 |
Glob Reg Health Technol Assess 2022; 9: 91-98 ISSN 2283-5733 | DOI: 10.33393/grhta.2022.2413 ORIGINAL RESEARCH ARTICLE |
 |
Budget Impact analysis of a new care system in patients with Parkinson’s disease
ABSTRACT
Objective: To estimate the economic impact of the introduction of a new care system based on apomorphine and Patient Support Program for motor fluctuations (“on-off” phenomena) in patients with Parkinson’s disease which are not sufficiently controlled by oral anti-Parkinson medication in Italy.
Method: A Budget Impact model was developed to evaluate the new care system in patients with Parkinson’s disease over a 3-years’ time horizon. The comparator treatments included in the analysis were treatments based on apomorphine and levodopa + carbidopa. The analysis was conducted from a National Health Service (NHS) perspective. Costs included in the analysis were acquisition costs and device costs. A deterministic sensitivity analysis was carried out to evaluate the uncertainty of the parameters used. A break-even analysis was conducted to identify the minimum number of subjects that would need to be treated with the new care system to obtain a positive Budget Impact (World With – World Without = 0).
Results: The analysis shows that the introduction of the new care system based on apomorphine could generate a cost saving incurred by the NHS of over € 5.7 million in 3 years. Break-even analysis shows that if it were possible to intercept with the new treatment at least 9 patients treated with apomorphine, there would not be an increase in costs for the NHS.
Conclusion: The new care system would respond to the unmet needs of patients with Parkinson’s disease by generating a reduction in the expenditure incurred by NHS.
Keywords: Apomorphine hydrochloride, Budget Impact Analysis, Economic Evaluation, Parkinson’s Disease
Received: April 14, 2022
Accepted: July 28, 2022
Published online: September 19, 2022
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Parkinson’s disease is a highly prevalent chronic degenerative disease with an extremely variable prevalence rate among the various populations of the world. It has a very significant economic impact from several perspectives: for society, for health systems, for patients and for their families. Worldwide, around 6.2 million people are affected by Parkinson’s disease, but this figure could actually be considerably higher as we know that many people go undiagnosed (1).
A recent nationwide meta-analysis conducted to estimate the prevalence of Parkinson’s disease in Italy showed that there is variability in prevalence rates according to age: 37.8/100,000 inhabitants in the 0-64 age group, 578.7/100,000 in the 65-75 age group and 1,235.7/100,000 in the 75 and over age group (2). The aggregate estimate was 193.7/100,000. Also within the same study, there was an association between the disease and male gender, but only in the older age groups (OR = 1.37, 95% CI 1.22-1.53, and OR = 1.31, 95% CI 1.21-1.42 for age groups 65-74 years and 75 years or older, respectively).
Approximately 1/3 of all patients with “advanced” Parkinson’s disease are not adequately controlled with the usual treatments but require the combination of different classes of treatment including apomorphine, duodopa and deep brain stimulation.
The recent NICE Guidelines indicate the therapy to be followed according to the status of Parkinson’s disease. In particular, for early-stage Parkinson’s disease the choice should be between dopamine agonists, levodopa or monoamine oxidase B (MAO-B) inhibitors for all those patients whose motor symptoms do not impact on their quality of life; for motor symptoms, the choice should be between dopamine agonists, MAO-B inhibitors or COMT inhibitors as an addition to levodopa therapy for all patients who have developed dyskinesias or motor fluctuations. For patients with advanced Parkinson’s disease, deep brain neurostimulation should only be considered when symptoms are not controlled with the best possible pharmacological treatment, which may include subcutaneous apomorphine administered intermittently or by continuous infusion. Apomorphine is also effective on non-motor symptoms (NMS) such as apathy, mood, hallucinations, attention, memory, and gastrointestinal and urinary problems. When “off” periods are associated with intractable pain, apomorphine may be considered an important option to alleviate patients’ discomfort (3).
Continuous treatment with apomorphine is useful in the advanced stages of Parkinson’s disease when oral treatments fail and when apomorphine bolus injections would be too frequent to appropriately manage so many “off” periods during the day. In addition to deep brain stimulation (DBS), apomorphine infusions and duodenal infusions of levodopa-carbidopa (duodopa) represent a concrete therapeutic option (4,5).
Recently, a new apomorphine-based therapy has been developed for the treatment of motor fluctuations (“on-off” phenomena) in patients with Parkinson’s disease who are insufficiently controlled by oral anti-Parkinson’s medication. This therapy, in addition to allowing the new apomorphine dosage in 20 mL (concentration 5 mg/mL) to be administered in a single vial/day (100 mg) to cover the patient’s entire daily requirement, is proposed not only as a new drug but also as a new “treatment system”, as it uses an innovative infusion pump and a “personalised” Patient Support Program (PSP) capable of simplifying the methods of administration and therapy management, resulting in improved compliance and adherence, and thus improved therapeutic results for the patient.
In particular, the PSP provides for:
– the provision of the infusion pump free of charge, as well as information on its management and logistics;
– free supply of all disposable materials (catheters, reservoirs), with guaranteed timely delivery to the patient’s home;
– a series of “homecare” services for the management of the pump and disposable materials for both patient and caregiver;
– in-hospital training courses for nurses, neurologists, patients and caregivers;
– presence of a “free phone number” available to the patient and the caregiver;
– follow-up visits with the nurses involved and calls with the patient;
– questionnaires to assess patient adherence and quality of life.
Although direct comparison studies between apomorphine and levodopa-carbidopa have not yet been conducted, when looking at randomised clinical trials of both infusion therapies, the size of the treatment effect between the two infusions is similar (6,7,8). Compared to patients treated with standard dopamine replacement therapies, levodopa-carbidopa intestinal gel and apomorphine with continuous infusion showed an increase in activation time with no troublesome dyskinesia of 1.9 h (95% CI 0.6-3.2; FU 3 months) (8) and 2.0 h (95% CI 0.7-3.4; FU 3 months) (7), respectively.
Considering the mode of administration, apomorphine infusion is easily reversible and less invasive than levodopa-carbidopa gel as the latter requires the insertion of a gastric tube. The provision of a “personalised” PSP in combination with apomorphine administration would further improve therapy management.
The aim of the study was to assess the economic impacts that could be generated by the introduction of the new apomorphine-based treatment and its PSP service for the treatment of motor fluctuations (“on-off” phenomena) in patients with Parkinson’s disease who are insufficiently controlled by oral anti-Parkinson’s medication.
The economic impact of the launch of the new apomorphine-based treatment on the market will be assessed by comparing the new treatment scenario with the scenario of the treatments currently available on the market.
Methods
The Budget Impact analysis was conducted from the perspective of the Italian National Health Service (NHS) and followed the Guidelines suggested by the International Society of Pharmacoeconomics and Outcome Research (ISPOR) (9,10).
This analysis is based on the comparison of two alternative scenarios: the scenario without the new apomorphine-based treatment, characterised by the presence of the therapies currently available on the market for the treatment of motor fluctuations in patients with Parkinson’s disease (apomorphine hydrochloride 50 mg/5 mL and levodopa + carbidopa 7 bags intestinal gel 100 mL 20 mg/mL + 5 mg/mL), and the scenario in which the introduction of the new therapy on the market is simulated.
The model took into account a time horizon of 3 years of analysis.
Eligible population and analysis scenarios
By applying the prevalence estimate for Parkinson’s disease obtained from the most recent national literature (Ricco et al. 2020 (2)) to the resident population in Italy, it was possible to estimate a number of patients with Parkinson’s disease in Italy amounting to approximately 114,250 subjects. The sub-analysis of the multi-country observational study named OBSERVE-PD, conducted by Stefani et al. in 2022 on the group of patients from 9 Italian centres (out of 128 centres worldwide), estimated a share of patients with advanced Parkinson’s disease of 42.9% (11); of those patients with advanced disease who were treated with oral/transdermal therapy (approx. 67%), 97.6% were not adequately controlled. Applying these estimates to the population with Parkinson’s disease in Italy, the number of patients with advanced Parkinson’s disease inadequately controlled with oral medication amounted to approximately 32,000 patients (Tab. I).
| Estimate | No. | Source | |||
|---|---|---|---|---|---|
| Resident population at January 1, 2022 | 58,983,122 | ISTAT (14) | |||
| Cases/100,000 inhabitants | 193.7 | 114,250 | Riccò et al. 2020 (2) | ||
| Patients with advanced Parkinson’s disease | 42.90% | 49,013 | |||
| Patients with advanced Parkinson’s disease treated with oral/transdermal therapy | 67.0% | 32,839 | Stefani et al. 2022 (11) | ||
| Patients with advanced Parkinson’s disease inadequately controlled with oral medication | 97.6% | 32,051 | |||
| IU 2018 | No. of patients 2018* | IU 2019 | No. of patients 2019* | ||
| Levodopa + carbidopa (intestinal gel) | 338,795 | 928 | 361,231 | 990 | |
| Apomorphine hydrochloride (continuous subcutaneous infusion) | 113,352 | 155 | 96,808 | 133 | IQVIA |
| Total | 1,083 | 1,123 | |||
| No. of patients 2020** | No. of patients 2021** | No. of patients 2022** | No. of patients 2023** | ||
| Levodopa + carbidopa (intestinal gel) | 1,055 | 1,125 | 1,200 | 1,279 | |
| Apomorphine hydrochloride (continuous subcutaneous infusion) | 113 | 97 | 83 | 71 | IQVIA |
| Total | 1,168 | 1,222 | 1,283 | 1,350 |
*Estimated assuming an annual number of International Units (IU) per patient of 365 for levodopa + carbidopa treatment and an annual number of IU per patient of 730 for apomorphine-based treatment.
**Estimated considering that estimated growth rate between 2018 and 2019 was 6.6% for levodopa + carbidopa and -15% for apomorphine-based hydrochloride.
The population eligible for the new apomorphine-based treatment was identified from an estimate of the number of patients on treatment with currently available therapies; this estimate was based on dispensation data for the period between January and December 2018 and January and August 2019 with a constant projection for the last four months of 2019 (IQVIA data, Tab. I).
Assuming an annual number of International Units (IU) per patient of 365 for levodopa + carbidopa treatment and an annual number of IU per patient of 730 for apomorphine-based treatment for 2018, approximately 928 patients treated with levodopa+ carbidopa and approximately 155 patients treated with apomorphine hydrochloride were estimated, respectively. An increase in patients treated with levodopa + carbidopa (+990 patients) and a decrease in patients treated with apomorphine (-133 patients) were estimated for 2019.
The number of patients potentially eligible for the new apomorphine-based treatment was 1,222, 1,282 and 1,350 in years 1, 2 and 3 (Tab. I). A proportional increase in the number of patients treated was assumed for the three simulated years compared to 2018-2019.
The shares of patients associated with each treatment for both scenarios were defined on the basis of Ever Pharma internal estimates. Table II shows the utilisation rates of the individual treatment options during the 3 years simulated.
The new apomorphine-based treatment is expected to gradually become the main therapeutic alternative together with levodopa + carbidopa in Parkinson’s disease patients affected by motor fluctuations (“on-off” phenomena) that are insufficiently controlled by oral anti-Parkinson’s medication.
| Treatments | Current Scenario | Alternative Scenario | |||||
|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | ||
| Levodopa + carbidopa | 92% | 94% | 95% | 89% | 86% | 83% | |
| Apomorphine hydrochloride | 8% | 6% | 5% | 7% | 4% | 1% | |
| Apomorphine hydrochloride hemihydrate + Electronic pump and consumables for continuous infusion + PSP | 0% | 0% | 0% | 4% | 10% | 16% | |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | |
In the scenario in which the introduction of the new treatment on the market is simulated, the percentage of patients treated with the new apomorphine-based drug at national level was considered to be 4%, 10% and 16% for years 1, 2 and 3 respectively.
Cost parameters
In this analysis, the cost of acquiring the treatment and the cost of the devices required for the use of the individual treatments were taken into account (Tab. III). The cost of the devices required for apomorphine administration (separate from the price of the drug and borne by the NHS) was calculated based on the assumption of one vertical needle and one syringe (730 units for each device) for each of the two daily administrations and taking into account the purchase of one pump every two years (duration as per the Canè pump data sheet).
Table IV shows the annual costs per patient associated with each specific cost item and the total annual cost per patient for each treatment option under analysis. In particular, for the administration of levodopa + carbidopa, the cost of the planned surgery for PEG placement was also taken into account. For the calculation of acquisition costs, a 100% adherence and compliance was assumed for each year of analysis. This assumption may not be plausible, but it was necessary in order to allow a direct comparison of different treatment strategies.
Sensitivity analysis
In order to identify different potential analysis scenarios over the years, a deterministic sensitivity analysis was conducted; this consists of varying one input parameter at a time in order to assess the impact of this variation on the results of the analysis. In particular, the following scenarios were evaluated for this model:
• Scenario 1: reduction in the price of levodopa + carbidopa as estimated by regional tenders (-31%)
• Scenario 2: reduction in the price of apomorphine-based treatment as estimated by regional tenders (-13%)
• Scenario 3: combined price variation of levodopa + carbidopa and apomorphine as estimated by regional tenders
• Scenario 4: change in penetration speed:
Base case: 50, 130 and 220 patients respectively to 2021, 2022 and 2023; Min 25, 65 and 110 patients and Max 75, 195 and 330 patients
• Scenario 5: change in prices of other devices not included in the price of the drug (±20% compared to the base case)
The results of the deterministic sensitivity analysis are shown by means of the tornado diagram.
Finally, a specific break-even analysis was conducted in order to identify the minimum number of patients that would need to be treated with the new apomorphine-based therapy (among those currently treated with apomorphine) in order to achieve a positive Budget Impact (World With – World Without = 0).
| Ex-Factory Price | IU Price | Daily dose | Total Annual Cost of Therapy | |
|---|---|---|---|---|
| Apomorphine hydrochloride hemihydrate + Electronic pump and consumables for continuous infusion + PSP | € 56.8 | € 42.6 | 1 | € 15,562.7 |
| Levodopa + carbidopa | € 682.3 | € 97.5 | 1 | € 35,576.5 |
| Apomorphine hydrochloride | € 29.5 | € 5.9 | 2 | € 4,314.3 |
| Additional devices for apomorphine hydrochloride | No. of devices per year | Unit cost | Total cost | Cost parameter source |
| Single Vertical Needles (2 units per day) | 730 | € 3.6 | € 2,628.0 | Canè 2018 price list |
| Infusion pumps (average duration 2 years) | 1 | € 1,314.0 | € 1,314.0 | Unit price net of discounts |
| Syringes (2 daily units) | 730 | € 5.1 | € 3,744.9 | (VAT included) |
| Drug | Surgery | Pump | Other devices¥ | Homecare | Total Annual Cost | |
|---|---|---|---|---|---|---|
| Levodopa + carbidopa | € 35,577 | € 1,129* | PSP | PSP | PSP | € 36,706 |
| Apomorphine hydrochloride | € 4,314 | – | € 1,314 | € 6,373 | ** | € 12,001 |
| Apomorphine hydrochloride hemihydrate + Electronic pump and consumables for continuous infusion + PSP | € 15,056 | – | PSP | PSP | PSP | € 15,563 |
*EGD scope (Reg. Cod. 45.17 rate € 738.55) + PEG placement (€ 345.54).
¥Sum of annual costs for needles and syringes (not included in the cost of the Duodopa system).
**No Homecare services offered.
Results
Table V shows the estimated direct healthcare costs for each scenario and for each year of analysis as well as the cost difference resulting from the comparison of the two scenarios. For both scenarios, the main expenditure item was characterised by the cost of the drug (98% of total expenditure). The introduction of the new apomorphine-based treatment on the Italian market with increasing shares of patients treated over the years would allow a cumulative reduction in NHS expenditure of more than € 5.7 million over 3 years (Fig. 1). This reduction in expenditure can be attributed to fewer patients being treated with levodopa + carbidopa and to savings in device purchases for patients treated with the new apomorphine-based therapy, as the cost of devices for this treatment is included in the price of the drug.
| ITALY | Expenditure | ||
|---|---|---|---|
| Year 1 results | World Without | World With | BUDGET IMPACT |
| Drug cost | € 40,444,219 | € 39,912,460 | € –531,759 |
| Surgery cost | € 177,846 | € 138,328 | € –39,518 |
| Cost of other devices | € 679,988 | € 574,540 | € –105,448 |
| TOTAL EXPENDITURE | € 41,302,054 | € 40,625,328 | € –676,726 |
| Year 2 results | World Without | World With | BUDGET IMPACT |
| Drug cost | € 43,034,011 | € 41,526,387 | € –1,507,623 |
| Surgery cost | € 189,623 | € 82,360 | € –107,263 |
| Cost of other devices | € 580,742 | € 334,696 | € –246,046 |
| TOTAL EXPENDITURE | € 43,804,377 | € 41,943,444 | € –1,860,933 |
| Year 3 results | World Without | World With | BUDGET IMPACT |
| Drug cost | € 45,808,229 | € 43,124,603 | € –2,683,626 |
| Surgery cost | € 202,181 | € 15,881 | € –186,299 |
| Cost of other devices | € 495,981 | € 109,337 | € –386,644 |
| TOTAL EXPENDITURE | € 46,506,392 | € 43,249,822 | € –3,256,570 |
| Results at 3 years | World Without | World With | BUDGET IMPACT |
| Drug cost | € 129,286,460 | € 124,563,451 | € –4,723,008 |
| Surgery cost | € 569,651 | € 236,569 | € –333,081 |
| Cost of other devices | € 1,756,713 | € 1,018,573 | € –738,139 |
| TOTAL EXPENDITURE AT 3 YEARS | € 131,612,824 | € 125,818,595 | € –5,794,229 |
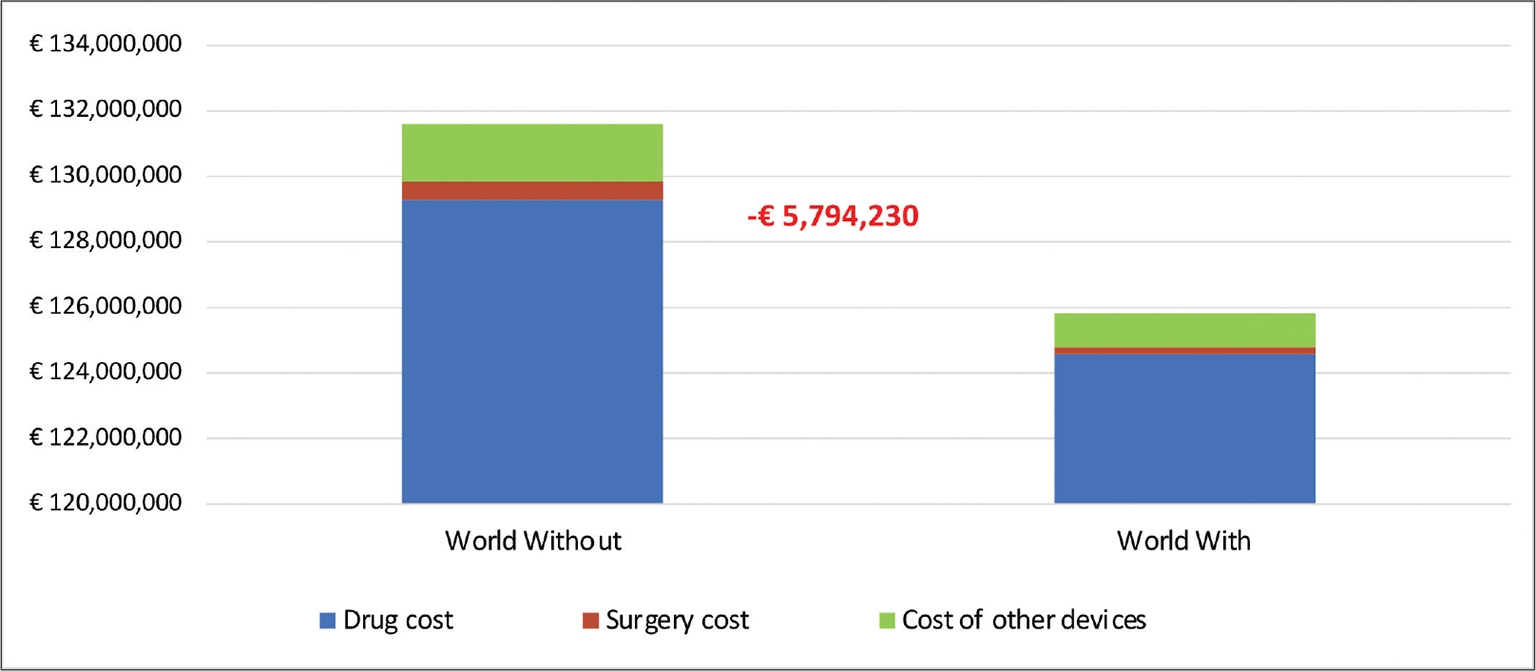
Fig. 1 - Composition of total expenditure at three years – Italy.
Looking at the results in each year of analysis, the NHS could achieve a reduction in expenditure of approximately € 676,726 in the first year of analysis, equivalent to over € 1.8 million in the second year of analysis and over € 3.2 million in the third year after the introduction of the new apomorphine-based treatment.
Figure 2 shows that the cumulative Budget Impact value estimated by the economic model is quite robust. In fact, in all scenarios simulated in the deterministic sensitivity analysis, the introduction of the new apomorphine-based treatment results in a cost reduction when compared to the current management of the patients under analysis. In particular, the parameter to which the greatest reduction in expenditure corresponds is the speed of penetration of the new apomorphine-based treatment. By simulating a number of patients treated with the new drug at 75, 195 and 330 in years 1, 2 and 3 respectively, a cumulative saving in NHS expenditure at 3 years of the analysis of approximately € 11.8 million could be achieved. However, it can be seen that in all pessimistic scenarios (MIN), reductions in expenditure would still be achieved.
Figure 3 shows the relationship between the number of patients treated with the new apomorphine-based therapy and the Budget Impact result one year after the introduction of the new apomorphine-based treatment. The analysis shows that the break-even level is 9 patients; therefore, if at least 9 patients on levodopa + carbidopa treatment could be treated, the NHS would begin to incur no additional costs.
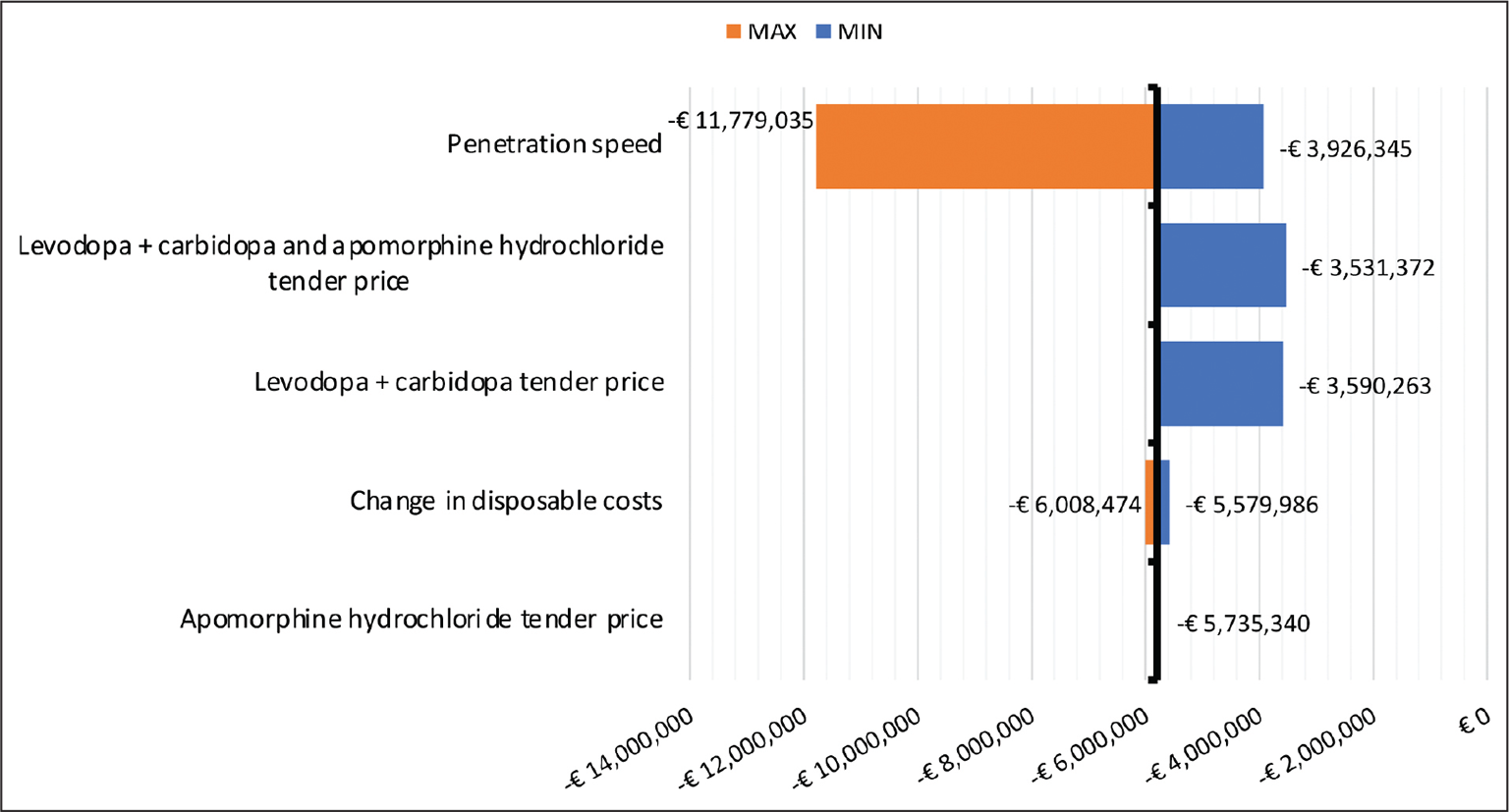
Fig. 2 - One-way sensitivity analysis – Cumulative estimates year 3.
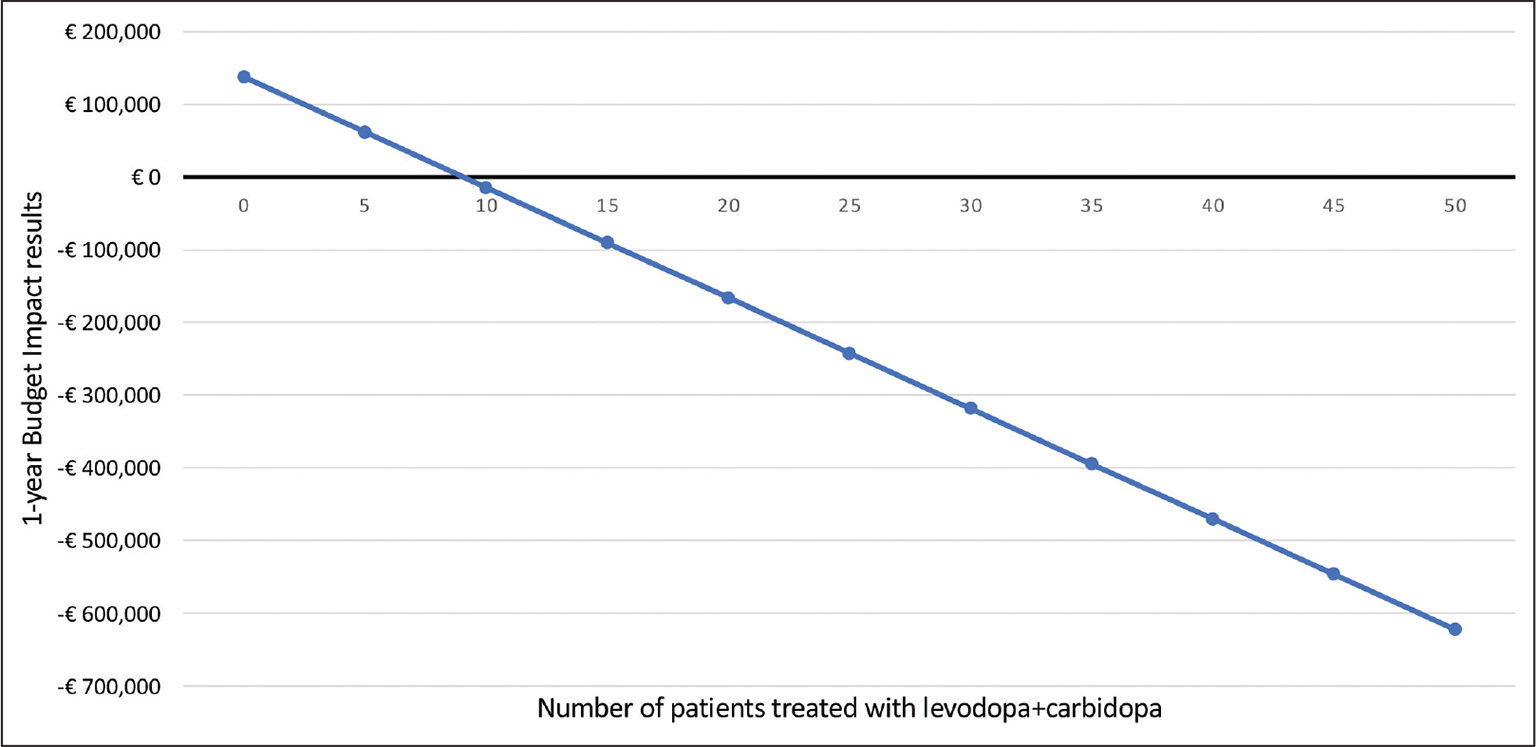
Fig. 3 - Break-even analysis.
Discussion
It is considered that the proposal of a “system of care” that guarantees “personalised” technologies and services (drug + device + PSP), and not just a “single drug”, can respond to still unmet needs of patients (difficulties in correct use and monitoring functions at the service of the patient and facilities), allowing a more correct allocation of available therapeutic resources and a reduction in healthcare costs generated by non-adherence.
Our analysis has four main limitations that must be taken into account. The Budget Impact has been estimated by only taking into account the acquisition and administration costs (in terms of devices and surgery) of the treatments under study, so it is reasonable to assume that this impact represents an underestimation of the real benefits that the introduction of the new treatment could generate in the national context. Furthermore, the analysis was conducted from the perspective of the NHS, which means that indirect costs, which account for more than 30% of the total expenditure associated with Parkinson’s disease, were not taken into account (12,13). A further limitation of the study relates to the estimated number of patients treated with the new apomorphine-based therapy. In fact, it is not easy to understand the real possibility of targeting patients in real clinical practice, but the break-even analysis showed that this percentage is sufficiently low that it does not represent a real barrier to accessing the use of the new apomorphine-based treatment. Finally, the assumption of 100% compliance is an assumption of the simulation model so that the three treatment options under analysis can have the same starting conditions in order to generate a bias-free result. Certainly, considering drug costs alone, compliance would be one variable in decreasing pharmaceutical expenditure; it would also be appropriate to assess the medium- to long-term effects in terms of subsequent management costs. Future and more in-depth analyses could develop this line of research in the field of pharmacoeconomics.
Taking into account the above-mentioned limitations, this analysis showed that the introduction of the new apomorphine-based treatment, including the cost of the device and consumables for continuous infusion, together with a personalised Patient Support Programme, could generate a cumulative reduction in NHS expenditure of more than € 5.7 million within 3 years after its introduction.
Improved patient access would not only allow proper management of Parkinson’s disease sufferers with motor fluctuations (“on-off” phenomena) who are insufficiently controlled by oral anti-Parkinson’s medication, but would also slow the progression of the levodopa + carbidopa treatment line. This would, on the one hand, avoid the need for highly invasive therapies and, on the other hand, reduce pharmaceutical expenditure.
Conclusions
The new apomorphine-based treatment, in combination with a personalised Patient Support Programme system and an innovative continuous infusion system, within the therapeutic options for the treatment of motor fluctuations (“on-off” phenomena) in patients with Parkinson’s disease who are insufficiently controlled by oral anti-Parkinson’s medication, could fill a therapy management gap that is particularly felt by clinicians today and, consequently, generate a reduction in expenditure by the NHS.
Disclosures
Conflict of interest: The Authors declare no conflict of interest.
Financial support: This study was supported by an unconditional grant from Ever Pharma Italia S.r.l.
References
- 1. European Parkinson’s Disease Association. What is Parkinson’s disease? Online Accessed April 2022.
- 2. Riccò M, Vezzosi L, Balzarini F, et al. Prevalence of Parkinson Disease in Italy: a systematic review and meta-analysis. Acta Biomed. 2020;91(3):e2020088. PubMed
- 3. Torti M, Bravi D, Vacca L, Stocchi F. Are All Dopamine Agonists Essentially the Same? Drugs. 2019;79(7):693-703. CrossRef PubMed
- 4. Jenner P, Katzenschlager R. Apomorphine – pharmacological properties and clinical trials in Parkinson’s disease. Parkinsonism Relat Disord. 2016;33(suppl 1):S13-S21. CrossRef PubMed
- 5. Djamshidian A, Poewe W. Apomorphine and levodopa in Parkinson’s disease: two revolutionary drugs from the 1950’s. Parkinsonism Relat Disord. 2016;33(suppl 1):S9-S12. CrossRef PubMed
- 6. Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares- da-Silva P; Bi-Park 1 investigators. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154-165. CrossRef PubMed
- 7. Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749-759. CrossRef PubMed
- 8. Olanow CW, Kieburtz K, Odin P, et al; LCIG Horizon Study Group. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141-149. CrossRef PubMed
- 9. Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis. Value Health. 2007;10(5):336-347. CrossRef PubMed
- 10. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5-14. CrossRef PubMed
- 11. Stefani A, Tessitore A, Tambasco N, et al. Criteria for identification of advanced Parkinson’s disease: the results of the Italian subgroup of OBSERVE-PD observational study. BMC Neurol. 2022;22(1):41. CrossRef PubMed
- 12. von Campenhausen S, Winter Y, Rodrigues e Silva A, et al. Costs of illness and care in Parkinson’s disease: an evaluation in six countries. Eur Neuropsychopharmacol. 2011;21(2):180-191. CrossRef PubMed
- 13. Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis. 2020;6(1):15. CrossRef PubMed
- 14. ISTAT. Popolazione residente al 1 gennaio 2022. Online Accessed April 2022.