Investig. 2019;39(12):1153-1174. CrossRef PubMed
- 47. Russell LB. The science of making better decisions about health: cost-effectiveness and cost-benefit analysis, Working Paper, No. 2014-06, Rutgers University, Department of Economics, New Brunswick, NJ, 2014. Accessed May 2022. Online
- 48. Planchard D, Popat S, Kerr K, et al. Metastatic non-small-cell lung cancer. ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Updated version published 15 September 2020 by ESMO Guidelines Committee. European Society for Medical Oncology; 2020:64.
- 49. Jefatura del Estado. Ley 14/2007, de 3 de julio, de Investigación biomédica. Ley 14/2007 Jul 4, 2007. Accessed March 2020. Online
- 50. Jefatura del Estado. Ley 41/2002, de 14 de noviembre, básica reguladora de la autonomía del paciente y de derechos y obligaciones en materia de información y documentación clínica. . Sec. Capítulo IV - Artículo 8, Ley 14/2007 Nov 15, 2002. Accessed March 2020. Online
- 51. Instituto Nacional de Estadística (INE). Encuesta Nacional de Salud. Microdatos. INE. Accessed April 2021. Online
- 52. Ohara G, Kurishima K, Nakazawa K, et al. Age-dependent decline in renal function in patients with lung cancer. Oncol Lett. 2012;4(1):38-42. CrossRef PubMed