 |
Glob Reg Health Technol Assess 2021; 8: 35-42 ISSN 2283-5733 | DOI: 10.33393/grhta.2021.2234 ORIGINAL RESEARCH ARTICLE |
 |
Costs of the management of hemophilia A with inhibitors in Spain
ABSTRACT
Introduction: Emicizumab is a first-in-class monoclonal antibody, recently authorized for the treatment of hemophilia A with inhibitors. This study aims to estimate the direct and indirect costs of the management of hemophilia A with inhibitors, in adult and pediatric patients, including the prophylaxis with emicizumab.
Methods: We calculated the costs of the on-demand and prophylactic treatments with bypassing agents (activated prothrombin complex concentrate and recombinant activated factor VII) and the emicizumab prophylaxis, from the societal perspective, over 1 year. The study considered direct healthcare costs (drugs, visits, tests, and hospitalizations), direct non-healthcare costs (informal caregivers), and indirect costs (productivity loss). Data were obtained from a literature review and were validated by an expert group. Costs were expressed in 2019 euros.
Results: Our results showed that the annual costs of the prophylactic treatment per patient varied between €543,062.99 and €821,415.77 for adults, and €182,764.43 and €319,826.59 for children, while on-demand treatment was €532,706.84 and €789,341.91 in adults, and €167,523.05 and €238,304.71 in pediatric patients. In relation to other prophylactic therapies, emicizumab showed the lowest costs, with up to a 34% and 43% reduction in the management cost of adult and pediatric patients, respectively. It reduced the bleeding events and administration costs, as this drug is less frequently administered by subcutaneous route. Emicizumab prophylaxis also decreased the cost of other healthcare resources such as visits, tests, and hospitalizations, as well as indirect costs.
Conclusion: In comparison to prophylaxis with bypassing agents, emicizumab reduced direct and indirect costs, resulting in cost savings for the National Health System and society.
Keywords: Activated prothrombin complex concentrate, Costs, Emicizumab, Hemophilia A, Recombinant factor VIIa
Received: January 21, 2021
Accepted: February 24, 2021
Published online: April 1, 2021
This article includes supplementary material
Global & Regional Health Technology Assessment - ISSN 2283-5733 - www.aboutscience.eu/grhta
© 2021 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
According to the Spanish National Registry, 2,595 patients with hemophilia A (HA) have been recorded in Spain: 80% are adults and 18% are children (1). One of the most severe complications of HA is the development of inhibitory antibodies to FVIII (2). Around 12% of patients with severe HA, 5% with moderate HA, and 1.5-3% with mild HA develop inhibitors (3,4).
The standard treatment of HA with inhibitors (HAwI) is the immune tolerance induction (ITI) therapy, which is expensive and is associated with low adherence and high failure rates (20-40%) (5-7). Therefore, other alternatives, such as bypassing agents (activated prothrombin complex concentrate [aPCC] and recombinant activated factor VII [rFVIIa]), have been widely used as on-demand or prophylactic treatments. Although rFVIIa is not licensed for prophylaxis, the most recent guidelines recommend this agent, as well as aPCC, as prophylactic therapy in patients with HAwI (6,8). Both agents are effective in preventing bleeding events. However, due to the short half-life of rFVIIa, aPCC may be preferred for prophylaxis, while the ease of reconstitution of rFVIIa and the small volume of the reconstituted product (that requires a shorter infusion time) may provide an advantage over aPCC. Nevertheless, these agents neither normalize thrombin generation nor fully correct hemostasis (6,9). Consequently, patients face a high risk of uncontrolled bleeding and subsequent complications, with a negative impact on quality of life and an increased mortality (6,10-12).
In 2018, emicizumab, a first-in-class monoclonal antibody, was authorized by the European Medicines Agency (13). Emicizumab connects activated factor IX and factor X to restore the function of the missing activated FVIII (5,14). Emicizumab does not induce the development of inhibitors against FVIII (14), and as it is administered subcutaneously once weekly, it could improve the patient’s quality of life (15,16).
The costs of the treatment of HAwI have been widely analyzed in Spain (7,17). However, the costs associated with this disorder still have not been analyzed from a societal perspective. Besides, as emicizumab has been recently authorized, it was not considered. Therefore, the aim of this study was to estimate the direct and indirect (ID) costs associated with the management of HAwI, in adult and pediatric patients (when ITI cannot be used or has failed), over 1 year.
Materials and methods
Data sources
Epidemiology data, healthcare resources utilization, and costs were obtained from a literature review. International references were used whenever national data were not available. Databases consulted were Medline/PubMed, Embase, Medes, and other official databases. When literature data differed from the current Spanish clinical practice, an expert group of three hematologists and one hospital pharmacist provided the necessary information. All extracted data were validated by the experts.
Strategies assessed
Bypassing agents and emicizumab were considered: aPCC and rFVIIa (prophylactic and on-demand regimens (18,19)), and emicizumab (prophylaxis regimen (14)).
The annual bleeding rate associated with each alternative and the relative risk of bleeding events were based on the results from the HAVEN 1 and 2 trials (20,21). The real-world annual bleeding rates of patients receiving bypassing agent therapies were considered (Tab. I) (17), as well as the frequency and duration of bleeding events by location and severity (Tab. II) (17,22,23). We also took into account the percentage of patients who required hospitalization due to the bleeding event and the length of the hospital stay (Tab. II) (7). These data were assumed for pediatric and adult patients.
| Treatment | Adult | Children | References | |
|---|---|---|---|---|
| Annual bleeding rate | ||||
| Emicizumab | Prophylaxis | 2.82 | 0.09 | (17,20,21) |
| Bypassing agents | On-demand | 21.72 | 15.43 | (17) |
| Prophylaxis | 9.48 | 8.72 | (17) | |
| Relative risk | ||||
| Emicizumab vs. bypassing agents | On-demand | 0.13 | – | (20) |
| Prophylaxis | 0.21 | 0.01 | (20,21) | |
| Bleeding site | Frequency (%) | Duration (days) | Hospitalization* | ||||
|---|---|---|---|---|---|---|---|
| Mild/mod. (17,23) | Severe (17,23) | Total (17,22) | Mild/mod. (17) | Severe (17) | Average length (days) (7) | Patients (%) (7) | |
| Joints | 55.00% | 45.00% | 65.84% | 2 | 7 | 3 | 15% |
| Muscle and soft tissues | 33.00% | 67.00% | 22.02% | 3 | 10 | 6 | 40% |
| Mucocutaneous tissues | 80.00% | 20.00% | 4.59% | 2 | 5 | 3 | 5% |
| Subcutaneous | 100.00% | 0.00% | 3.93% | 1 | 0 | 3 | 5% |
| Intracranial | 0.00% | 100.00% | 0.30% | 0 | 30 | 30 | 100% |
| Other areas | 33.00% | 67.00% | 3.32% | 2 | 7 | 3 | 40% |
The data were provided and validated by an advisory board.
mod. = moderate.
*Percentage of patients who require hospitalization due to the bleeding event and the length of hospital stay.
Costs and resource use
Costs were expressed in 2019 euros. Since the study was developed from a societal perspective, direct healthcare (DHC), direct non-healthcare (DNHC), and ID costs were considered. As costs came from different years, they were updated to 2019 using the corresponding inflation rate: a medicine consumer price index (CPI) for DHC (except for pharmaceutical costs) and a general CPI for DNHC and ID costs (24).
DHC costs included drugs, visits, tests, and hospitalizations. In the prophylactic regimens, doses were 1.5 mg of emicizumab per kg of body weight (bw)/week; 60 U of aPCC per kg of bw, three times/week; and 90 µg of rFVIIa per kg of bw, three times/week (14,17). Doses in on-demand regimens are shown in Suppl. table S1 (17-19). It was considered that the average bw was 27.6 kg in pediatric and 72.9 kg in adult patients (25). The study also took into account that if a bleeding event occurred in spite of the prophylaxis with emicizumab, on-demand treatment with rFVIIa would be administered (14). In case of a bleeding event during the prophylactic treatment with aPCC or rFVIIa, these drugs would be respectively administered in an on-demand regimen (18,19).
Drug costs were estimated using ex-factory prices (26) and the Royal Decree Law 8/2010 deduction rate (27) (Suppl. table S2). It was assumed that the content of the vials is optimized, which would decrease the global cost of treatments.
In prophylactic regimens, bypassing agents are usually intravenously self-administered by patients or injected by caregivers at home, so only 5% of patients come to the hospital. The cost of administration for each drug was estimated based on the time of preparation (reconstitution of the medicinal product) plus the time of administration in the day hospital (€0.57 per minute) (24,28). A 25-minute preparation and the maximum infusion rate (2 U per kg of bw per minute) was considered for aPCC (18), while the preparation and administration of rFVIIa and emicizumab were estimated in 10 and 8 minutes overall, respectively.
The management of HAwI includes medical visits and tests, which differ in pediatric and adult patients (Tab. III). In addition, bleeding events require special management (Tab. IV). Unit costs of visits and tests were the median value of the unit costs for each Autonomous Community in Spain (Suppl. table S3) (28). The study also included the hospital admissions; the length of hospital stays, and the percentage of hospitalized patients by bleeding site (Tab. II). Hospitalization costs came from the Hospital Discharge Records in the National Health System registry (29).
| Adults | Children | |||
|---|---|---|---|---|
| Bypassing agents | Emicizumab | Bypassing agents | Emicizumab | |
| Visits | ||||
| Hematology | 6 | 3 | 12 | 4 |
| Nurse | 6 | 3 | 12 | 4 |
| Physiotherapy | 4 | 4 | 4 | 4 |
| Psychology | 2 | 2 | 2 | 2 |
| Pharmacy | 5.25 | 6 | 10.5 | 6 |
| Tests | ||||
| Hemogram and biochemistry | 6 | 4 | 11 | 4 |
| Ultrasound | 1 | 1 | 1 | 1 |
Reference: Advisory board.
| Joints | Muscle and soft tissues | Mucocutaneous tissues | SC | IC | Other areas | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Visits | |||||||||||||
| Hematology | 3 | 60% | 3 | 90% | 1 | 30% | 1 | 30% | 14 | 100% | 3 | 80% | (7) |
| Maxillofacial surgery | 0 | 0% | 0 | 0% | 1 | 20% | 1 | 20% | 0 | 0% | 0 | 0% | (7) |
| Rehabilitation | 2 | 50% | 3 | 40% | 0 | 0% | 0 | 0% | 4 | 100% | 0 | 0% | (7) |
| Traumatology | 1 | 20% | 2 | 10% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | (7) |
| Neurology | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 2 | 100% | 0 | 0% | (7) |
| Primary care physician | 2 | 30% | 2 | 50% | 2 | 5% | 2 | 5% | 2 | 100% | 2 | 50% | (7) |
| Nurse | 3 | 60% | 3 | 90% | 1 | 30% | 1 | 30% | 14 | 100% | 3 | 80% | (7) |
| Physiotherapy | 5 | 20% | 5 | 30% | 0 | 0% | 0 | 0% | 14 | 100% | 0 | 0% | (7) |
| Emergency room | 1 | 5% | 1 | 40% | 1 | 5% | 1 | 5% | 1 | 100% | 1 | 50% | (7) |
| Tests | |||||||||||||
| Coagulation test | 1 | 30% | 1 | 45% | 1 | 10% | 1 | 10% | 3 | 100% | 1 | 80% | (7) |
| Ultrasound | 1 | 20% | 2 | 90% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 50% | (7) |
| X-rays | 0 | 0% | 0 | 0% | 1 | 5% | 1 | 5% | 0 | 0% | 0 | 0% | (7) |
| Hemogram and biochemical | 1 | 30% | 3 | 60% | 1 | 10% | 1 | 10% | 3 | 100% | 3 | 80% | (7) |
| Cranial CT scan | 0 | 0% | 0 | 0% | 1 | 5% | 1 | 5% | 1 | 100% | 0 | 0% | (7) |
| Abdominal CT scan | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 5% | (7) |
| Chest-abdominal-pelvis CT scan | 0 | 0% | 1 | 10% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | (7) |
| Red blood cell transfusion | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 10% | (7) |
Data were provided or validated by an advisory board.
% = percentage of patients that attend to the visits/tests; CT = computed tomography; IC = intracranial; n = number of visits/tests; Ref. = reference; SC = subcutaneous.
To estimate the DNHC costs, it was considered that 30% of the adult patients received an average of 2.08 hours of daily care, while all pediatric patients received 4 hours/day of care (30). The hourly wage for informal and formal caregivers was assumed to be the same, according to the proxy good method (31). Therefore, as the average annual salary for formal caregivers is €15,889.56 (24,28), and since caregivers spend 31 hours of work weekly (24), it was estimated that the salary per hour is €9.83.
ID costs (productivity losses) were only estimated for adult patients, as it was assumed that the main informal caregiver of pediatric patients is unemployed. According to the advisory board, the study considered that only 10% of adult patients were employed, and that their productivity losses were due to absenteeism or sick leave. Besides, to estimate the costs due to absenteeism, the duration of tests and visits was considered to be: hemogram (1.5 hours), hematology and nurse visit, psychology, and ultrasound (2 hours each), and physiotherapy (3 hours). The costs of sick leave were estimated based on the length of bleeding events. Since the average annual salary for men is €27,006.96 (24), with an average of 36.4 hours of effective working time weekly (24), a salary of €14.23 per hour was considered.
Sensitivity analysis
A univariate sensitivity analysis was conducted to examine the influence of the most sensitive parameters. According to the advisory board, different scenarios were built based on the possible variation of length of bleeding events (±20%); annual bleeding rate (±10%); patients’ weight (±10%); dose of aPCC (85 U/kg, 3 times weekly) (18); cost of visits, tests, and hospitalizations (±10%); employed patients (+30%); and caregiver salary (±20%).
Results
Adults
The cost of management of HAwI in patients on prophylaxis was between €543,062.99 and €821,415.77, while in those receiving on-demand treatment it accounted from €532,706.84 to €789,341.91 (Fig. 1 and Tab. V). Most of the costs for each alternative were pharmaceutical costs (>98%). As can be seen, the drug costs in prophylaxis implied between €538,756.93 (emicizumab) and €815,146.67 (rFVIIa); while in those receiving the on-demand strategy, costs were €523,947.87 and €780,772.37 for aPCC and rFVIIa, respectively. Therefore, emicizumab implied cost savings of 17% and 34% in comparison to other prophylactic treatments (aPCC and rFVIIa, respectively). In addition, emicizumab showed a 31% cost reduction, compared with the on-demand therapy with rFVIIa. The on-demand strategy with aPCC implied the lowest costs associated with the management of HAwI (2% lower than the prophylaxis with emicizumab).
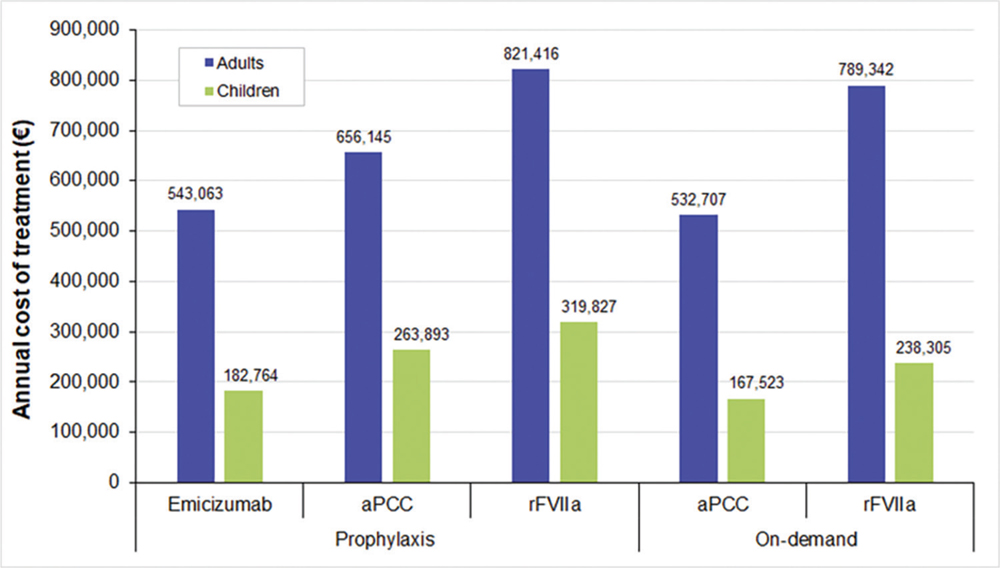
Fig. 1 - Annual average cost per patient in prophylactic and on-demand treatments. aPCC = activated prothrombin complex concentrate; rFVIIa = recombinant activated factor VII.
Regarding other DHC costs, on-demand treatments showed higher costs associated with visits, tests, and hospitalizations than prophylaxis, due to the higher incidence of bleeding events. Besides, aPCC and rFVIIa showed higher administration costs than emicizumab, especially in prophylaxis (€308.17, €51.78, and €3.93, respectively). The treatment with emicizumab showed the lowest administration and monitoring costs, in comparison to other treatments (Tab. V).
| Prophylaxis | On-demand | ||||
|---|---|---|---|---|---|
| Emicizumab | aPCC | rFVIIa | aPCC | rFVIIa | |
| Adults | |||||
| Direct healthcare costs | €540,677.17 | €653,354.58 | €818,599.83 | €529,471.38 | €786,106.45 |
| Drug | €538,756.93 | €649,645.03 | €815,146.67 | €523,947.87 | €780,772.37 |
| Administration | €3.93 | €308.17 | €51.78 | €219.63 | €30.20 |
| Visits | €1,485.17 | €2,529.85 | €2,529.85 | €3,833.27 | €3,833.27 |
| Tests | €332.03 | €538.76 | €538.76 | €708.17 | €708.17 |
| Hospitalizations | €99.12 | €332.78 | €332.78 | €762.44 | €762.44 |
| Direct non-healthcare costs (informal care) | €2,240.38 | €2,240.38 | €2,240.38 | €2,240.38 | €2,240.38 |
| Indirect costs (productivity loss) | €145.44 | €549.66 | €575.56 | €995.08 | €995.08 |
| Total costs | €543,062.99 | €656,144.62 | €821,415.77 | €532,706.84 | €789,341.91 |
| Children | |||||
| Direct healthcare costs | €168,403.03 | €249,531.21 | €305,465.19 | €153,161.65 | €223,943.31 |
| Drug | €166,777.90 | €244,388.22 | €300,576.54 | €147,120.69 | €218,041.43 |
| Administration | €0.12 | €305.52 | €51.18 | €160.53 | €21.45 |
| Visits | €1,327.79 | €3,716.64 | €3,716.64 | €4,431.18 | €4,431.18 |
| Tests | €294.15 | €814.74 | €814.74 | €907.61 | €907,61 |
| Hospitalizations | €3.06 | €306.10 | €306.10 | €541.64 | €541.64 |
| Direct non-healthcare costs (informal care) | €14,361.40 | €14,361.40 | €14,361.40 | €14,361.40 | €14,361.40 |
| Total costs | €182,764.43 | €263,892.61 | €319,826.59 | €167,523.05 | €238,304.71 |
aPCC = activated prothrombin complex concentrate; rFVIIa = recombinant activated factor VII.
DNHC costs were the same in prophylaxis and on-demand treatments (€2,240.38). However, the latter showed higher ID costs, because the higher rate of bleeding events implied patients were off work more often. It is worth noting that among the prophylactic alternatives, emicizumab showed the lowest ID costs (€145.44), as it requires lower monitoring visits and tests, compared to bypassing agents, resulting in less absenteeism and productivity losses.
Children
The management of HAwI accounted between €182,764.43 and €319,826.59 in prophylaxis and €167,523.05 and €238,304.71 in on-demand treatments for pediatric patients (Fig. 1 and Tab. V). As can be seen, drug costs represented more than 80% of the total costs. Since most of the drugs are weight-dosed, the costs of the treatment in pediatric patients were lower than in adults. Drug costs in prophylaxis varied between €166,777.90 (emicizumab) and €300,567.54 (rFVIIa), while in on-demand treatments costs were between €147,120.69 (aPCC) and €218,041.43 (rFVIIa). In comparison to prophylactic treatments, emicizumab reduced 31% and 43% the cost associated with the management of HAwI (aPCC and rFVIIa, respectively). Furthermore, the emicizumab treatment was 23% less expensive than the on-demand treatment with rFVIIa. The cost savings associated with the emicizumab treatment were higher in the pediatric population than in adult patients. However, the prophylaxis with emicizumab was 9% costlier than the on-demand treatment with aPCC in children.
In agreement with the results in adults, the costs associated with visits, tests, and hospitalizations were higher in the on-demand strategies, as they required a closer monitoring and implied a higher incidence of bleeding events than the prophylactic treatments. Besides, the costs of visits and tests were higher in pediatric than adult patients (Tab. V), due to the higher frequency of monitoring in children (Tab. III). However, hospitalization costs were higher in adult patients (Tab. V), because of the higher bleeding rates registered in these patients (Tab. I).
DNHC costs were the same in all strategies (€14,361.40), but they were higher than those for adult patients, as pediatric patients require more care than adults.
Sensitivity analysis
The sensitivity analysis results can be found in Online Resource (Suppl. tables S4 and S5). Tornado diagrams show those parameters which implied a variation on base case results of at least ±0.1% (Suppl. figure S1 and Suppl. figure S2). As can be seen, the most influential parameters for adult patients were weight, length of bleeding events, and annual bleeding rate. However, as could be expected, the length and the annual bleeding rate had a higher effect in the cost of on-demand treatments (±19.8 and ±10.0%) compared to prophylaxis (±6.6% and ±3.3%, respectively). It should be noted that increasing the dose of aPCC up to 85 U/kg resulted in a 27.0% increase of the cost of the prophylaxis with aPCC.
In agreement with the results in the adult population, the most influential parameters in children were patients’ weight, length of bleeding events, and annual bleeding rate. However, the length of bleeding events had almost no influence on the treatment with emicizumab (±0.1%), but it increased in other prophylactic (±6.2%) and on-demand treatments (±18.4%). Accordingly, the annual bleeding rate showed a light influence on the cost of emicizumab treatment (±0.1%) that increased to ±3.2% in other prophylactic treatments and ±9.3% in on-demand therapies. The variations in other parameters were patients’ weight (±9.4%), caregiver salary (±1.7%), and cost of healthcare resources (±0.3%) (Suppl. tables S4 and S5).
Discussion
Our results showed that the annual costs of the prophylaxis for patients with HAwI varied between €543,062.99 and €821,415.77 for adults, and €182,764.43 and €319,826.59 for children, while on-demand treatment was €532,706.84 and €789,341.91 in adults, and €167,523.05 and €238,304.71 in pediatric patients. The on-demand treatment with aPCC was the least expensive alternative. However, emicizumab showed the lowest costs among the prophylactic alternatives—with up to a 34% and 43% reduction in adults and children, respectively—as it required lower administration and monitoring costs than bypassing agents. The sensitivity analysis showed that the most influential parameters were length and annual bleeding rate, and patients’ weight.
Two studies previously estimated the cost of the treatment of HAwI in Spain. The first study included the DHC costs of the management of the disease over 1 year. The cost of the prophylaxis with aPCC was €524,387.52/patient, while the on-demand treatment with rFVIIa amounted to €627,876.47/patient. In agreement with our results, this study showed that drug costs represented more than 85% of the DHC cost (7). In the most recent study, the researchers estimated the annual drug costs of prophylactic and on-demand treatments with bypassing agents (aPCC and rFVIIa) in adult and pediatric patients. Regarding their results, if the same market share was assumed for both agents (50%), the average annual cost of the prophylaxis and on-demand treatments would be €661,518 and €621,293 in adult patients and €247,307 and €172,998 in children, respectively (17). However, taking into account both agents, our results would be €738,780 and €661,024 in adult patients and €291,860 and €202,914 in children, respectively. As can be seen, their results were 5–10% lower than ours, mostly due to the differences in the price of aPCC, as they estimated €0.65/U (€2017) and we considered €0.70/U (€2019) (26). Besides, they assumed the use of aPCC for the treatment of breakthrough bleeding events—those that occur in spite of the prophylactic treatment (26), while we considered rFVIIa or aPCC, according to the drug administered in the prophylactic treatment (18,19).
Despite its low incidence, HA implies an important burden for society, especially for patients who develop inhibitors (32,33). Therefore, one of the strongest findings of our research is estimating the DNHC and ID costs in Spain. Regardless of study designs and populations, prophylactic treatments have shown a reduced incidence of bleeding events vs. on-demand regimens (34-37), improving survival and quality of life. According to the data used in our estimations, prophylaxis requires lower medical visits and monitoring tests than on-demand therapies, resulting in lower DHC costs. Furthermore, as the former are usually home-administered, they reduce productivity losses and ID costs.
Recently, several economic studies evaluated emicizumab for the treatment of HAwI. The cost-effectiveness ratio of emicizumab was estimated from the National Health System perspective in Italy. The emicizumab treatment improved the patients’ quality of life by 0.94 quality-adjusted life-years (QALYs) vs. bypassing agents. In line with our results, it reduced the DHC costs, resulting in the least expensive alternative (€12 million) compared to aPCC (€32 million) or rFVIIa (€37 million) in a lifetime horizon. Therefore, the authors concluded that emicizumab is cost-effective, considering a cost-utility threshold of €100,000/QALY (38). Besides, the budget impact of emicizumab was estimated in Italy and the United States, from the payer’s perspective. The former concluded that the progressive introduction of emicizumab resulted in a budget reduction of €45.4 million (€0.27 million per patient) in a simulated time period from 2019 to 2021 (38). In the United States, the prophylaxis with emicizumab showed cost savings of $1,945,480 (around €1,748,700) per patient vs. the FVIII treatment, over a 20-year time horizon (39).
Our study is not without limitations. First, due to the lack of information about the management of HAwI in Spain, the resource use was provided and validated by an advisory board. As some of the parameters may not represent the real-world situation in our country, they were included in the sensitivity analysis. Second, although the patients’ response to these agents may differ, we used the same annual bleeding rate and relative risk of bleeding events for both bypassing agents, to estimate the average cost of the treatment of HAwI per patient. Third, our study considered that patients with HAwI are adherent to the treatment; however, if patients on prophylaxis were not 100% compliant, drug costs would be lower, and the cost of other healthcare resources would be increased. Four, a 1-year time horizon may be too short to capture less-frequent serious bleeding events such as intracranial ones, usually associated with fatal outcomes and higher costs. Therefore, the results of the present study would be higher, in case a longer time horizon was taken into account (7,17). Fifth, despite their high cost, we did not consider hemophilia-related surgeries, such as orthopedic surgery or joint replacement, because of their low incidence and their small contribution to the annual overall costs associated with the HAwI management (7). Finally, this study did not include other ID costs, such as the negative impact of bleeding events on the quality of life. If those costs were considered, the global results would be higher, especially in on-demand strategies.
Despite these limitations, our study updated the calculations about the cost of the management of HAwI in Spain, including new alternatives, as the prophylaxis with emicizumab. A multicriteria decision analysis was recently developed to evaluate the value of emicizumab in our country. The authors concluded that emicizumab may change the clinical course of the disease, as it showed better efficacy than the current alternatives. Besides, as emicizumab can be self-administered subcutaneously once weekly, it could improve the patient’s quality of life, and patients’ and caregivers’ working life would be less affected due to reduction of the hospital attendance (15). In agreement with previous studies, our study confirms that the emicizumab therapy is the least expensive prophylactic alternative, as it reduces the cost of other healthcare resources, as well as ID costs.
Future economic evaluations should aim at comparing the efficiency of the prophylaxis treatment vs. on demand treatments, from the Spanish social perspective.
Conclusion
Our study shows that the reduction in the bleeding events and the frequency of administration of emicizumab, that can be self-administered at home, to patients with HAwI result in cost savings for the National Health System and society, compared to the prophylaxis with other alternatives.
Authors’ contributions
B.G., E.R.B., and A.G.D. conceived and designed this study. A.D., Y.I., I.P.R., and A.G.D. developed the study and interpreted the results. A.D., Y.I., I.P.R., A.G.D., B.G., and E.R.B. wrote the drafts of this manuscript. S.B., M.T.A., R.N., and J.L.P. critically revised the manuscript. All authors approved the submitted version of the manuscript.
Disclosures
Funding: This study was funded by Roche Farma, although Roche did not influence the results of the study.
Financial disclosure: B.G. and E.R.B. are employees of Roche Farma; A.D., Y.I., I.P.R., and A.G.D. work in Weber, company that received fees from Roche Farma.
S.B., M.T.A., R.N., and J.L.P. have received honorarium from Roche Farma during the conduct of the study. M.T.A. has also received honoraria for speaking and consulting, and funds for research from Takeda, Bayer, CSL-Behring, Grifols, Novo Nordisk, Sobi, Octapharma, Roche Farma, Amgen, Novartis, and Pfizer. R. N. has also received honoraria for speaking in conferences sponsored by Novo Nordisk, Takeda, Grifols, Roche, Pfizer, Octapharma, CSL-Behring and Sobi.
References
- 1. Ministerio de Sanidad, Servicios Sociales e Igualdad: Hemofilia. Online Accessed May 8, 2019.
- 2. Grupo de hemostasia y trombosis: Guía asistencial de hemofilia en Castilla y León. Online Accessed May 8, 2019.
- 3. Aznar JA, Altisent C, Álvarez-Román MT, Bonanad S, Mingot-Castellano ME, López MF. Moderate and severe haemophilia in Spain: an epidemiological update. Haemophilia. 2018;24(3):e136-e139. CrossRef PubMed
- 4. Federación Española de Hemofilia (FEDHEMO). Online Accessed June 3, 2020.
- 5. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS): Informe de Posicionamiento Terapéutico de emicizumab (Hemlibra®) en hemofilia A con inhibidores del factor VIII. Online (2019). Accessed June 20, 2019.
- 6. López-Fernández MF, Altisent Roca C, Álvarez-Román MT, et al. Spanish Consensus Guidelines on prophylaxis with bypassing agents in patients with haemophilia and inhibitors. Thromb Haemost. 2016;115(5):872-895. CrossRef PubMed
- 7. Villarrubia R, Oyagüez I, Álvarez-Román MT, Mingot-Castellano ME, Parra R, Casado MA. Cost analysis of prophylaxis with activated prothrombin complex concentrate vs. on-demand therapy with activated factor VII in severe haemophilia A patients with inhibitors, in Spain. Haemophilia. 2015;21(3):320-329. CrossRef PubMed
- 8. Mingot-Castellano ME, Álvarez-Román MT, López-Fernández MF, et al. Spanish consensus guidelines on prophylaxis with bypassing agents for surgery in patients with haemophilia and inhibitors. Eur J Haematol. 2016;96(5):461-474. CrossRef PubMed
- 9. Leissinger CA. Prevention of bleeds in hemophilia patients with inhibitors: emerging data and clinical direction. Am J Hematol. 2004;77(2):187-193. CrossRef PubMed
- 10. Hay CR, Ludlam CA, Colvin BT, et al; UK Haemophilia Centre Directors Organisation. Factor VIII inhibitors in mild and moderate-severity haemophilia A. Thromb Haemost. 1998;79(4):762-766. CrossRef PubMed
- 11. Leissinger C, Cooper DL, Solem CT; HTRS Investigators. Assessing the impact of age, race, ethnicity and inhibitor status on functional limitations of patients with severe and moderately severe haemophilia A. Haemophilia. 2011;17(6):884-889. CrossRef PubMed
- 12. Walsh CE, Soucie JM, Miller CH; United States Hemophilia Treatment Center Network. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90(5):400-405. CrossRef PubMed
- 13. European Medicines Agency (EMA). First-in-class medicine to prevent bleeding in haemophilia A patients with inhibitors. Online Accessed March 12, 2020.
- 14. European Medicine Agency: Hemlibra. Ficha técnica o resumen de las características del producto. Online Accessed June 25, 2019.
- 15. Álvarez-Román MT, Cuervo-Arango I, Pérez-Santamarina R, et al. Determining the value contribution of emicizumab (Hemlibra®) for the prophylaxis of haemophilia A with inhibitors in Spain by multi-criteria decision analysis. Glob Reg Health Technol Assess. 2019;1-8. CrossRef
- 16. Skinner M. Emicizumab prophylaxis improves long-term health-related quality of life (HRQoL) in Persons with Haemophilia A (PwHA) with and without inhibitors: update from the HAVEN 3 and HAVEN 4 studies. Online Accessed February 25, 2020.
- 17. Jiménez-Yuste V, Álvarez Román MT, Mingot-Castellano ME, Fernández Mosteirin N, Mareque M, Oyagüez I. Análisis de costes del tratamiento para pacientes con hemofilia A con inhibidor en España. PharmacoEcon Span Res Artic. 2018;15(1-4):25-34. CrossRef
- 18. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS): CIMA. Feiba 25 U/mL polvo y disolvente para solución para perfusión. Ficha técnica o resumen de las características del producto. Online Accessed May 28, 2019.
- 19. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS): CIMA. NovoSeven. Online Accessed November 6, 2019.
- 20. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809-818. CrossRef PubMed
- 21. Young G, Oldenburg J, Liesner R, et al. HAVEN 2: efficacy, safety and pharmacokinetics of once-weekly prophylactic emicizumab (ACE910) in pediatric patients (<12 years) with hemophilia A with inhibitors: interim analysis of single-arm, multicenter, open-label, phase 3 study. American Society of Hematology. 60th ASH Annual Meeting and Exposition; 2018 Dec 1; San Diego, California.
- 22. Mahlangu J, Oldenburg J, Callaghan MU, et al. Bleeding events and safety outcomes in persons with haemophilia A with inhibitors: a prospective, multi-centre, non-interventional study. Haemophilia. 2018;24(6):921-929. CrossRef PubMed
- 23. Dimichele D, Négrier C. A retrospective postlicensure survey of FEIBA efficacy and safety. Haemophilia. 2006 Jul;12(4):352-362. CrossRef PubMed
- 24. National Statistics Institute. Working market. Online Accessed November 4, 2019.
- 25. Aznar JA, Lucía F, Abad-Franch L, et al. Haemophilia in Spain. Haemophilia. 2009;15(3):665-675. CrossRef PubMed
- 26. Consejo General de Colegios Oficiales de Farmacéuticos: Bot Plus 2.0 Base de Datos de Medicamentos. Online Accessed February 27, 2019.
- 27. Ministerio de Sanidad: Listado de Medicamentos afectados por las deducciones del RD 8/2010. Online Accessed February 27, 2019.
- 28. Median value of the unit costs for Autonomous Communities (2019).
- 29. Ministerio de Sanidad, Consumo y Bienestar Social. Subdirección General de Información Sanitaria. Registro de Actividad de Atención Especializada – RAE-CMBD. Online Accessed August 29, 2019.
- 30. Bastida JL, Linertová R, Aguilar PS, Hens Pérez M, Posada de la Paz M, Oliva Moreno J. Los costes socioeconómicos y la calidad de vida relacionada con la salud en pacientes con enfermedades raras en España. IMSERSO. 114 (2012). Online Accessed March 5, 2020.
- 31. van den Berg B, Brouwer WBF, Koopmanschap MA. Economic valuation of informal care. An overview of methods and applications. Eur J Health Econ. 2004;5(1):36-45. CrossRef PubMed
- 32. Zhou Z-Y, Hay JW. Efficacy of bypassing agents in patients with hemophilia and inhibitors: a systematic review and meta-analysis. Clin Ther. 2012;34(2):434-445. CrossRef PubMed
- 33. O’Hara J, Hughes D, Camp C, Burke T, Carroll L, Diego DG. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106. CrossRef PubMed
- 34. Oak B, Nambiar S, Springs S, Eguale T. A comparison of prophylactic versus on-demand treatment regimens of coagulation factor VIII for bleeding and joint outcomes in Hemophilia A patients without inhibitors: a systematic review and meta-analysis. Value Health. 2018;21:S247. CrossRef
- 35. Nugent D, O’Mahony B, Dolan G; International Haemophilia Access Strategy Council. Value of prophylaxis vs on-demand treatment: application of a value framework in hemophilia. Haemophilia. 2018;24(5):755-765. CrossRef PubMed
- 36. Castaman G, Linari S. Prophylactic versus on-demand treatments for hemophilia: advantages and drawbacks. Expert Rev Hematol. 2018;11(7):567-576. CrossRef PubMed
- 37. Earnshaw SR, Graham CN, McDade CL, Spears JB, Kessler CM. Factor VIII alloantibody inhibitors: cost analysis of immune tolerance induction vs. prophylaxis and on-demand with bypass treatment. Haemophilia. 2015;21(3):310-319. CrossRef PubMed
- 38. Cortesi PA, Castaman G, Trifirò G, et al. Cost-effectiveness and budget impact of emicizumab prophylaxis in haemophilia A patients with inhibitors. Thromb Haemost. 2020;120(2):216-228. CrossRef PubMed
- 39. Patel AM, Corman SL, Chaplin S, Raimundo K, Sidonio RF. Economic impact model of delayed inhibitor development in patients with hemophilia a receiving emicizumab for the prevention of bleeding events. J Med Econ. 2019;22(12):1328-1337. CrossRef PubMed