 |
Glob Reg Health Technol Assess 2022; 9: (Suppl. 2): 14-19 ISSN 2283-5733 | DOI: 10.33393/grhta.2022.2420 REVIEW |
 |
New evidence in adjunctive treatment of focal-onset seizures in adults: a critical appraisal
ABSTRACT
Anti-seizure medications (ASMs) represent the pillar of the treatment of epilepsy. The rate of drug-resistant epilepsy remained substantially unchanged over time and there is still the need for new and more effective treatment options. Brivaracetam, cenobamate, eslicarbazepine acetate, lacosamide and perampanel are ‘third-generation’ ASMs.
The aim of this article is to summarize the currently available evidence about the relative efficacy and tolerability of the ‘third-generation’ ASMs as adjunctive treatment of focal-onset seizures in adults.
So far, no randomized controlled study directly compared these ASMs, and their comparative efficacy and tolerability have been indirectly evaluated by one network meta-analysis. Sixteen trials were included in the network meta-analysis. The efficacy endpoints were the rates of seizure response and seizure freedom, defined as ≥ 50% and 100% reduction in baseline monthly seizure frequency. The tolerability endpoints were the rate of patients who developed any treatment emergent adverse events (TEAEs) and any TEAE leading to drug discontinuation. Cenobamate had the greatest likelihood of being the best option for the ≥ 50% and 100% seizure frequency reduction. Brivaracetam and lacosamide had the greatest likelihood to rank as the best-tolerated treatments for the occurrence of any TEAE and TEAE leading to discontinuation.
Although network meta-analyses are not substitutes of direct comparisons, they can provide valuable evidence about the hierarchy of interventions. Additional real-world data can be useful complement to characterize the clinical profile and therapeutic potentialities of third-generation ASMs.
Keywords: Anti-seizure medications, Epilepsy, Focal seizures
Received: May 9, 2022
Accepted: May 30, 2022
Published online: June 28, 2022
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Background
Epilepsies are a group of neurological disorders characterized by recurrent, unprovoked seizures, which can be either focal or generalized. Focal-onset seizures are the most common type of seizures experienced by people with epilepsy, and they can be associated with impaired awareness. With approximately 70 million people affected worldwide, epilepsy accounts for a significant proportion of the global disease burden (1). The estimated proportion of the general population with active epilepsy, that is, continuing seizures or with the need for treatment at a given time, is between 4 and 10 per 1,000 people (2).
Epilepsy can have significant social impact and economic implications. People with epilepsy can experience reduced access to educational opportunities and barriers to enter some occupations. Uncontrolled epilepsy is often associated with significant psychological dysfunction and impaired quality of life and carries the risk of premature death (3,4). Further, stigma and discrimination still surround epilepsy in different countries across the world. The economic impact of epilepsy varies significantly depending on the disease duration and severity, response to treatment, and the health-care setting. Out-of-pocket costs and productivity losses create substantial burdens on households.
The treatment of epilepsy is mainly symptomatic, and anti-seizure medications (ASMs) represent the pillar. Most people with epilepsy can become seizure free with appropriate use of one or more ASMs. However, seizures are not controlled in more than one-third of the patients (5,6). Despite the increased availability of ASMs, the rate of drug-resistant epilepsy remained substantially unchanged over time and there is still the need for new and more effective treatment options (7). Over the last decade, five ‘third-generation’ ASMs, namely brivaracetam (BRV), cenobamate (CNB), eslicarbazepine acetate (ESL), lacosamide (LCM), and perampanel (PER), have been licensed for adjunctive treatment of focal-onset seizures in adult patients (8).
The aim of this article is to summarize the currently available evidence about the relative efficacy and tolerability of any of these ‘third-generation’ ASMs to one another as adjunctive treatments of focal-onset seizures in adults and suggest implications for clinical practice and future research.
The evidence from the literature
There are no randomized controlled studies that directly compared the ‘third-generation’ ASMs. So far, the comparative efficacy and tolerability of these drugs have been evaluated by one systematic review with network meta-analysis (9). Database and trial register including MEDLINE, the Cochrane Central Register of Controlled Trials, and the US National Institutes of Health Clinical Trials Registry were searched to identify randomized, double-blinded, controlled trials comparing add-on BRV, CNB, ESL, LCM, and PER versus any comparator in adult patients with focal epilepsy uncontrolled by one or more concomitant ASMs (9). Only trials with a maintenance period or a period of stable dose of 12 weeks or longer were considered (9).
The efficacy endpoints were the rates of seizure response and seizure freedom, defined as a ≥ 50% and 100% reduction in baseline monthly seizure frequency during the maintenance treatment period. When information over the maintenance phase was not available, the treatment period was considered (9). The ‘pragmatic intent-to-treat’ approach was used for defining the seizure freedom, whenever available. According to this approach, only patients who were seizure free and completed the entire study were considered as seizure free (10). This is a more conservative methodology to measure seizure freedom and it provides more reliable information about the actual treatment efficacy in comparison to the ‘observation carried forward’ strategy, which considers as being seizure free those patients who dropped out of a study and were free from seizures at the last available assessment (10).
The tolerability endpoints were the rate of patients who developed any treatment emergent adverse events (TEAEs) and any TEAE leading to drug discontinuation. For any drug, only licensed maintenance doses for adjunctive treatment were considered in accordance with the prescribing information. The daily doses were 50-200 mg for BRV, 200-400 mg for CNB, 800-1200 mg for ESL, 200-400 mg for LCM, and 4-12 mg for PER (11-15).
The comparative efficacy and safety of the included ASMs were estimated through network meta-analyses within a frequentist framework (16). The hierarchy of competing interventions was established through the surface under the cumulative ranking curve (SUCRA) and mean ranks.
The randomized, controlled trials included in the quantitative synthesis were sixteen (17-32): three for add-on BRV, one for add-on CNB, four for add-on ESL, four for add-on LCM, and four for add-on PER. The trials enrolled 6,753 participants: 4,507 were assigned to active treatments (BRV = 803, CNB = 221, ESL = 990, LCM = 1,104, and PER = 1,389) and 2,246 to placebo (9).
Efficacy
The rates of participants with ≥ 50% and 100% reduction in baseline monthly seizure frequency were provided by all the included trials. For the seizure freedom endpoint, the ‘pragmatic intention-to-treat (ITT)’ data were available in most studies; in three trials, the status at the time of treatment withdrawal was used to impute the freedom from seizure for the remainder of the study (21,24,32). The network meta-analyses showed that all ASMs were associated with higher rates of seizure response than placebo, and CNB was associated with a higher probability of ≥50% reduction in baseline seizure frequency than BRV, ESL, LCM, and PER (Fig. 1) (9). In the analysis of seizure freedom outcome, BRV, CNB, ESL, and PER were more efficacious than placebo, whereas there were no statistically significant differences between the ASMs (Fig. 2) (9). According to SUCRA, CNB had the greatest likelihood to rank as the best treatment option for both the seizure response and seizure freedom endpoints (Tab. I) (9).
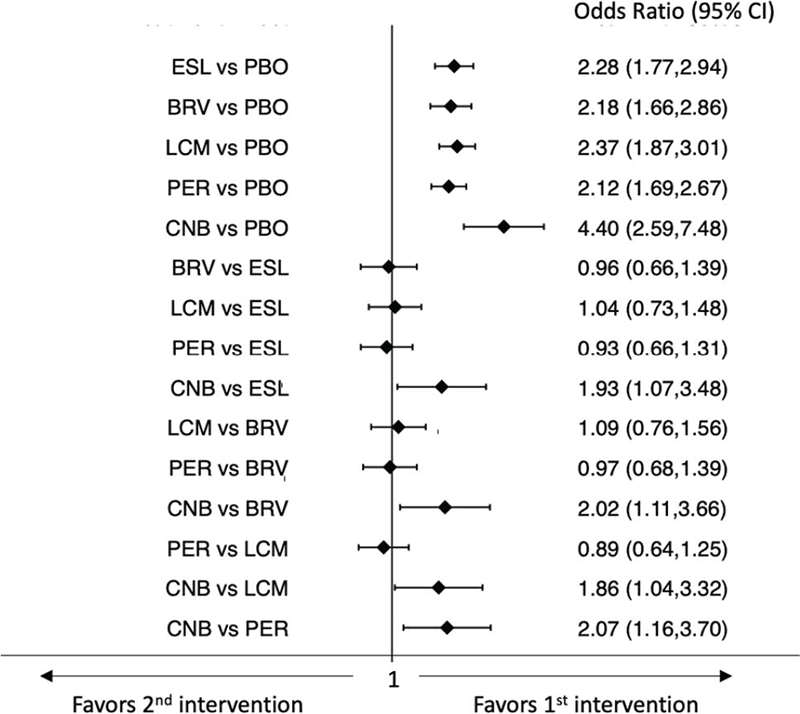
Fig. 1 - Interval plot for the seizure response outcome.
BRV = brivaracetam; CI = confidence interval; CNB = cenobamate; ESL = eslicarbazepine acetate; LCM = lacosamide; PBO = placebo; PER = perampanel.
Tolerability
The rates of participants who experienced at least one TEAE were available from all the included trials except two (26,27). The rates of participants who experienced at least one TEAE leading to discontinuation were available from all the included trials except one LCM study (27).
The network meta-analysis showed that all ASMs were associated with higher rates of participants who experienced at least one TEAE than placebo. Further, BRV and LCM were associated with a lower risk of the occurrence of TEAEs compared to ESL; PER was associated with a higher risk of the occurrence of TEAEs compared to BRV (Fig. 3) (9). In the analysis of the rates of patients who experienced at least one TEAE leading to discontinuation, all ASMs were less tolerated than placebo, whereas there were no statistically significant differences between the ASMs (Fig. 4) (9). According to SUCRA, BRV and LCM had the greatest likelihood to rank as the best-tolerated treatments for both the endpoints of the occurrence of any TEAE and TEAE leading to discontinuation (Tab. II) (9).
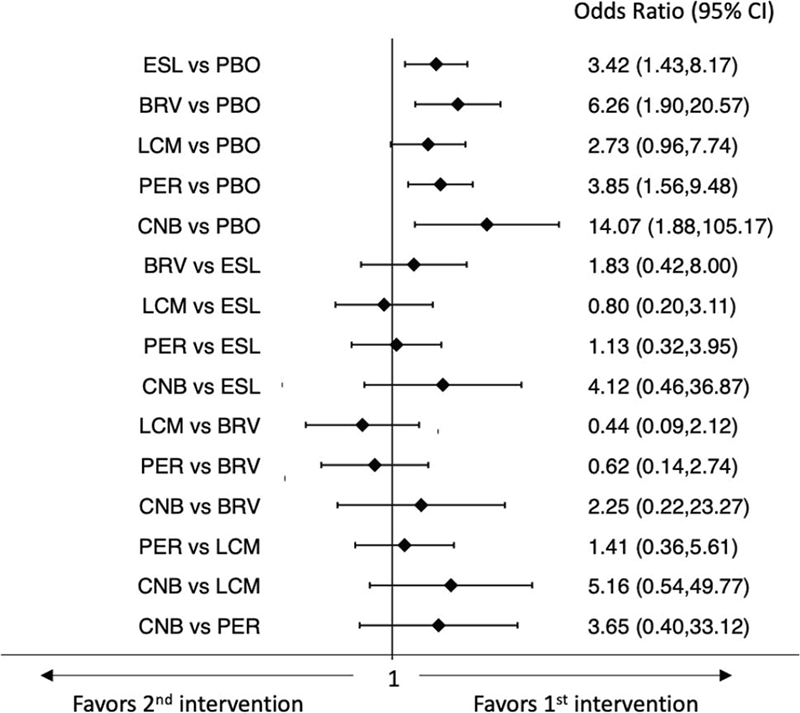
Fig. 2 - Interval plot for the seizure freedom outcome.
BRV = brivaracetam; CI = confidence interval; CNB = cenobamate; ESL = eslicarbazepine acetate; LCM = lacosamide; PBO = placebo; PER = perampanel.
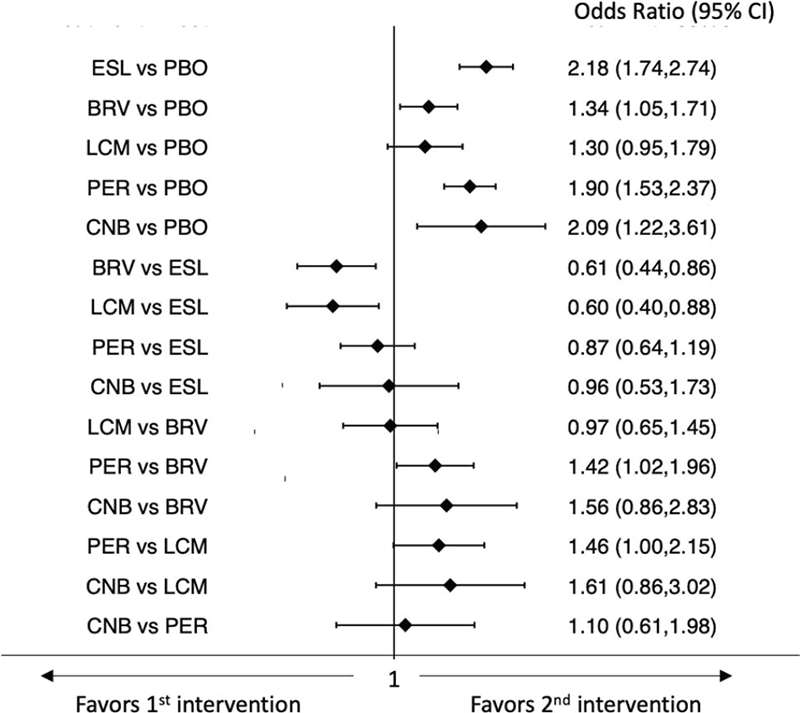
Fig. 3 - Interval plot for the occurrence of at least one treatment-emergent adverse event.
BRV = brivaracetam; CI = confidence interval; CNB = cenobamate; ESL = eslicarbazepine acetate; LCM = lacosamide; PBO = placebo; PER = perampanel.
| Treatment | Surface under the cumulative ranking curve | Mean rank |
|---|---|---|
| Seizure response | ||
| Brivaracetam | 46.2 | 3.7 |
| Cenobamate | 99.0 | 1.1 |
| Eslicarbazepine acetate | 53.4 | 3.3 |
| Lacosamide | 60.8 | 3.0 |
| Perampanel | 40.7 | 4.0 |
| Placebo | 0.0 | 6.0 |
| Seizure freedom | ||
| Brivaracetam | 72.4 | 2.4 |
| Cenobamate | 88.8 | 1.6 |
| Eslicarbazepine acetate | 47.2 | 3.6 |
| Lacosamide | 37.8 | 4.1 |
| Perampanel | 53.0 | 3.4 |
| Placebo | 0.8 | 6.0 |
Higher values of surface under the cumulative ranking curve correspond to higher probabilities of better efficacy.
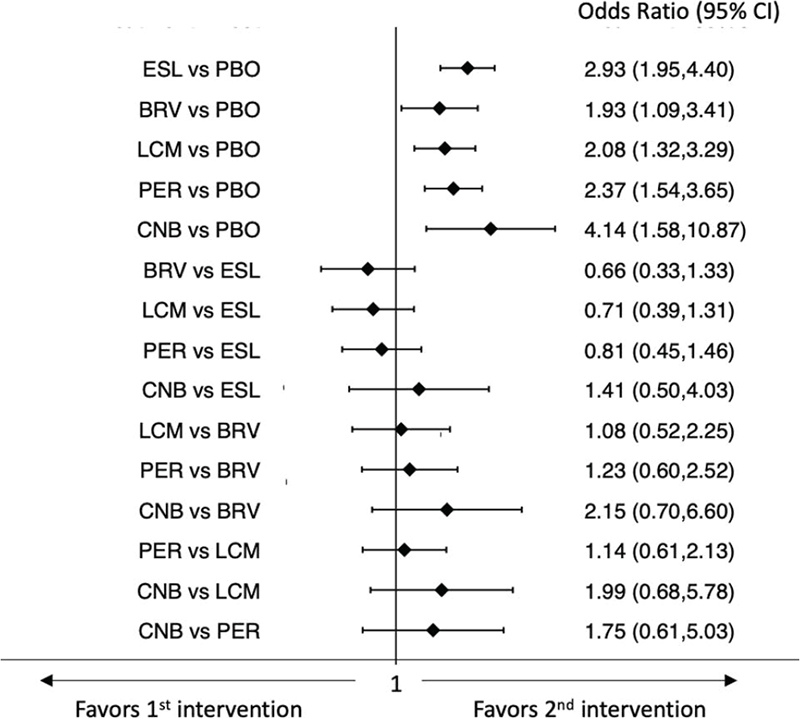
Fig. 4 - Interval plot for the occurrence of at least one treatment-emergent adverse event leading to discontinuation.
BRV = brivaracetam; CI = confidence interval; CNB = cenobamate; ESL = eslicarbazepine acetate; LCM = lacosamide; PBO = placebo; PER = perampanel.
| Treatment | Surface under the cumulative ranking curve | Mean rank |
|---|---|---|
| At least one treatment-emergent adverse event | ||
| Brivaracetam | 67.0 | 2.6 |
| Cenobamate | 21.5 | 4.9 |
| Eslicarbazepine acetate | 12.8 | 5.4 |
| Lacosamide | 70.2 | 2.5 |
| Perampanel | 29.6 | 4.5 |
| Placebo | 98.8 | 1.1 |
| At least one treatment-emergent adverse event leading to discontinuation | ||
| Brivaracetam | 62.3 | 2.9 |
| Cenobamate | 11.9 | 5.4 |
| Eslicarbazepine acetate | 24.8 | 4.8 |
| Lacosamide | 56.8 | 3.2 |
| Perampanel | 44.5 | 3.8 |
| Placebo | 99.7 | 1.0 |
Higher values of surface under the cumulative ranking curve correspond to higher probabilities of better tolerability.
Third-generation ASMs for focal seizures: comparative efficacy and safety
The currently available comparative analysis of the ‘third-generation’ ASMs suggested that CNB given as adjunctive treatment of focal-onset seizures in adult patients is associated with a higher rate of seizure response and a greater likelihood to rank best for seizure freedom outcome compared to add-on BRV, ESL, LCM, and PER (9).
Among the third-generation ASMs, CNB is the most recently approved for treating focal seizures, and these findings bring promise for people with epilepsy whose seizures are difficult to control.
The Food and Drug Administration in the USA approved CNB for the treatment of focal-onset seizures in adults in 2019 (33). The European Medicines Agency in the EU approved CNB for the adjunctive treatment of focal-onset seizures with or without secondary generalization in adult patients with epilepsy who have not been adequately controlled despite a history of treatment with at least two anti-epileptic medicinal products in 2021 (12).
CNB is a novel tetrazole-derived carbamate compound with a unique dual complementary mechanism of action. It decreases excitatory currents by preferentially inhibiting the persistent component of the sodium current and enhancing the inactivated state of voltage-gated sodium channels (34). In addition, it enhances inhibitory currents by acting as a positive allosteric modulator of high-affinity γ-aminobutyric acid (GABA)A receptors at a non-benzodiazepine binding site (35). The unique dual mechanism of action of CNB suggests that it has the potential to both prevent seizure initiation and limit seizure spread (36).
The network meta-analysis suggested better tolerability of BRV and LCM against the other third-generation ASMs: these two compounds were associated with the greatest likelihood to be the best-tolerated options for both the endpoints of the occurrence of any TEAE and the occurrence of TEAEs leading to treatment withdrawal (9).
Among the considered ASMs, CNB ranked as the drug linked with the greatest probability of the occurrence of TEAEs. In this regard, the rapid uptitration of CNB by 100 mg for a week from the daily dosage of 200 mg to the daily dosage of 400 mg, and the impossibility to modify the concomitant therapeutic regimen during the trial might have played a role in the incidence of TEAEs. Importantly, drug–drug interactions may occur when CNB is administered. CNB can inhibit the cytochrome P450 (CYP) 2C19, and drugs like phenytoin and phenobarbital, which are metabolized, in part, by this isoenzyme may have their levels increased (37). Following multiple doses of adjunctive CNB, the plasma exposures of phenobarbital and phenytoin have been shown to increase by a mean of 37% and 84%, respectively (38). The elevation of drug levels may lead to increased risk of adverse events. The coadministration of clobazam with a CYP2C19 inhibitor has also been demonstrated to increase by two to six times the plasma levels of N-desmethylclobazam, which is the active metabolite of clobazam and is mainly metabolized by the CYP2C19 enzyme (39,40). Proactive reductions or dose alterations of concomitant ASMs should be considered according to the potential risk of drug–drug interactions to minimize the risk of treatment failure. In this regard, the effects of dose adjustments of concomitant ASMs have been explored in a post hoc analysis of a phase 3, multicenter, open-label study of adjunctive CNB for the treatment of uncontrolled focal seizures (38). Patients continuing CNB had greater mean reductions and percent changes of doses of concomitant ASMs from baseline compared to patients who discontinued the treatment. Doses of phenytoin, phenobarbital, clobazam, valproate, and LCM were decreased early, when patients were in the titration phase, while carbamazepine, oxcarbazepine, and eslicarbazepine had their doses decreased later, during the maintenance phase (38). Dose decreases were mostly due to the occurrence of adverse events related to the central nervous system, like somnolence, dizziness, and balance disorders. For example, phenytoin doses were reduced by a mean of 60.8% and phenobarbital doses by a mean of 40.0% in patients continuing CNB (38).
Direct head-to-head trials represent the most rigorous methodology to ascertain and compare the relative efficacy and tolerability of treatments. These studies, however, are costly and they are not required by regulatory authorities for ASM approval. It is unlikely that similar randomized controlled trials will be ever planned and conducted. In the absence of direct comparisons, network meta-analyses can use indirect evidence to estimate how ASMs measure up to each other and provide a hierarchy of competing interventions.
Importantly, the validity of the results of a network meta-analysis is strongly influenced by the degree of similarity and the methodological quality of the trials that are included in the comparisons (41). The network meta-analysis comparing the third-generation ASMs adopted rigid inclusion criteria with the aim to reduce the source of heterogeneity across the trials and minimize as much as possible the influence of potential confounding variables on the estimates of treatment effect (9). All the studies included in the analyses were overall clinically and methodologically homogeneous and none was judged at high risk of bias. Despite their similarities, however, a certain degree of diversity may exist among the studies, even if not explicitly recognized by heterogeneity testing. Some differences in the design of the trials and the baseline characteristics of the study cohorts may have affected the findings. It is also worth noting that the low event rates and scarcity of patients achieving some of the endpoints were associated with wide confidence intervals and such imprecision in the estimates can limit the sensitivity to identify differences across the ASMs and influence the rankings of treatments (9). Importantly, all trials included in the network meta-analysis were sponsored by pharmaceutical companies, and evidence about the efficacy and tolerability of CNB is obtained from one single study (9).
Conclusion
Network meta-analyses cannot be considered as substitutes of direct comparisons. Nonetheless, under certain assumptions, they can provide valuable evidence about the hierarchy of interventions and offer guidance for clinical practice and decision-making (42-44).
The comparative analyses of data from randomized, placebo-controlled trials of third-generation ASMs suggested that CNB is associated with the highest probability to be the best treatment option for efficacy outcomes, and BRV and LCM are associated with the greatest probabilities of being the best-tolerated drugs (9). Additional data obtained in real-world practice can overcome the limits of the randomized, controlled trials and be a useful complement to better characterize the clinical profile and therapeutic potentialities of the third-generation ASMs for the treatment of focal seizures in adult patients.
Disclosures
Conflicts of interest: SL has received speaker’s or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, and UCB Pharma and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, and GW Pharmaceuticals.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Payment of publication fees was supported by Angelini Pharma S.p.A.
References
- 1. Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy – a review. Epilepsy Res. 2009;85(1):31-45. CrossRef PubMed
- 2. Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the ‘common’ neurologic disorders? Neurology. 2007;68(5):326-337. CrossRef PubMed
- 3. Josephson CB, Patten SB, Bulloch A, et al. The impact of seizures on epilepsy outcomes: a national, community-based survey. Epilepsia. 2017;58(5):764-771. CrossRef PubMed
- 4. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. CrossRef PubMed
- 5. Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet. 1995;346(8968):140-144. CrossRef PubMed
- 6. Lattanzi S, Zaccara G, Giovannelli F, et al. Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta-analysis. Acta Neurol Scand. 2019;139(1):33-41. CrossRef PubMed
- 7. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279-286. CrossRef PubMed
- 8. Kwok CS, Johnson EL, Krauss GL. Comparing safety and efficacy of ‘Third-Generation’ antiepileptic drugs: long-term extension and post-marketing treatment. CNS Drugs. 2017;31(11):959-974. CrossRef PubMed
- 9. Lattanzi S, Trinka E, Zaccara G, et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. 2022;82(2):199-218. CrossRef PubMed
- 10. Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48(7):1303-1307. CrossRef PubMed
- 11. Brivaracetam. Summary of product characteristic. Online (Accessed April 2022).
- 12. Cenobamate. Summary of product characteristic. Online (Accessed April 2022).
- 13. Eslicarbazepine acetate. Summary of product characteristic. Online (Accessed April 2022).
- 14. Lacosamide. Summary of product characteristic. Online (Accessed April 2022).
- 15. Perampanel. Summary of product characteristic. Online (Accessed April 2022).
- 16. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80-97. CrossRef PubMed
- 17. Biton V, Berkovic SF, Abou-Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55(1):57-66. CrossRef PubMed
- 18. Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47-56. CrossRef PubMed
- 19. Klein P, Schiemann J, Sperling MR, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890-1898. CrossRef PubMed
- 20. Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38-48. CrossRef PubMed
- 21. Elger C, Halász P, Maia J, Almeida L, Soares-da-Silva P; BIA-2093-301 Investigators Study Group. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50(3):454-463. CrossRef PubMed
- 22. Gil-Nagel A, Lopes-Lima J, Almeida L, Maia J, Soares-da-Silva P; BIA-2093-303 Investigators Study Group. Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol Scand. 2009;120(5):281-287. CrossRef PubMed
- 23. Ben-Menachem E, Gabbai AA, Hufnagel A, Maia J, Almeida L, Soares-da-Silva P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010;89(2-3):278-285. CrossRef PubMed
- 24. Sperling MR, Abou-Khalil B, Harvey J, et al; 304 Study Team. Eslicarbazepine acetate as adjunctive therapy in patients with uncontrolled partial-onset seizures: results of a phase III, double-blind, randomized, placebo-controlled trial. Epilepsia. 2015;56(2):244-253. CrossRef PubMed
- 25. Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308-1317. CrossRef PubMed
- 26. Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, et al; SP755 Study Group. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443-453. CrossRef PubMed
- 27. Chung S, Sperling MR, Biton V, et al; SP754 Study Group. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958-967. CrossRef PubMed
- 28. Hong Z, Inoue Y, Liao W, et al; EP0008 Study Group. Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: A randomized, double-blind, placebo-controlled study. Epilepsy Res. 2016;127:267-275. CrossRef PubMed
- 29. French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79(6):589-596. CrossRef PubMed
- 30. French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54(1):117-125. CrossRef PubMed
- 31. Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78(18):1408-1415. CrossRef PubMed
- 32. Nishida T, Lee SK, Inoue Y, Saeki K, Ishikawa K, Kaneko S. Adjunctive perampanel in partial-onset seizures: Asia-Pacific, randomized phase III study. Acta Neurol Scand. 2018;137(4):392-399. CrossRef PubMed
- 33. Cenobamate FDA. Full prescribing information. Online (Accessed April 2022).
- 34. Nakamura M, Cho JH, Shin H, Jang IS. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. 2019;855:175-182. CrossRef PubMed
- 35. Sharma R, Nakamura M, Neupane C, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879:173117. CrossRef PubMed
- 36. Guignet M, Campbell A, White HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. 2020;61(11):2329-2339. CrossRef PubMed
- 37. Roberti R, De Caro C, Iannone LF, Zaccara G, Lattanzi S, Russo E. Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug-drug interactions and tolerability. CNS Drugs. 2021;35(6):609-618. CrossRef PubMed
- 38. Rosenfeld WE, Abou-Khalil B, Aboumatar S, et al. Post hoc analysis of a phase 3, multicenter, open-label study of cenobamate for treatment of uncontrolled focal seizures: effects of dose adjustments of concomitant antiseizure medications. Epilepsia. 2021;62(12):3016-3028. CrossRef PubMed
- 39. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246-1251. CrossRef PubMed
- 40. Lattanzi S, Trinka E, Striano P, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia. 2020;61(6):1090-1098. CrossRef PubMed
- 41. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472-475. CrossRef PubMed
- 42. Thieffry S, Klein P, Baulac M, et al. Understanding the challenge of comparative effectiveness research in focal epilepsy: a review of network meta-analyses and real-world evidence on antiepileptic drugs. Epilepsia. 2020;61(4):595-609. CrossRef PubMed
- 43. Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417-428. CrossRef PubMed
- 44. Lattanzi S, Trinka E, Del Giovane C, Nardone R, Silvestrini M, Brigo F. Antiepileptic drug monotherapy for epilepsy in the elderly: A systematic review and network meta-analysis. Epilepsia. 2019;60(11):2245-2254. CrossRef PubMed