 |
Glob Reg Health Technol Assess 2020; 7(1): 40-49 DOI: 10.33393/grhta.2020.2113 ORIGINAL RESEARCH ARTICLE |
 |
Budget impact analysis of extended half-life recombinant factor IX (rFIXFc) in the treatment of haemophilia B
ABSTRACT
Introduction: Prophylaxis with factor IX (FIX) concentrates, produced by recombinant DNA technology (rFIX) or human plasma-derived concentrates, is the treatment of choice for haemophilia B (HB); rFIX covalently fused to the Fc domain of human immunoglobulin G1 (rFIXFc) allows for prophylaxis/treatment with one infusion every 7-14 days. The purpose of this study is to quantify the financial impact of prophylaxis with rFIXFc vs. other approved rFIX and reimbursed for treatment of HB in Italy.
Methods: The number of patients was estimated according to Italian epidemiological data and use of rFIX. Dose and frequency of administration used for weekly prophylaxis were those recommended in the Summary of Product Characteristics (SPC), while clinical trials and literature data were used to calculate bleeding rates and management. Drug costs were calculated using regional ex-factory net prices. In the model, a reference scenario (Reference) vs. an alternative scenario (Alternative) were created to account for introduction of rFIXFc, estimating an increasing trend of the market share of rFIXFc in a 3-year timeframe. The analysis was developed in the perspective of the National Health Service and included healthcare costs related to rFIX for prophylaxis and resolution of bleeding events.
Results: The model estimated an overall cumulative expenditure (years 1-3) of €209,453,646 for the Reference and €207,465,568 for Alternative scenarios, with calculated cumulative savings of €1,988,068.
Conclusions: The increasing use of rFIXFc as a substitute for other rFIX concentrates in the treatment of HB can represent a financially viable choice for the Italian National Health Service while ensuring effective control of bleeding.
Keywords: Budget impact analysis, Haemophilia B, rFIXFc
Received: April 2, 2020
Accepted: June 10, 2020
Published online: July 14, 2020
© 2020 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Any commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Haemophilia B is a rare, potentially disabling congenital haemorrhagic disease caused by deficiency of coagulation factor IX (FIX). The condition mainly affects male subjects and its natural history in the absence of adequate treatment is marked by muscle and joint haemorrhages that lead to disabling arthropathy characterized by functional limitations and chronic pain (1). Severity of bleeding symptoms is related to residual plasma levels of FIX, and development of arthropathy is more frequent in severe forms of the disease (2).
Haemophilia is classified as mild when the biological activity of FIX is between 5% and 40%, with symptoms like bleeding secondary to minor trauma, surgery, or invasive procedures (e.g. dental extraction), although spontaneous bleeding is also possible. When biological activity of FIX is between 1% and 5%, this is considered to be moderate haemophilia with pathological bleeding secondary to minor trauma, surgery, or invasive procedures, while spontaneous bleeding is rare (2).
With biological activity of FIX <1%, haemophilia is considered severe, being characterized by frequent spontaneous bleeding as well as joint bleeding secondary to minor trauma, surgery, or invasive procedures (2). Haemophilia B has an incidence of 3 cases per 100,000 live male births. According to the Associazione Italiana Centri Emofilia (AICE) national registry data, of all patients with a known diagnosis of haemophilia B in Italy in 2017, 43.9% were affected by mild form, 21.1% by moderate form, and 35.0% by severe form (3).
The treatment of haemophilia is based on replacement therapy with administration of FIX concentrates according to prophylactic or on-demand regimens. According to the latest estimates, in Italy, prophylaxis is the most adopted therapeutic regimen in patients with severe haemophilia B (85.1%) and is prescribed in about 40% of patients with moderate haemophilia (3).
The primary goal of prophylaxis is prevention of joint damage. Prophylaxis entails regular intravenous infusions of FIX concentrates in order to prevent the onset of spontaneous bleeding (especially in the joints) by maintaining a minimum level of FIX in plasma (trough level) in the range of moderate to mild haemophilia (1). With prophylaxis, it is possible to prevent the onset of joint damage or at least to slow its progression and to reduce the number of bleeding episodes, which gives patients the opportunity to lead a relatively normal life (1,4,5,6,7,8,9,10).
Given the greater ease of use (e.g. reduced infusion volumes, faster preparation times) and better safety profile (11,12), whenever possible, recombinant products (rFIX) are preferred over plasma-derived factors (pFIX) (3). Indeed, in Italy, rFIX is more commonly used than pFIX (3).
Given the need for repeated intravenous administrations, adherence to prophylaxis can be problematic, which can lead to missed administrations and/or under-dosing (13,14,15,16,17,18). Poor adherence can thus be associated with an increase in bleeding rates due to the reduced protection conferred by intermittent treatment (19,20,21,22). The main barriers to treatment adherence include the time needed for infusion, planning of the infusion, and problems in venous access (19,22).
In recent years, new rFIX molecules were registered and reimbursed, with improved pharmacokinetic profile in order to ensure good haemostatic coverage with a lower number of infusions (3,23,24,25,26). On average, half-life of these new molecules is 4-5 times the normal physiological value, allowing prophylactic infusions every 7, 10, or ≥14 days. This is in contrast to prophylaxis with standard products, requiring 2-3 infusions per week (27,28,29,30).
Thanks to these characteristics, the extended half-life of rFIXs offers greater therapeutic flexibility, with better adherence, greater access to prophylaxis, better long-term outcomes, and potential cost savings (10,17).
Considering the economic impact of these drugs on the National Health Service (NHS), the most recent data published by the National Observatory on the use of Medicines (OsMed) reported that in Italy, in 2018, coagulation factors were among the drug categories with the greatest impact on healthcare expenditures (31).
Among these new molecules, rFIX covalently fused to the Fc domain of human immunoglobulin G1 (rFIXFc) has been available in Italy for the treatment of patients with haemophilia B since 2017 (3,32). Prophylaxis with rFIXFc has been shown to be safe and effective for the prevention and treatment of bleeding events in both paediatric and adult haemophilia B populations (23,25). Fusion with the Fc fragment of human IgG1 leaves the functional capacity of FIX intact, with no observed increase in immunogenicity. Pharmacokinetic and efficacy data support the potential for treatment at prolonged intervals, while providing excellent bleeding control vs. shorter half-life rFIX molecules in patients of all ages (23,25).
The aim of this study was to estimate the economic impact of the use of prophylaxis with rFIXFc compared to other rFIX that are available in Italy for the treatment of moderate to severe haemophilia B.
Methods
The analysis was carried out from the perspective of the NHS considering a 3-year period, including only direct healthcare costs related to use of rFIX for prophylaxis and resolution of bleeding episodes.
The number of eligible patients was estimated from Italian population data and prevalence of the disease (3,33). The haemophilia B population was then stratified by age (<6, 6-11, 12-17, and ≥18 years).
In each age group, the mean per patient body weight was considered: (a) 20 kg in subjects <6 years; (b) 30 kg for ages 6-11 years; (c) 55 kg for ages 12-17 years; (d) 70 kg in adults.
Percentage of haemophilia B adults treated with rFIX prophylaxis was based on consumption data from the AICE national registry (3).
Calculation of percentage of paediatric patients treated with rFIX prophylaxis was based on the following assumptions:
a)In patients <6 years and patients aged 12-18 years, prophylaxis is used in 50% of patients with moderate haemophilia and in 80% of patients with severe haemophilia.
b)In the 6-11 year age group, prophylaxis is used in 70% of patients with moderate haemophilia and in 85% of patients with severe haemophilia.
Two extended half-life rFIX concentrates were included in the model: (1) rFIXFc (eftrenonacog alfa; Alprolix®) and (2) rIX-FP, a recombinant fusion protein containing rFIX fused with recombinant albumin (rIX-FP – albutrepenonacog alfa; Idelvion®); two standard half-life rFIX concentrates were included: (1) nonacog gamma (Rixubis®) and (2) nonacog alfa (Benefix®). Market shares for each rFIX were estimated based on 2018 market data and projected for the next 3 years.
Doses and frequencies of administration by age (<12 years vs. ≥12 years) in prophylaxis were obtained from the respective Summary of Product Characteristics (SPC) of each rFIX concentrate included in the model (27,28,29,30). For extended half-life rFIX, weekly prophylactic administrations were considered in the base case scenario, that is, one administration per week (27,28).
Bleeding rates and the amount of rFIX used for each product were based on literature data (12,34,35). According to clinical practice and guidelines, bleeding episodes are treated with an additional dose of FIX products (1). rFIXFc and rIX-FP bleeding rates and the amount of rFIX used to manage bleedings were retrieved from the pivotal clinical trials (23,24,25,26).
Additional data were estimated as follows:
a)rIX-FP, albutrepenonacog alfa: in order to estimate the amount of drug used to treat a bleeding episode in adults and children, the mean number of administrations derived from the clinical study was multiplied by the dose indicated for prophylaxis (24,26).
b)Nonacog gamma: to estimate the amount of drug used in children <12 years of age, bleeding rates were taken from the EPAR (36), and rFIX dose was considered to be the same as administered in adults (12).
c)Nonacog alfa: mean dose used for prophylaxis was applied to management of bleeding episodes, multiplied by the mean number of infusions needed to treat a bleeding episode (34).
Unit cost for each rFIX was calculated as average price from published regional tenders in seven Italian regions (Piedmont, Veneto, Tuscany, Lazio, Campania, Puglia and Sicily) (37).
Two scenarios are compared in the model: a reference scenario (Reference) and an alternative scenario (Alternative) in which increased rFIXFc market shares replace the other rFIX considered.
The base case analysis considered rFIXFc and rIX-FP prophylaxis to be administered once every 7 days. Sensitivity analysis was also carried out in adult patients, considering administrations once every 10 or 14 days as reported in the respective SPC (27,28). Specifically, administration every 14 days was considered for rIX-FP, and every 10 or 14 days for rFIXFc. This also allowed to identify, given a sample of 100 patients for each arm, in what percentage of patients receiving rFIXFc every 10 days the two treatments reached parity price.
Results
Base case analysis
Based on population data, epidemiology, and drug consumption in Italy (3,33) and on the hypotheses mentioned for paediatric patients, it was estimated that 308 patients (245 adults) at the beginning of the 3-year period had haemophilia B and were receiving prophylaxis with rFIX; and 311 patients (247 adults) in in the third year (Tab. I). The number of patients treated with the different rFIX therapies was estimated on the basis of annual market shares, with constant shares for therapies over the 3-year period in the reference scenario (Reference) and a share of 27.4% for rFIXFc. In the alternative scenario (Alternative), an increase in the market share for rFIXFc (ranging from 33.5% in year 1 to 46.0% in year 3) and a decrease in the other therapies were considered; it seems reasonable to forecast that the market may progressively move towards an increased use of the less expensive alternative as prophylaxis with rFIXFc was less expensive than prophylaxis with rIX-FP. Market shares were assumed to be the same across all patient age groups (Tab. II).
To calculate the costs of prophylaxis and management of a single bleeding episode, data on body weight by age group, mean dose, and number of administrations needed for prophylaxis and total dose in international units (IU) per kg per patient were considered (Tab. III). These quantities were multiplied by the average regional tender prices to account for local territorial specificities (37); therefore, the mean costs per patient were estimated for prophylaxis and for management of a single bleeding episode by treatment and by patient age/body weight (Tab. IV). Overall, the mean annual costs for weekly prophylaxis with rFIXFc was €69,392 in children <6 years and €220,792 in adults. This cost was slightly higher than the cost of the two standard half-life products, with small differences vs. gamma nonacog for which an even higher cost was estimated in paediatric patients (Tab. IV). The lower consumption of rFIXFc vs. standard half-life products largely compensated its higher unit price, ultimately resulting in comparable overall cost per patient. Conversely, rIX-FP prophylaxis, considering the higher unit price, was more costly than the other rFIX. For example, additional costs vs. rFIXFc ranged from +€18,124 (+26.1%) in patients <6 years to +€85,514 (+38.7%) in adult patients.
| Age group (years) | <6 years | 6-11 years | 12-17 years | ≥18 years | Total | REF |
|---|---|---|---|---|---|---|
| Male population | ||||||
| Year 1 | 1,497,652 | 1,740,807 | 1,783,819 | 24,509,294 | 29,531,572 | (33) |
| Year 2 | 1,477,937 | 1,714,713 | 1,791,778 | 24,585,947 | 29,570,375 | (33) |
| Year 3 | 1,461,599 | 1,683,090 | 1,803,123 | 24,657,668 | 29,605,480 | (33) |
| Data on disease | ||||||
| Prevalence % | 0.003 | 0.003 | 0.003 | 0.003 | (3) | |
| Moderate % | 21.1 | 21.1 | 21.1 | 21.1 | (3) | |
| Severe % | 35.0 | 35.0 | 35.0 | 35.0 | (3) | |
| Consumption | ||||||
| Recombinant % | 100.0 | 100.0 | 100.0 | 86.8 | (3)* | |
| Prophylaxis in moderate patients % | 50.0 | 70.0 | 50.0 | 40.0 | (3)* | |
| Prophylaxis in severe patients % | 80.0 | 85.0 | 80.0 | 85.1 | (3)* | |
| Estimated patients | ||||||
| Year 1 | 18 | 24 | 21 | 245 | 308 | |
| Year 2 | 18 | 24 | 22 | 246 | 310 | |
| Year 3 | 18 | 24 | 22 | 247 | 311 | |
rFIX = recombinant factor IX; *Model assumption, see text for details.
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Reference Scenario | |||
| rFIXFc
eftrenonacog alfa Alprolix® |
27.40% | 27.40% | 27.40% |
| rIX-FP
albutrepenonacog alfa Idelvion® |
29.30% | 29.30% | 29.30% |
| Nonacog gamma
Rixubis® |
6.70% | 6.70% | 6.70% |
| Nonacog alfa
Benefix® |
36.60% | 36.60% | 36.60% |
| Alternative Scenario | |||
| rFIXFc
eftrenonacog alfa Alprolix® |
33.50% | 38.60% | 46.00% |
| rIX-FP
Albutrepenonacog alfa Idelvion® |
27.00% | 25.00% | 22.20% |
| Nonacog gamma
Rixubis® |
5.80% | 4.90% | 4.10% |
| Nonacog alfa
Benefix® |
33.70% | 31.50% | 27.70% |
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
| Age group (years) | <6 | 6-11 | 12-17 | ≥18 | REF | |
| Mean weight | 20 | 30 | 55 | 70 | * | |
| Drug | ||||||
| rFIXFc
eftrenonacog alfa Alprolix® |
Prophylaxis: mean dose IU/kg | 55.00 | 55.00 | 50.00 | 50.00 | (27) |
| Prophylaxis: no. weekly infusions | 1.00 | 1.00 | 1.00 | 1.00 | (27) | |
| Annual bleeding rate | 1.10 | 2.10 | 3.12 | 3.12 | (23,25) | |
| Total dose to resolve bleeding event IU/kg | 68.20 | 68.20 | 51.74 | 51.74 | (23,25) | |
| rIX-FP albutrepenonacog alfa Idelvion® | Prophylaxis: mean dose IU/kg | 42.50 | 42.50 | 42.50 | 42.50 | (28) |
| Prophylaxis: no. weekly infusions | 1.00 | 1.00 | 1.00 | 1.00 | (27) | |
| Annual bleeding rate | 4.09 | 3.44 | 2.22** | 2.22** | (24,26) | |
| Total dose to resolve bleeding event IU/kg | 52.33 | 52.33 | 43.44 | 43.44 | (24,26) | |
| nonacog gamma
Rixubis® |
Prophylaxis: mean dose IU/kg | 60.00 | 60.00 | 50.00 | 50.00 | (29) |
| Prophylaxis: no. weekly infusions | 2.00 | 2.00 | 2.00 | 2.00 | (29) | |
| Annual bleeding rate | 2.70 | 2.70 | 4.26 | 4.26 | (12,36) | |
| Total dose to resolve bleeding event IU/kg | 83.38 | 83.38 | 83.38 | 83.38 | (12) | |
| nonacog alfa
Benefix® |
Prophylaxis: mean dose IU/kg | 63.70 | 63.70 | 40.00 | 40.00 | (30) |
| Prophylaxis: no. weekly infusions | 1.40 | 1.40 | 2.00 | 2.00 | (30) | |
| Annual bleeding rate | 3.90 | 3.90 | 3.78 | 3.78 | (34,35) | |
| Total dose to resolve bleeding event IU/kg | 65.85 | 65.85 | 50.80 | 50.80 | (34,35) | |
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
*Model assumption, see text for details; **The same annual bleeding rate was assumed for patients switching from on-demand to prophylaxis.
| <6 years | 6-11 years | 12-17 years | ≥18 years | REF | ||
|---|---|---|---|---|---|---|
| rFIXFc
eftrenonacog alfa Alprolix® |
Mean price IU | € 1.21 | (37) | |||
| Mean cost prophylaxis | €69,392 | €104,088 | €173,479 | €220,792 | ||
| Mean costs for resolution of bleeding event | €1,655 | €2,482 | €3,452 | €4,393 | ||
| rIX-FP
Albutrepenonacog alfa Idelvion® |
Mean price IU | €1.98 | (37) | |||
| Mean cost prophylaxis | €87,516 | €131,274 | €240,669 | €306,306 | ||
| Mean costs for resolution of bleeding event | €2,072 | €3,108 | €4,731 | €6,021 | ||
| Nonacog gamma
Rixubis® |
Mean price IU | €0.60 | (37) | |||
| Mean cost prophylaxis | €74,358 | €111,538 | €170,405 | €216,879 | ||
| Mean costs for resolution of bleeding event | €994 | €1,490 | €2,732 | €3,478 | ||
| Nonacog alfa
Benefix® |
Mean price IU | €0.68 | (37) | |||
| Mean cost prophylaxis | €63,329 | €94,993 | €156,227 | €198,834 | ||
| Mean costs for resolution of bleeding event | €899 | €1,349 | €1,908 | €2,428 | ||
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
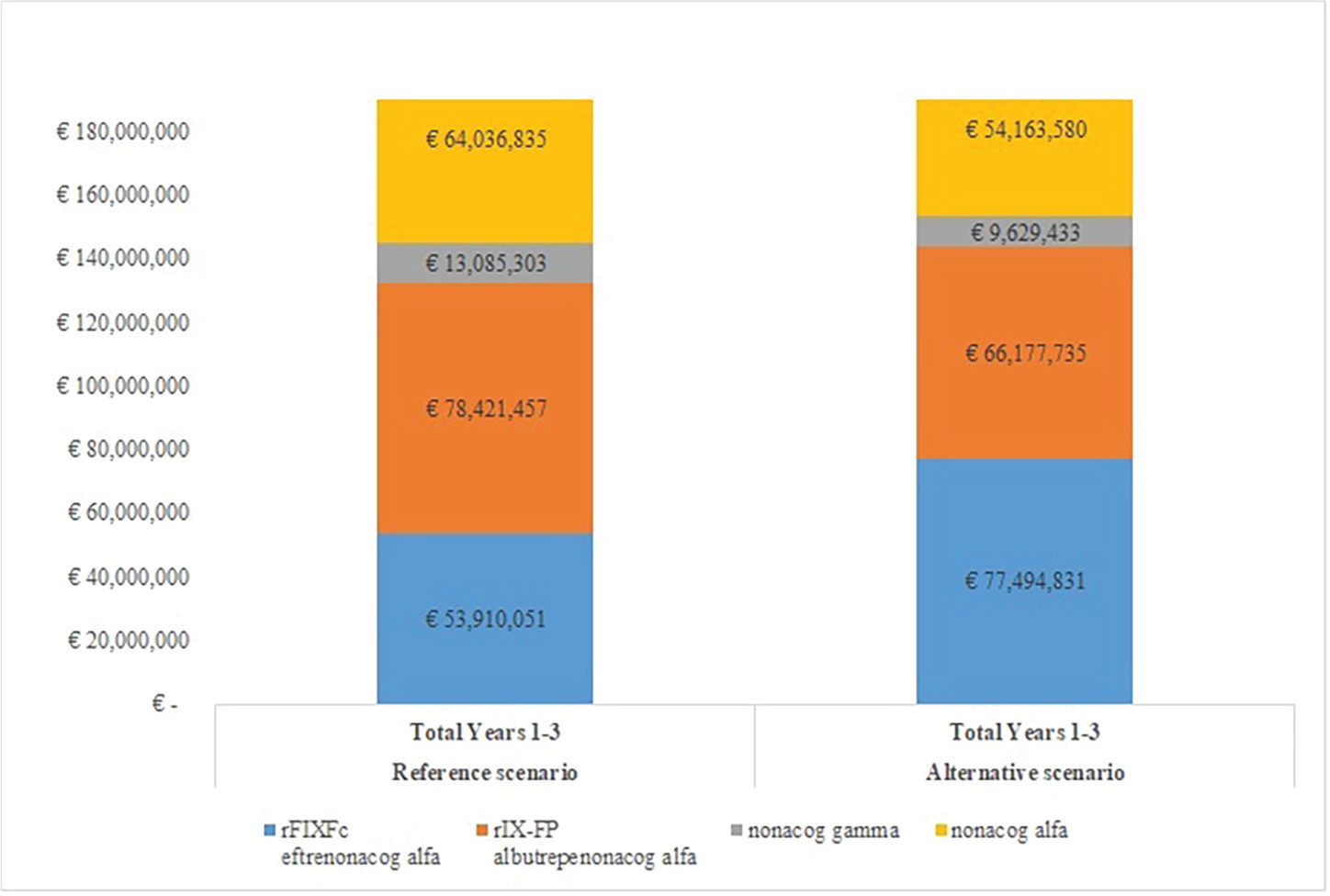
Budgetary impact was calculated from the number of patients being treated with each drug (Tabs. I and II), multiplied by the cost of prophylaxis with each rFIX. The cost for management of bleeding episodes was then added to the costs of prophylaxis: this was calculated by multiplying the number of patients by the annual bleeding rate, costs for managing a single bleeding episode by treatment used, and age/body weight class. The results are detailed in Table V and Figures 1 and 2. The model showed a total cumulative cost over 3 years of €209,453,646 in the Reference scenario and €207,465,578 in the Alternative scenario (Tab. V; Fig. 1). The costs of prophylaxis are thus the vast majority of costs, that is, 95.12% in the Reference scenario and 95.00% in the Alternative scenario.
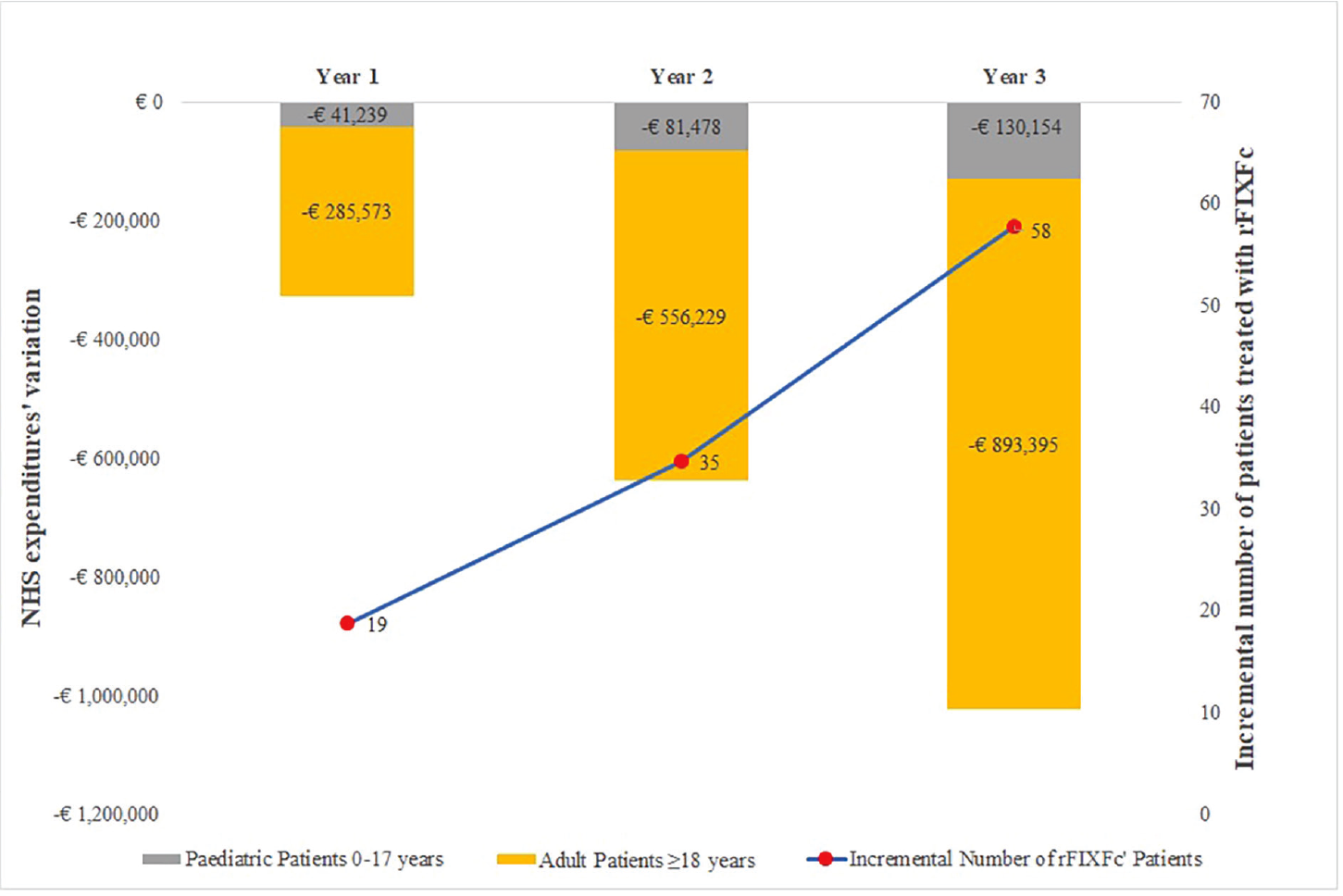
Considering the greater number of patients being treated with rFIXFc, that is, 19 patients in year 1 and 58 in year 3, together with an increase in spending for rFIXFc ranging from €3,978,827 for the first year to €12,253,665 for the third, the overall decrease in spending was estimated to be −€326,811 in year 1 to −€1,023,549 in year 3 (Tab. V; Fig. 2). The overall decrease in costs in the 3-year period was estimated to be −€1,988,068 (Fig. 2), considering the weekly prophylactic dosages reported in the respective SPC for rFIX and data from clinical studies and the literature to estimate the number of bleeding episodes and management (12,17,23,24,25,26,27,28,29,30,34,36,38).
Sensitivity analysis
In sensitivity analysis, changes in expenditure (prophylaxis only) were calculated for extended half-life drugs in a hypothetical cohort of 200 adult patients, 100 treated with rFIXFc and 100 with rIX-FP. For rIX-FP, it was assumed that all patients were treated with 75 IU/kg every 14 days, while for rFIXFc, at a dose of 100 IU/kg, the percentage of patients with infusions every 10 or 14 days was considered. This hypothesis, which considers that no patient treated with rIX-FP undergoes administrations every 10 days, is unfavourable towards rFIXFc and was chosen to validate the robustness of the model.
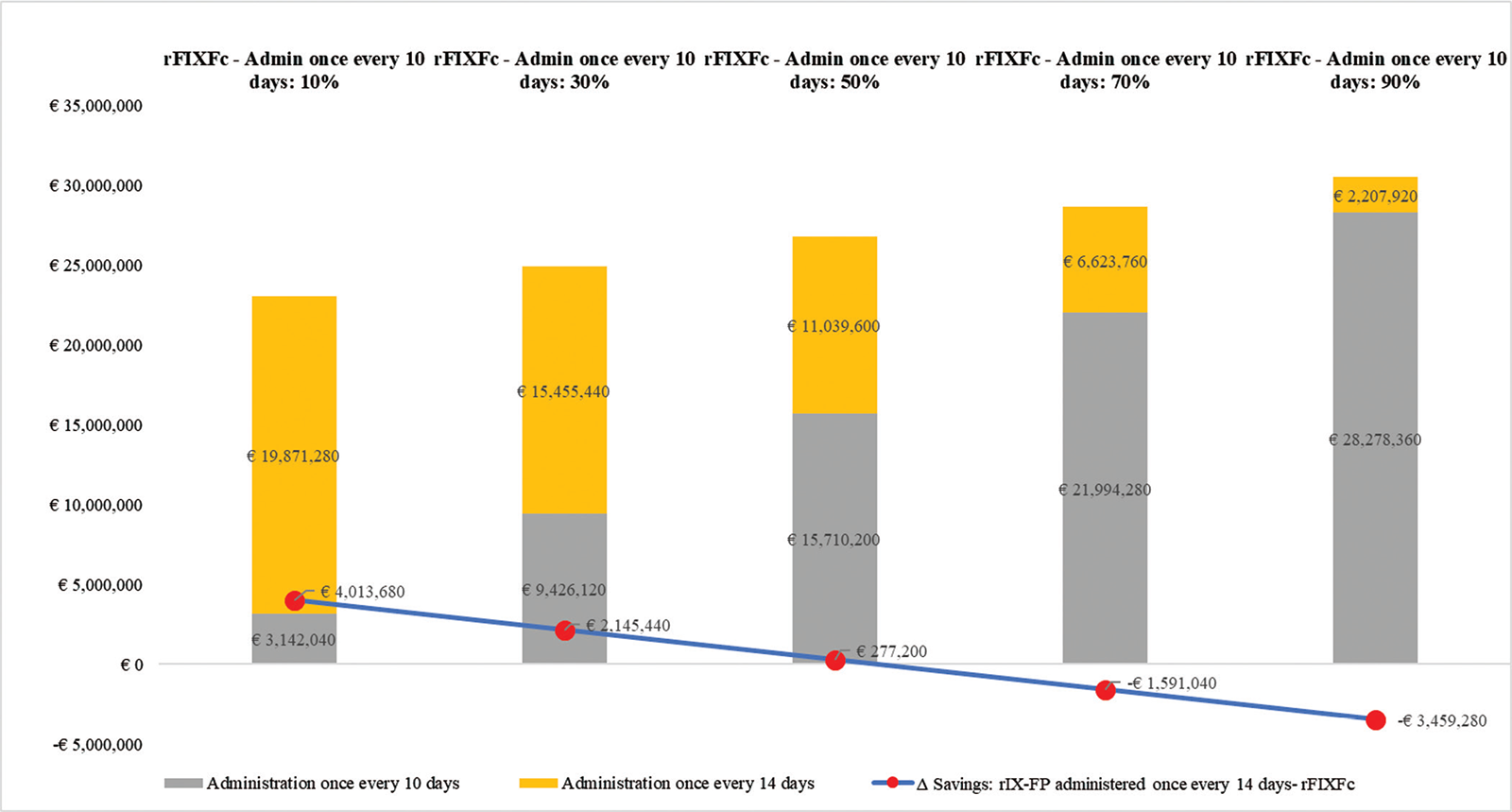
Data show that at the doses recommended in the SPC (27), if one administration every 14 days is considered, the cost of rFIXFc is lower (−€49,478) than the costs of rIX-FP at a dose of 75 IU/kg every 14 days. If rFIXFc is administered as prophylaxis once every 10 days, this results in higher costs per patient per year for prophylaxis compared to rIX-FP (+€43,934). These differences are influenced by the number of administrations considered for rFIXFc (Tab. VI).
The sensitivity analysis, shown in Figure 3, demonstrates that overall costs in the theoretical cohort are influenced by the percentage of patients in the rFIXFc arm with administrations every 10 or 14 days. The parity price is reached when 53% of patients with rFIXFc receive an administration every 10 days; therefore, at any lower patient percentage, rFIXFc therapy would be cost-saving for the NHS.
| Year 1 | Year 2 | Year 3 | Total | |
|---|---|---|---|---|
| Reference Scenario | ||||
| rFIXFc
eftrenonacog alfa Alprolix® |
€17,872,108 | €17,986,845 | €18,051,098 | €53,910,051 |
| rIX-FP
albutrepenonacog alfa Idelvion® |
€25,997,759 | €26,165,017 | €26,258,681 | €78,421,457 |
| nonacog gamma
Rixubis® |
€4,338,113 | €4,365,833 | €4,381,357 | €13,085,303 |
| nonacog-alfa
Benefix® |
€21,229,600 | €21,365,551 | €21,441,684 | €64,036,835 |
| Total | €69,437,580 | €69,883,246 | €70,132,819 | €209,453,646 |
| Alternative Scenario | ||||
| rFIXFc
eftrenonacog alfa Alprolix® |
€21,850,935 | €25,339,132 | €30,304,763 | €77,494,831 |
| rIX-FP
albutrepenonacog alfa Idelvion® |
€23,956,979 | €22,325,100 | €19,895,656 | €66,177,735 |
| nonacog gamma
Rixubis® |
€3,755,381 | €3,192,923 | €2,681,129 | €9,629,433 |
| nonacog alfa
Benefix® |
€19,547,473 | €18,388,384 | €16,227,722 | €54,163,580 |
| Total | €69,110,769 | €69,245,539 | €69,109,270 | €207,465,578 |
| Δ Alternative scenario – Reference scenario | ||||
| rFIXFc
eftrenonacog alfa Alprolix® |
+€3,978,827 | +€7,352,287 | +€12,253,665 | +€23,584,779 |
| rIX-FP
albutrepenonacog alfa Idelvion® |
−€2,040,780 | −€3,839,917 | −€6,363,025 | −€12,243,722 |
| nonacog gamma
Rixubis® |
−€582,732 | −€1,172,910 | −€1,700,228 | −€3,455,870 |
| nonacog alfa
Benefix® |
−€1,682,127 | −€2,977,167 | −€5,213,961 | −€9,873,255 |
| Total | −€326,811 | −€637,707 | −€1,023,549 | −€1,988,068 |
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
Discussion
Phase III trials for rFIXFc showed that prophylaxis with rFIXFc is safe and effective and associated with significant reductions in bleeding episodes, which represents the desired target in the management of haemophilia B (23,25).
Treatment with extended half-life rFIX has the advantage of fewer administrations, an aspect that is very important for both patients and caregivers (39). To date, only a limited number of studies have evaluated the pharmacoeconomic impact of recombinant therapies in patients with haemophilia B in Italy; only one has assessed the impact of new extended half-life rFIX, with different conclusion compared to our results (34). Pradelli et al. estimated that rFIXFc had higher costs than rIX-FP, since the doses and treatment regimens used were different from those considered in the present analysis (34). In that study, they estimated rIX-FP prophylaxis at a dose of 50 IU/kg, which represents the minimum dose of rIX-FP for once every 14 days administration. This dose was derived from a pharmacokinetic model published by Zhang et al. in 2016 (40); indeed, data were not taken from a clinical trial with efficacy based on patient outcomes (40). In the main Phase III trial in adults (26), the dose investigated was 75 IU/kg administered every 10 or 14 days; if this latter dose had been used, it would likely have given different results, that is, an increase in costs due to an increase in the costs of prophylaxis with rIX-FP 30% higher or more (considering a dose of 50 IU/kg vs. 75 IU/kg) (34).
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
NHS = National Health Service; rFIXFc = recombinant extended half-life factor IX produced with Fc technology.
In the present analysis we used a conservative approach, considering the weekly dosages from the SPC in the base case scenario and 14-day administrations from the SPC in the sensitivity analysis, in which the number of patients receiving rFIXFc and undergoing infusions every 10 or 14 days was assessed, evaluating the impact on mean costs (27,28). As demonstrated in a previous study, over 90% of the costs of haemophilia are attributable to drug therapy (98% of direct healthcare costs and 92% of total costs); accordingly, substantial changes in doses and drug prices would impact the results (41).
| rFIXFc – eftrenonacog-alfa | rIX-FP
albutrepenonacog-alfa Infusion every 14 days |
REF | ||
|---|---|---|---|---|
| Infusion every 10 days | Infusion every 14 days | |||
| Weight of patient | 70 kg | * | ||
| Dose IU/kg | 100 | 100 | 75 | (27,28) |
| Price per IU | €1.21 | €1.21 | €1.98 | (37) |
| Costs of infusion | €8,492 | €8,492 | €10,395 | |
| No. infusions/year | 37 | 26 | 26 | |
| Total costs/year | €314,204 | €220,792 | €270,270 | |
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
*Model assumption, see text for details.
rFIXFc = recombinant extended half-life factor IX produced with Fc technology; rIX-FP = recombinant fusion protein consisting of FIX with albumin moiety.
In light of the difficulty in carrying out direct comparative studies in a rare disease, to reach the higher numbers needed for statistically and clinically significant evidence, and considering the potential consumption of the two extended half-life drugs, we believe that our approach provides conservative estimates and solid results; the sensitivity analysis, based on an unfavourable hypothesis for rFIXFc, is particularly robust and fully supports the economic benefits of rFIXFc.
The major limitations of our analysis are the lack of direct comparison between therapies, although we used the doses from the SPC and bleeding rates from Phase III trials, which even if dissimilar due to the different populations studied still contribute to the robustness of the economic model (27,28,29,30). A detailed review of the Phase III studies of rFIXFc and rIX-FP clearly demonstrates several differences (23,24,25,26):
a)Patient numbers differ significantly in the rIX-FP and rFIXFc Phase III trials.
b)Different pre-study treatment regimens and baseline study populations in both trials may have influenced medical status.
c)The PROLONG-9FP study design included selection of patients.
d)Pharmacokinetic evaluations have been performed with different methodologies, different pre-study products, sampling time points, and at different timelines during the trials.
Real-world evidence data presented at the American Society of Hematology (ASH) in December 2019 by Malec et al. found important differences in bleeding control for rIX-FP vs. rFIXFc in patients with severe haemophilia B (90 patients including 67 treated with extended half-life products). In 26 patients receiving rIX-FP, 16 (62%) had unexpected or poorly controlled bleeding events, while in 37 patients receiving rFIXFc there were no such events (42). In light of this evidence, our assessment appears conservative and possibly underestimates the potential savings that could be obtained with rFIXFc.
Another possible limitation of this study is that the costs of hospitalization, instrumental/laboratory tests, and management of intracranial bleeding were not included; moreover, the costs directly sustained by patients and caregivers (especially relevant in paediatric patients) and work days lost were not taken into account. Since the study by Kodra et al. reported that 92% of total costs and 98% of all healthcare costs are attributable to drugs, we believe our analysis reflects the true economic impact of greater utilization of rFIXFc over other rFIX (41). In addition, it should be emphasized that the model does not take into account advantages that could be possibly obtained with extended half-life drugs compared to standard half-life drugs in terms of adherence (and therefore efficacy) and quality of life. In fact, considering fewer administrations, extended half-life factors, especially in some patient groups, could provide greater adherence and thus greater efficacy in real-life management; therapies with poor adherence may be less effective, leading to unnecessary and unjustified costs for the NHS and the society as a whole.
Lastly, the sensitivity analysis validated the results by using scenarios that are particularly unfavourable to rFIXFc by assuming infusion every 10 days, while for rIX-FP administration every 14 days was deliberately considered. Administration of rIX-FP every 10 days would produce a cost of prophylaxis in adults of €384,615 per patient per year, well above the cost of the every 14 days option considered in the model. The 10 days administration interval considered for rFIXFc is certainly the most unfavourable for the product: in fact, in the pivotal study, 53.8% of patients, in the group with an interval-adjusted prophylaxis, had reached an interval of 14 days or more in the last 3 months of therapy (25). This further confirms the validity of the present economic analysis: despite the unfavourable hypotheses for rFIXFc, there is still economic benefit for the NHS compared to the other factors with extended half-life.
Conclusions
In conclusion, an increase in the use of rFIXFc, replacing other rFIX concentrates for treatment of haemophilia B, can represent an economically advantageous choice for the NHS with overall savings, at the national level, over the 3-year period of €1,988,068, while ensuring effective control of bleedings. Sensitivity analyses confirmed the robustness of the results.
Disclosures
Conflict of Interest: AA and BP are employees of Certara Italy S.r.l.; MME reports personal fees from Swedish Orphan Biovitrum (Sobi), Bayer Healthcare, CSL Behring, Grifols, Kedrion, Novo Nordisk, Octapharma, Pfizer, Roche; CA and TC are employees of Sobi S.r.l., Milan, Italy.
Financial support: Sobi S.r.l., Milan, Italy, has provided financial support to cover the cost of this project and the editorial assistance, but has not influenced the manuscript content.
References
- 1. World Federation of Haemophilia. Guidelines for the management of Haemophilia. 2nd ed. 2013. Available at: https://www1.wfh.org/publications/files/pdf-1472.pdf accessed 12/06/2020.
- 2. ORPHANET. Emofilia B. Available at: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=IT&Expert=98879 accessed 12/06/20
- 3. Abbonizio F, Hassan HJ, Riccioni R, Arcieri R, Associazione Italiana Centri Emofilia (AICE), Giampaolo. Registro Nazionale delle Coagulopatie Congenite. Rapporto 2017. Roma: Istituto Superiore di Sanità 2019 (Rapporti ISTISAN 19/8).
- 4. Budde U, Drewke E. Von Willebrand factor multimers in virus-inactivated plasmas and F VIII concentrates. Beitr Infusionsther Transfusionsmed 1994;32:408-414.
- 5. Chin S, Williams B, Gottlieb P, et al. Virucidal short wavelength ultraviolet light treatment of plasma and factor VIII concentrate: protection of proteins by antioxidants. Blood 1995 Dec 1;86(11):4331-4336.
- 6. Chuansumrit A, Isarangkura P, Chantanakajornfung A, et al. The efficacy and safety of lyophilized cryoprecipitate in haemophilia A. J Med Assoc Thai 1999 Nov;82 Suppl 1:S69-S73.
- 7. El-Ekiaby M, Sayed MA, Caron C, et al. Solvent-detergent filtered (S/D-F) fresh frozen plasma and cryoprecipitate minipools prepared in a newly designed integral disposable processing bag system. Transfus Med 2010 Feb;20(1):48-61.
- 8. Franchini M, Rossetti G, Tagliaferri A, et al. Dental procedures in adult patients with hereditary bleeding disorders: 10 years experience in three Italian haemophilia centers. Haemophilia 2005;11:504-509.
- 9. Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood 1997 Oct 1;90(7):2515-2521.
- 10. World Federation of Hemophilia. What is prophylaxis. Last Updated December 2014. Available at: https://elearning.wfh.org/elearning-centres/prophylaxis/#what_is_prophylaxis accessed 12/06/2020.
- 11. Roth DA, Kessler CM, Pasi KJ, Rup B, Courter SG, Tubridy KL. Human recombinant factor IX: safety and efficacy studies in haemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood 2001 Dec 15;98(13):3600-3606.
- 12. Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicentre phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level </=2%) haemophilia B. Haemophilia 2014 Jan;20(1):15-24.
- 13. De Moerloose P, Urbancik W, Van Den Berg HM, Richards M. A survey of adherence to haemophilia therapy in six European countries: results and recommendations. Haemophilia 2008 Sep;14(5):931-938.
- 14. du Treil S, Rice J, Leissinger CA. Quantifying adherence to treatment and its relationship to quality of life in a well-characterized haemophilia population. Haemophilia 2007 Sep;13(5):493-501.
- 15. Duncan N, Kronenberger W, Roberson C, Shapiro A. VERITAS-Pro: a new measure of adherence to prophylactic regimens in haemophilia. Haemophilia 2010 Mar;16(2):247-255.
- 16. Geraghty S, Dunkley T, Harrington C, Lindvall K, Maahs J, Sek J. Practice patterns in haemophilia A therapy – global progress towards optimal care. Haemophilia 2006 Jan;12(1):75-81.
- 17. Iorio A, Krishnan S, Myren KJ, et al. Continuous prophylaxis with recombinant factor IX Fc fusion protein and conventional recombinant factor IX products: comparisons of efficacy and weekly factor consumption. J Med Econ 2017 Apr;20(4):337-344.
- 18. Schrijvers LH, Uitslager N, Schuurmans MJ, Fischer K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: a systematic review. Haemophilia 2013 May;19(3):355-361.
- 19. Hacker MR, Geraghty S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia 2001 Jul;7(4):392-396.
- 20. Krishnan S, Vietri J, Furlan R, Duncan N. Adherence to prophylaxis is associated with better outcomes in moderate and severe haemophilia: results of a patient survey. Haemophilia 2015 Jan;21(1):64-70.
- 21. McLaughlin JM, Witkop ML, Lambing A, Anderson TL, Munn J, Tortella B. Better adherence to prescribed treatment regimen is related to less chronic pain among adolescents and young adults with moderate or severe haemophilia. Haemophilia 2014 Jul;20(4):506-512.
- 22. Schrijvers LH, Kars MC, Beijlevelt-van der Zande M, Peters M, Schuurmans MJ, Fischer K. Unravelling adherence to prophylaxis in haemophilia: a patients’ perspective. Haemophilia 2015 Sep;21(5):612-621.
- 23. Fischer K, Kulkarni R, Nolan B, et al. Recombinant factor IX Fc fusion protein in children with haemophilia B (Kids B-LONG): results from a multicentre, non-randomised phase 3 study. Lancet Haematol 2017 Feb;4(2):e75-e82.
- 24. Kenet G, Chambost H, Male C, et al. Long-acting recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in children. Results of a phase 3 trial. Thromb Haemost 2016 Sep 27;116(4):659-668.
- 25. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in haemophilia B. N Engl J Med 2013 Dec 12;369(24):2313-2323.
- 26. Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in haemophilia B: results of a phase 3 trial. Blood 2016 Apr 7;127(14):1761-1769.
- 27. European Medicine Agency (EMA). Alprolix: EPAR – Product Information. Last updated 10/04/2019. Available at: https://www.ema.europa.eu/en/documents/product-information/alprolix-epar-product-information_it.pdf accessed 12/06/2020.
- 28. European Medicine Agency (EMA). Idelvion: EPAR – Product Information. Last updated 28/05/2020. Available at: https://www.ema.europa.eu/en/documents/product-information/idelvion-epar-product-information_it.pdf accessed 12/06/2020.
- 29. European Medicine Agency (EMA). Rixubis: EPAR – Product Information. Last updated 16/03/2020. Available at: https://www.ema.europa.eu/en/documents/product-information/rixubis-epar-product-information_it.pdf accessed 12/06/2020.
- 30. European Medicine Agency (EMA). Benefix: EPAR – Product Information. Last updated 18/01/2019. Available at: https://www.ema.europa.eu/en/documents/product-information/benefix-epar-product-information_it.pdf accessed 12/06/2020.
- 31. Osservatorio Nazionale sull’impiego dei Medicinali. L’uso dei farmaci in Italia. Rapporto Nazionale Anno 2018. Roma: Agenzia Italiana del Farmaco 2019.
- 32. Gazzetta Ufficiale della Repubblica Italiana. Available at: http://95.110.157.84/gazzettaufficiale.biz/emittenti/elencoEmittente9.htm accessed 12/06/2020.
- 33. Istituto Nazionale di Statistica (ISTAT). Previsioni della Popolazione anno 2017-2065. Available at: http://demo.istat.it/ accessed 12/06/2020.
- 34. Pradelli L, Villa S, Castaman G. Albutrepenonacog alfa (Idelvion®) for the treatment of Italian patients with haemophilia B: a budget impact model. Farmeconomia. Health Econ Therapeut Pathways 2018;19(1):1-10.
- 35. Valentino LA, Rusen L, Elezovic I, Smith LM, Korth-Bradley JM, Rendo P. Multicentre, randomized, open-label study of on-demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in haemophilia B subjects. Haemophilia 2014 May;20(3):398-406.
- 36. European Medicine Agency (EMA). Rixubis: EPAR European Public Assessment Report. Last updated 16/03/2020. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/rixubis#product-information-section accessed 12/06/2020.
- 37. Prezzi da aggiudicazione da Gara Regionale: Piemonte, Veneto, Toscana, Lazio, Campania, Puglia e Sicilia. Data on file. 2019.
- 38. Kavakli K, Smith L, Kuliczkowski K, et al. Once-weekly prophylactic treatment vs. on-demand treatment with nonacog alfa in patients with moderately severe to severe haemophilia B. Haemophilia 2016 May;22(3):381-388.
- 39. Furlan R, Krishnan S, Vietri J. Patient and parent preferences for characteristics of prophylactic treatment in haemophilia. Patient Prefer Adherence 2015;9:1687-1694.
- 40. Zhang Y, Roberts J, Bensen-Kennedy D, et al. Population pharmacokinetics of a new long-acting recombinant coagulation factor IX albumin fusion protein for patients with severe haemophilia B. J Thromb Haemost 2016 Nov;14(11):2132-2140.
- 41. Kodra Y, Cavazza M, Schieppati A, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus 2014 Apr;12 Suppl 3:s567-s575.
- 42. Malec LM, Croteau SE, Callaghan M, Matino D, Friedman KD, Sidonio RF, Jr. Spontaneous bleeding and poor bleeding response with extended half-life factor IX products: a survey of select US and Canadian haemophilia treatment centers. Blood 2019 Nov 13;134(Suppl 1):2407.