 |
Drug Target Insights 2024; 18: 94-104 ISSN 1177-3928 | DOI: 10.33393/dti.2024.3219 ORIGINAL RESEARCH ARTICLE |
 |
Investigating the combinatory effect of Sclerocarya birrea with doxorubicin against selected colorectal cancer cell lines
ABSTRACT
Introduction: Colorectal cancer incidences continue to increase annually, worldwide. Herbal plants with antiproliferative properties received research interest as agents that can be adjuvant therapies with chemotherapy drugs to enhance their efficacy and reverse drug resistance.
Methods: Sclerocarya birrea ethanolic (SBE) and aqueous (SBW) extracts combined with doxorubicin (DOX) against drug-sensitive and drug-resistant colorectal cancer cells were investigated for their potential adjuvant and drug resistance reversal. The extracts were assessed for their potential anticancer activities on HCT15 and HT29 cell lines as well as their doxorubicin potentiating and drug resistance reversal effects respectively. The extracts were assessed for their cytotoxicity on normal 3T3-L1 fibroblast cells.
Results: Both SBE and SBW extracts exhibited no toxicity against normal 3T3 cells and showed low activity on the HT29 cell line. Contrarily, resistant HCT15 cells showed moderate to low activity with significantly higher inhibitory concentration (IC)50 values. The combination of SBE with DOX and SBW with DOX resulted in antagonistic interactions, causing an increase in IC50 values for HT29 and HCT15 cells. In contrast, the combination of DOX and verapamil (VER) produced an additive effect, with no change in their IC50 values.
Conclusion: Based on the findings from the combination treatment, the SBE and SBW extracts demonstrated higher efficacy and synergistic effects combined with DOX at IC75 compared to the combination of DOX and VER, suggesting their potential as anticancer agents. However, further research on both the SBE and SBW extracts’ mechanisms of action and in vivo effects is warranted.
Keywords: Colorectal cancer, Doxorubicin drug resistance, Sclerocarya birrea, Synergy, Verapamil
Received: July 23, 2024
Accepted: September 19, 2024
Published online: December 11, 2024
This article includes supplementary material
Corresponding author:
Motlalepula G. Matsabisa
email: MatsabisaMG@ufs.ac.za
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Colorectal cancer (CRC) is ranked third in cancer incidences and second in terms of cancer fatalities, worldwide (1). According to the South African statistics, CRC is mostly ranked second in men and fourth in women. It tends to occur most frequently in White people (52%-54%), subsequently followed by African people (26%-28%), coloured people (14%-15%), and Indian people (4%-7%) (2). Conventional chemotherapy drugs, like doxorubicin (DOX) used for treating CRC, require higher doses for increased efficacy, which often leads to severe side effects in patients, drug resistance, and decreased treatment effectiveness (3). First-generation P-glycoprotein (P-gp) inhibitors, such as verapamil (VER), have been shown to increase intracellular DOX accumulation; however, their usage also at higher doses produces unfavourable side effects, limiting their ability to treat patients with cancer (4). As a result, medicinal plants acquired attention as agents that can be used to potentiate the effect of conventional drugs at lower doses, thus minimizing the occurrence of drug resistance (5). The use of natural products in managing and treating colon cancer is extensively reported across various scientific studies that looked into in vitro and in vivo models (6-8). However, there is insufficient data on the effects of natural products to reverse or mitigate drug resistance, particularly doxorubicin resistance in colon cancer (9).
Sclerocarya birrea, also known as the Marula tree, is an African indigenous, dioecious tree belonging to the Anacardiaceae family (10). The fruits of the tree are commonly used for making food and alcoholic beverages, the leaves are traditionally used to treat heartburns, and the bark decoction of the tree is used to treat diarrhoea and abdominal pains (11). A study by Masoko et al showed that the ethanol extracts of the plant possess antifungal properties when used together with standard antifungals (12). Other studies revealed that the bark extracts of this plant had other pharmacological activities such as anticancer, antidiabetic, anti-inflammatory, anti-atherogenic as well as antioxidant activities (11,13). However, research on the combinatory effect of this plant with DOX is still lacking in CRC.
Therefore, the present study examined the possible antitumour effects of ethanolic (SBE) and water (SBW) extracts of S. birrea bark. The synergistic, additive, or antagonistic effects of DOX when combined with ethanolic and water extracts from S. birrea bark have been evaluated in relation to selected human CRC cell lines. The effects have also been evaluated for reversing drug resistance against chemoresistant CRC cell lines.
Methods
Plant material collection and identification
The plant was collected by Rangers of Zuka in the Northern KwaZulu-Natal Private Conservancy, KwaZulu-Natal, South Africa. The South African National Biodiversity Institute documented the plant’s identification, which was indicated by its national tree number of 360. Following the harvest, the plant was washed to remove debris, dried at room temperature, and grounded into fine powder using an electric hammermill (Roff, Kroonstad, South Africa).
Preparation of S. birrea extract
The extraction process was performed according to Mohammed et al (14) and Nyoni et al (15) with slight modifications. Accurately weighed 40 g of the dried plant material was extracted (24 hours × 3) in ethanol and distilled water, respectively, at a ratio of 1:5 w/v, at room temperature using a horizontal shaker (ABC Hansen Africa, South Africa). The extract was filtered using a Whatman filter paper and the filtrate was stored at 4°C. Thereafter, SBE extract was concentrated using a rotary evaporator (Buchi, South Africa) and SBW extract was dried using freeze-dryer (Buchi, South Africa). Both dried extracts were stored away from direct sunlight and moisture for further use.
The percentage yield of both plant extracts was calculated using the below equation:
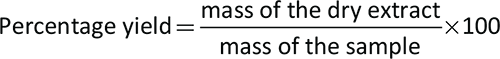
Analysis of S. birrea extracts using thin layer chromatography
To determine the chemical fingerprint of the extracts, thin layer chromatography (TLC) analysis was conducted according to methods adopted from Masoko et al (12) and Abdulhamid et al (16). For each crude extract, 1 mg/mL stock solution was prepared by reconstituting each extract with the solvent of extraction. The following mobile phases were used for the analysis:
A. Benzene:methanol:ammonium hydroxide (90:10:1, v/v/v)
B. Dichloromethane:ethyl acetate:hexane (5:2:1 v/v/v)
TLC analysis
TLC was performed on aluminium TLC plates precoated with silica gel 60 PF254 (Thermo Fisher Scientific, South Africa). The plates were spotted with 20 µL of S. birrea extracts approximately 1 cm from the bottom edge of the plates. Both plates were kept into presaturated Shandon chromatographic tanks containing mobile phases at room temperature. All the samples were left to run for 30 minutes. Ultraviolet light was used to visualize the spots on TLC plates at 254 and 366 nm. The sample and solvent fronts of the separated spots were marked and measured, and a retention factor (Rf) was calculated using the equation below:

High-performance liquid chromatography analysis
The analysis was performed on an Agilent 1100 high-performance liquid chromatography (HPLC) system equipped with a diode array detector. In brief, 1 mg/mL stock solution was prepared by reconstituting each extract with the solvent of extraction. The stock solution was then filtered through a 0.45 µm filter. The liquid chromatography (Agilent 1100 HPLC with a diode detector) analysis was performed on a Phenomenex Luna 5u C18 (2) column of 100 Å (150 × 4.6 mm, 5 μm) with gradient elution and peaks measured at wavelength of 280 nm. The column oven temperature was set to 30°C, and the flow rate was 1.0 mL/min. The injection volume was 10 μL, and the dwell volume of the HPLC system was 1.8 mL. Distilled water served as the mobile phase A and acetonitrile served as mobile phase B. The absolute run time was 40 minutes using the following multistep linear gradient: 0 minute, 95% A and 5% B; 7 minutes, 65% A and 35% B; 12 minutes, 55% A and 45% B; 17 minutes, 50% A and 50% B; 27 minutes, final conditioning cycle of 95% A and 5% B for 5 minutes was included before the next analysis. An OpenLab CDS ChemStation Edition Software was used for the result analysis.
Bioanalysis of S. birrea bark extracts, DOX, and VER
S. birrea extracts, DOX, and VER were evaluated against a normal human embryonic fibroblast cell line, drug-sensitive human colorectal adenocarcinoma cell line (HT29) and drug-resistant human colorectal adenocarcinoma cell line (HCT15), to determine their cytotoxicity, anticancer, and resistant reversal effects.
Cell culture
The cell lines 3T3-L1, HT-29, and HCT-15 were purchased from the American Type Cell Culture (ATCC, Manassas, Virginia). All the cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% foetal bovine serum (FBS) and incubated in a humidified incubator at 37°C with 5% CO2 and 95% atmosphere until 70%-80% confluency was reached. Details of cell lines used during the study are tabulated in Supplementary Table 1.
Sample preparation
DOX and VER (positive controls) were prepared as stock solutions in dimethyl sulfoxide (DMSO) at concentrations of 5 and 2 mg/mL, respectively. DOX working stock solutions were prepared at 5.0, 4.0, 3.0, 2.0, 1.0, 0.5, 0.25, 0.13, 0.06, and 0.03 µg/mL while VER working concentration range was 200, 150, 100, 50, 25, 12.5, 6.25, 3.13, 1.56, and 0.78 µg/mL.
Ethanol (60 mg/ml) and water (50 mg/ml) extracts stock solutions were prepared in DMSO and DMSO:H2O at a ratio of 1:1 (v/v), respectively. A range of working concentrations were prepared for ethanol extract at 400, 300, 200, 100, 50, 25, 12.5, 6.25, 3.13, and 1.56 µg/mL while 500, 450, 400, 300, 200, 100, 50, 25, 12.5, and 6.25 µg/mL were prepared for the H2O extract. All samples and positive controls were prepared in culture medium.
Cytotoxicity assay and anticancer activity of the extracts
The cytotoxicity of S. birrea extracts, DOX, and VER was studied to determine their inhibitory and anticancer activity on HT29 and HCT15 cells, as well as their influence on embryonic 3T3 cell growth (normal cells) by MTT assay. The cells were treated with single treatment of SBE and SBW extracts and/or DOX and VER at various concentrations and incubated for a period of 72 hours.
MTT assay
MTT assay was performed to examine the cell viability. After 72 hours incubation period, 100 µL of MTT solution contained in 200 µL of fresh medium was added into each well and incubated for 3 hours at 37°C. After the incubation period was complete, purple formazan salts appeared in the bottom of the wells. Thereafter, a microplate reader (Multiskan GO, Thermo Fisher Scientific, South Africa) was used to read the absorbances of each plate at 570 nm, which were used to calculate growth inhibition values and further determine the inhibitory concentration (IC)50 values of the test samples.
Selectivity index
The selectivity index (SI) is an estimate of the ratio of the toxic concentrations in the test sample relative to its optimal bioactive concentration. Establishing the SI value is important for determining whether further work can be continued. It is given by the equation below and an SI value ≥3 indicates that the test sample can be further investigated (17):
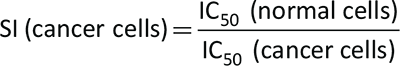
Combination treatment of the selected cell lines with S. birrea extracts and the control drugs
Combination treatment in HT29 cells
The HT29 cells were seeded in a 96-well microplate (1.5 × 104 cells/well), and exposed, in duplicates, to each agent alone (100 µL) and both (50 µL/agent) in combination. The cells were subjected to a total of five concentrations of half-fold serial dilutions, with two concentration points above and below the IC50 values of each S. birrea extract, DOX, and VER. The treated plates were then incubated for 72 hours followed by MTT assay for cell growth inhibition determination. The IC50 of combination treatment was then determined to calculate the combination index (CI).
Resistance reversal assay on HCT15 cells
A resistance reversal assay was performed on the drug-resistant cell line HCT15 to determine cell growth inhibition after exposure to a combination of SBE extract + DOX, SBW extract + DOX, as well as the combination of VER + DOX. HCT15 cells were seeded in a 96-well microplate (1.5 × 104 cells/well) and treated with 100 µL of DOX or VER and/or S. birrea extracts alone and also exposed to 50 µL of DOX combined with 50 µL of VER. The cells were also treated with 50 µL of DOX combined with 50 µL SBE as well as 50 µL of DOX combined with 50 µL SBW followed by 72 hours incubation.
Analysis of combination cell treatment
Calculation of CI
One of the most used ways to evaluate whether the combined effect of S. birrea extracts and DOX is effective is to determine a CI that is calculated from Chou-Talalay’s method on CompuSyn software (Online) using absorbance values from MTT assay (18). The computer software CompuSyn and the equation is used to calculate the CI.

Calculation of dose reduction index
Dose reduction index (DRI), also known as the reversal ratio or the cytotoxicity enhancement ratio, is a measure of how many times the dose may be reduced when compared to the doses of each drug when used separately (18), which is calculated as follows:

Calculation of cell growth inhibition percentage
The following equation was used to calculate the cell growth inhibition (19) percentage:

where Ab = absorbance value of the blank, At = absorbance value of the test compound, and Ac = absorbance value of the control.
Drug combination evaluation using Bliss independence model
To determine whether the anticancer effect of combining two drugs targeting different biological pathways shows a synergistic effect of drug combinations, a bliss independent model was used, which employs average response measurements at each combination dosage. The bliss independence model was accessed using Online.
Statistical analysis
The data was expressed as means ± standard error of the mean (SEM) of three independent experiments. Results were analysed using Microsoft Excel for anticancer and resistance reversal activity and graphs for anticancer were generated from GraphPad Prism version 8, 2008 software (GraphPad Software, Inc., La Jolla, CA, USA). For CI interactions and the dose-response index, the computer software CompuSyn was used. Synergy finder was used to determine the synergy dose points at 95% confidence interval and the level of significance was determined at p values ≤0.05.
Results and discussion
Plant extraction
S. birrea bark was extracted using cold ethanol and distilled water. The SBE extract yield was found to be 12.75% and resulted in a sticky, dark brown, dry extract, while the SBW extract resulted in a brown, spongy powder with a percentage yield of 11.43%.
TLC analysis
TLC analysis was performed to obtain a chemical fingerprint of different compounds that separated in the S. birrea extracts using different mobile phases. Supplementary Figure 1 shows TLC bands obtained from the SBE extract using two mobile phases. The mobile phase as depicted in Supplementary Figure 1A resulted in yielding a total of four bands with Rf values ranging from 0.4 to 0.9, while the mobile phase as shown in Supplementary Figure 1B resulted in yielding five bands with Rf values ranging from 0.2 to 0.9 (Supplementary Tab. 3). SBW extract couldn’t give good separation on TLC plates in aforementioned mobile phase.
High-performance liquid chromatography
HPLC was performed to obtain the chemical fingerprint of the separated compounds from S. birrea bark extracts. Figure 1 shows the chromatogram of the SBE extract superimposed with the standard chromatogram of gallic acid at 220 nm, while Figure 2 shows the chromatogram peaks of the SBW extract superimposed with the chromatogram of gallic acid at 220 nm. Therefore, chromatograms indicate the possible presence of gallic acid in both plant extracts.
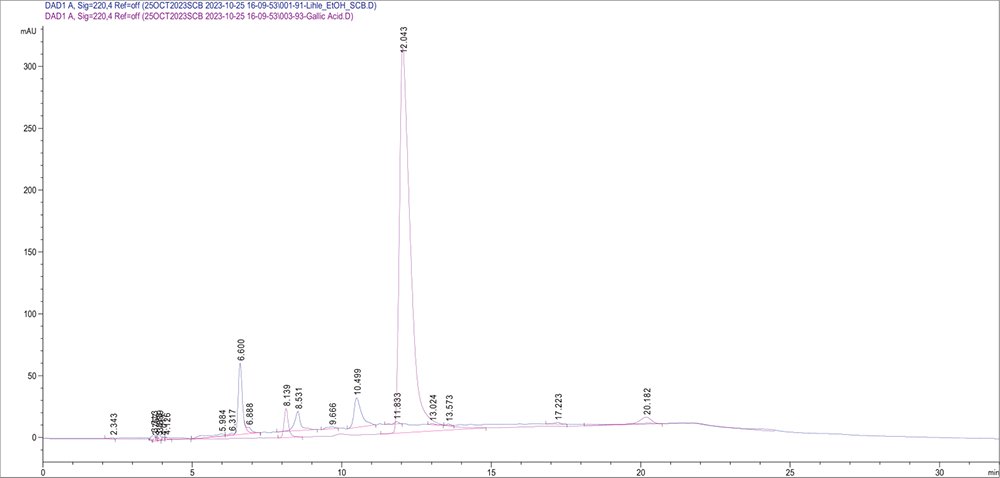
FIGURE 1 - Chromatogram peaks of the SBE extract superimposed with the chromatogram of gallic acid. The HPLC conditions were as follows: The column oven temperature was set to 30°C; flow rate was 1.0 mL/min; injection volume was 10 μL; and the dwell volume of the HPLC system was 1.8 mL. HPLC = high-performance liquid chromatography; SBE = Sclerocarya birrea ethanol.
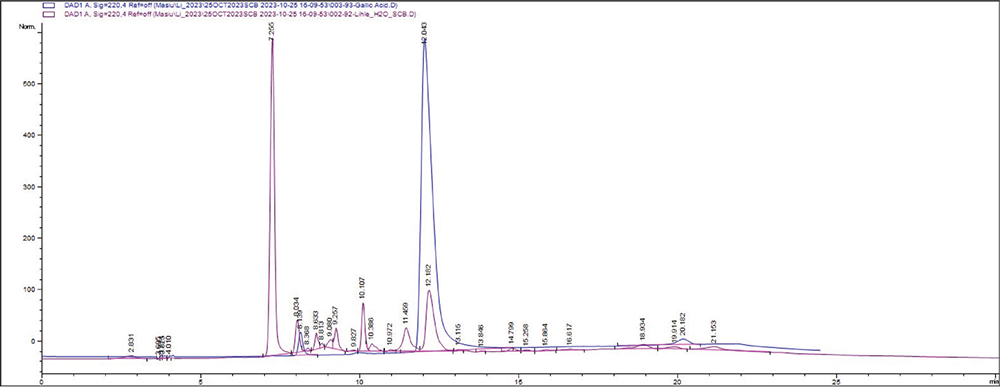
FIGURE 2 - Chromatogram peaks of the SBW extract superimposed with the chromatogram of gallic acid. The HPLC conditions were as follows: The column oven temperature was set to 30°C; flow rate was 1.0 mL/min; injection volume was 10 μL; and the dwell volume of the HPLC system was 1.8 mL. HPLC = high-performance liquid chromatography; SBW = Sclerocarya birrea water.
Anticancer effect of VER, DOX, and S. birrea bark extracts
S. birrea extracts, VER, and DOX were tested for their cytotoxicity against drug-sensitive HT29 and drug-resistant HCT15 cell lines to determine their anticancer activity by MTT assay.
The SBE extract induced an anticancer effect on both HT29 and HCT15 cells in a dose-dependent manner with the higher inhibition of ~70% and ~80% at 400 µg/mL and lowest percentage inhibition of ~1% and ~15% at 1.56 µg/mL (Figs. 3A and 4A) with an IC50 value of 157.46 ± 0.23 and 50.67 ± 1.61 µg/mL, respectively (Tab. 1). In addition, the SBW extract also induced growth inhibitory effects in HT29 and HCT15 cell lines in a dose-dependent manner with the highest percentage inhibition of ~80% and ~60% at 500 µg/mL and lowest percentage inhibition of ~5% and ~10% recorded at concentrations of 6.25 µg/mL (Figs. 3B and 4B). The IC50 value of the SBW extract was 181.80 ± 0.41 µg/mL in HT29 cells and 438.42 ± 0.12 µg/mL in HCT15 cells as shown in Table 1.
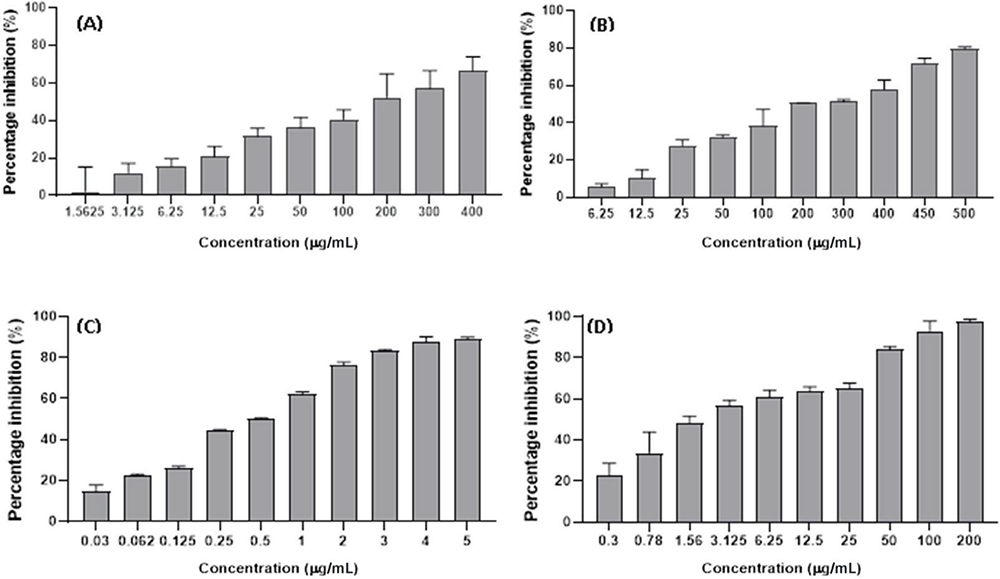
FIGURE 3 - Anticancer effect of SBE (A), SBW (B), DOX (C), VER (D) on HT29 cell line. DOX = doxorubicin; SBE = Sclerocarya birrea ethanol; SBW = Sclerocarya birrea water; VER = verapamil.
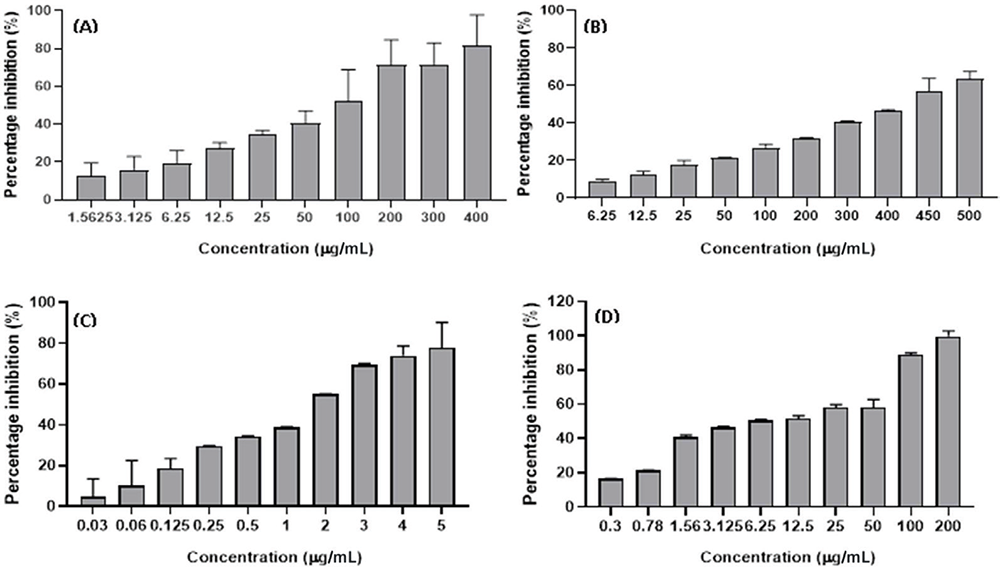
FIGURE 4 - Anticancer effect of SBE (A), SBW (B), DOX(C), VER (D) on HCT15 cell line. DOX = doxorubicin; SBE = Sclerocarya birrea ethanol; SBW = Sclerocarya birrea water; VER = verapamil.
DOX demonstrated anticancer effect on both HT29 and HCT15 cells with the higher percentage inhibition of 80% on both cell lines at 5 µg/mL and lowest percentage inhibition of 19% (on HT29) and 17% (on HCT15) at 0.03 µg/mL (Figs. 3C and 4C) with an IC50 value of 0.45 ± 0.10 and 1.34 ± 0.1 µg/mL, respectively (Tab. 1). In addition, VER also exhibited an anticancer effect on both the aforementioned cell lines (Figs. 3D and 4D) with the highest percentage of ~99% inhibition at a concentration of 200 µg/mL whereas the lowest percentage inhibition was ~20% at a concentration of 0.3 µg/mL for HT29 and HCT15, respectively. Furthermore, the IC50 of VER on HT29 cells obtained was 2.78 ± 0.46 µg/mL while VER on HCT15 cells was 8.75 ± 2.03 µg/mL (Tab. 1).
| Cell type | DOX (µg/mL) | VER (µM) | SBE extract (µg/mL) | SBW extract (µg/mL) |
|---|---|---|---|---|
| HT29 | 0.45 ± 0.10 | 2.78 ± 0.46 | 157.46 ± 0.23 | 181.80 ± 0.41 |
| HCT15 | 1.34 ± 0.1 | 8.75 ± 2.03 | 50.67 ± 1.61 | 438.42 ± 0.12 |
DOX = doxorubicin; IC = inhibitory concentration; SBE = Sclerocarya birrea ethanol; SBW = Sclerocarya birrea water; VER = verapamil.
Combination treatment of the S. birrea extracts with DOX and DOX with VER on HT29 cell line
SBE and SBW extracts were also subjected to combination treatment with DOX and DOX with VER (control) to examine their interaction against HT29 cells. The nature of interaction was evaluated using CI values from CompuSyn, which gives a quantitative definition of an additive interaction, that is, when the CI value is 1.00; a synergistic interaction when the CI value is ˂1; and an antagonistic interaction when the CI value is ˃1.15 at IC50, IC75, IC90, and IC95. The combination treatment was also performed to determine the DRI of the test samples at IC50, IC75, IC90, and IC95 in order to determine how much the doses of each test samples in combination were reduced to achieve effect levels that were comparable with those achieved with single test samples. The IC and CI values of the combined treatment of VER and DOX, S. birrea extracts, and DOX against HT29 cells are shown in Supplementary Table 4. The combined treatment was also evaluated using synergy finder to determine synergistic dose points and their synergy scores from their percentage inhibition.
As seen in Supplementary Table 4, the combined treatment of VER and DOX resulted in an additive effect with CI value of 1.0, while the IC50 values decreased from 3.04 to 1.31 µg/mL for VER and 0.45 to 0.26 µg/mL for DOX. The decreased IC50 values were achieved by DRI with a magnitude 2.3-fold and 1.7-fold for VER and DOX, respectively. However, IC75, IC90, and IC95 demonstrated normal to strong synergistic effects coupled with a significant decrease in the concentrations of VER and DOX with greater DRI ratios, which indicated that there was reduced toxicity.
Three synergistic dose points were obtained with their synergy scores as well as their inhibition scores in the combination treatment of DOX and VER against HT29 cells. The highest synergy score recorded was 6.84 yielded by the combination of 0.11 µg/mL of DOX and 4.76 µg/mL of VER with a cell growth inhibition percentage of 57.85% as shown in Figure 5. The second synergistic dose points were 1.19 µg/mL of VER and 1.8 µg/mL of DOX with a synergy score of 2.56 and an inhibition percentage of 82.07%. The third synergistic dose points were found from the combination of 0.6 µg/mL of VER and 1.18 µg/mL of DOX with a synergy score of 0.99 and an inhibition percentage of 73.2%. Other dosage points resulted in antagonistic scores and one additive score of 0.43.
SBE extract and DOX combinations exhibited normal to strong antagonistic effects from IC50 to IC95 as depicted in Supplementary Table 5. The DRI values of both the drug and the ethanol extract decreased drastically while synergy finder analysis showed no synergistic scores obtained from the combination of the ethanol extract and DOX as shown in Figure 5.
Combinations of the SBW extract and DOX yielded antagonistic interactions (CI of 2.5) while both IC50 values were increased by a magnitude of 0.3-fold and 0.4-fold for SBW extract and DOX, respectively (Supplementary Table 6). Contrarily, IC75, IC90, and IC95 resulted in normal synergistic to strong synergistic interactions with CI values of 0.97, 0.37, and 0.19, further attributed by a significant decrease in the concentrations of both the drug and the extract. Synergy finder analysis showed that this combination yielded no synergistic scores as shown in the bliss heatmap diagram in Figure 5.
Combination treatment of the S. birrea extracts with DOX and DOX with VER on HCT15
SBE and SBW extract of S. birrea were also subjected to combination treatment with DOX and DOX with VER (control) to examine their interaction against HCT15 cells. The nature of interaction was evaluated using CI values (as mentioned above) from CompuSyn. The combination treatment was also performed to determine the DRI of the test samples at IC50, IC75, IC90, and IC95 in order to determine how much the doses of each test samples in combination were reduced to achieve effect levels that were comparable with those achieved with single test samples. The IC and CI values of the combined treatment of VER and DOX, S. birrea extracts, and DOX against HCT15 cells are shown in Supplementary Table 7 and Supplementary Table 8, respectively. The combined treatment was also evaluated using synergy finder to determine synergistic dose points and their synergy scores from their percentage inhibition.
The combined treatment of VER and DOX resulted in an antagonistic effect with CI value of 1.33 coupled with decreased IC50 values of VER (6.6 to 0.38 µg/mL) and DOX (2.99 to 0.33 µg/mL) by DRI magnitudes of 2.64-fold and 0.46-fold as shown in Supplementary Table 7. At IC75, IC90, and IC95, a strong synergistic interaction was observed when the two drugs were used against HCT15 cells (CI = between 0.3 and 0.09), which indicates that resistance reversal activity was observed from the combination treatment of these control drugs. According to the bliss heatmap model, there were ten synergy scores obtained from different concentrations of the combination treatment of DOX and VER as shown in Figure 6.
Combination of the SBE extract and DOX yielded an antagonistic effect (CI of 3.75) with increased IC50 value of SBE extract (49.35 to 89.86 µg/mL) and DOX (0.68 to 1.32 µg/mL) by DRI ratios of 0.54-fold and 0.51-fold, respectively, as depicted in Supplementary Table 8. Moreover, strong synergistic interactions were demonstrated from IC75 to IC95, with CI values ranging from 0.001 to 0.26, which suggests that drug resistance was reversed. Additionally, two synergistic dose points were obtained from this combination (Fig. 6), one from 40.84 µg/mL of the extract and 0.312 µg/mL of DOX with a synergy score of 4.7 and a highest synergy score of 6.63 was yielded by the combination of 40.84 µg/mL of the extract and 0.612 µg/mL of DOX with an inhibitory percentage of 73.57%.
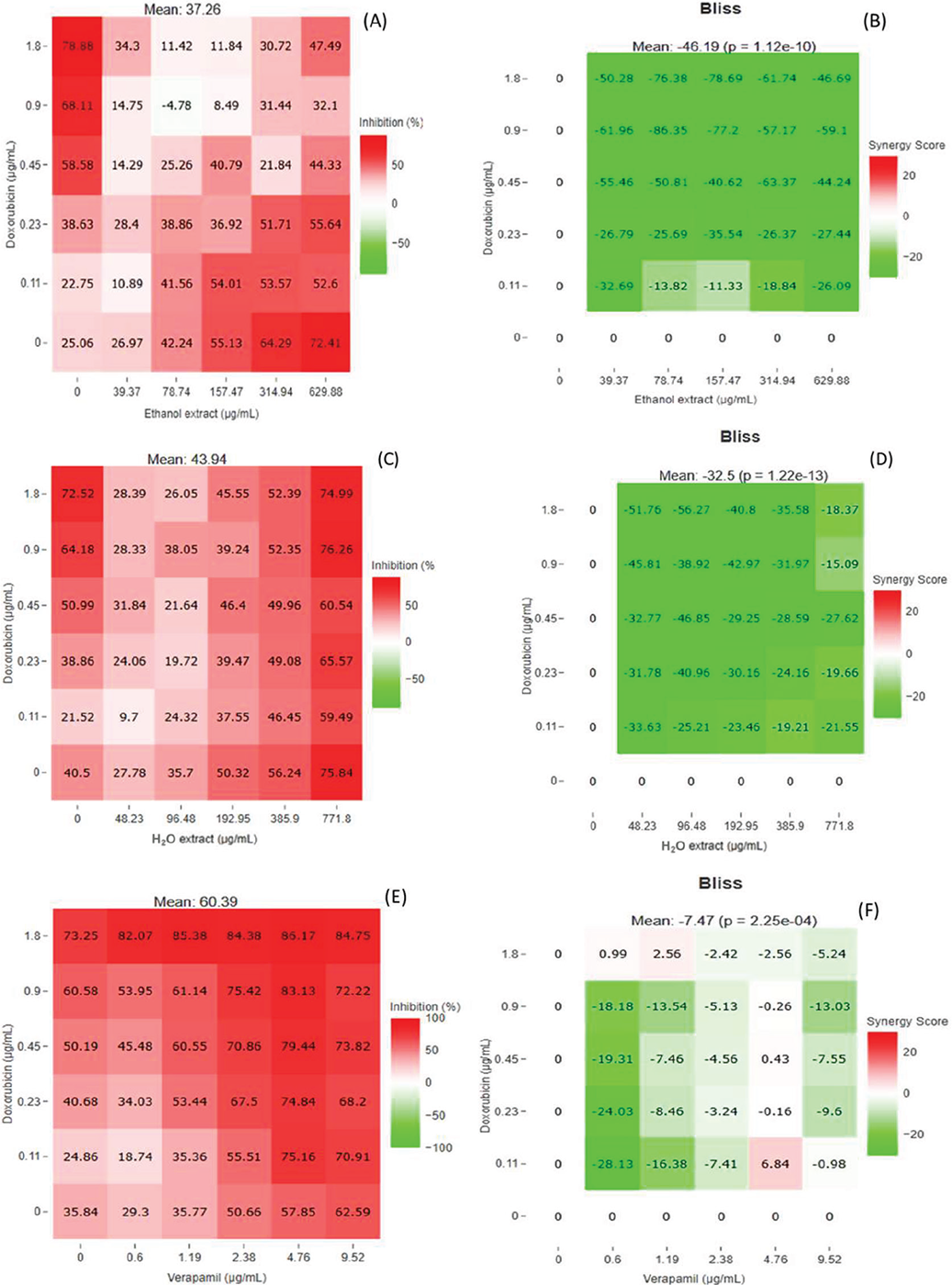
FIGURE 5 - Heatmap diagrams generated using synergy finder. Heatmap diagram illustrating the inhibition percentages of the combination of the SBE extract and DOX (A), SBW extract and DOX (C), and VER and DOX (E) on HT29 cell line, while the bliss heatmap diagram shows synergistic, antagonistic, and additive scores between the dose points of the combination of SBE extract and DOX (B), combination of SBW extract and DOX (D), and combination of VER and DOX (F) on HT29 cell line. DOX = doxorubicin; SBE = Sclerocarya birrea ethanol; SBW = Sclerocarya birrea water; VER = verapamil.
The combinatory effect of the SBW extract and DOX yielded strong antagonistic interactions from IC50 to IC95. The DRI ratios were decreased drastically while no synergistic dose points were recorded using synergy finder analysis as shown in Supplementary Table 9 and Figure 6.
The dose-response curve illustrates the combined effect of the drugs and S. birrea extracts in the supplementary data sheet depicted as the bliss independence model synergy map for each combination treatment.
Cytotoxic effects of test samples against 3T3 cell line
To comprehend the cytotoxic effects of the experimental samples, 3T3 cells were subjected to escalating doses of DOX, VER, SBE, and SBW extract. The assessment of cytotoxicity for the test samples was conducted through the MTT assay during a 72-hour incubation period. In general, the results indicated that the SBE and SBW extracts exhibited a greater impact on normal cell susceptibility compared to DOX and VER. Notably, cell viability demonstrated a dose-dependent decrease across all the test samples. DOX decreased the cell viability of normal cells when treated with a concentration range of 1-5 µg/mL and showed significant toxic effects on 3T3 cells with an IC50 value of <1 (Tab. 2). Similarly, 3T3 cells treated with VER (at concentration range of 25-100 µg/mL) also showed toxic effects with an IC50 of 0.14 µg/mL (Tab. 2). SBE did not show toxic effects on the normal cell lines as it had an IC50 of 80.38 ± 4.09 µg/mL (Tab. 2). Moreover, the SBW extract showed less toxicity (IC50 in 290.62 ± 48.37 µg/mL) on the normal cells compared to the SBE extract (Tab. 2).
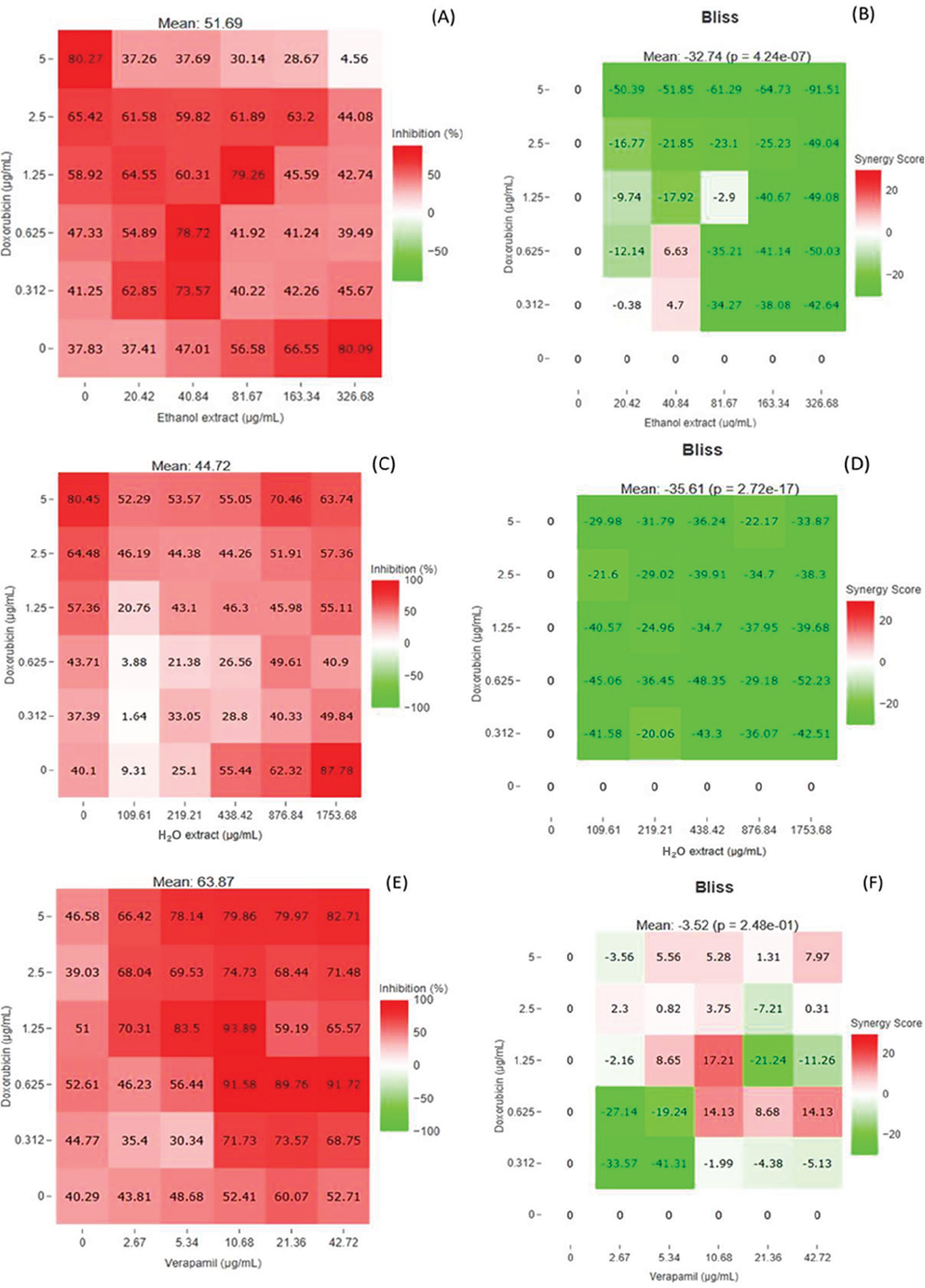
FIGURE 6 - Heatmap diagrams generated using synergy finder. Heatmap diagram illustrating the inhibition percentages of the combination of the SBE extract and DOX (A), SBW extract and DOX (C), and VER and DOX (E) on HCT15 cells, while the bliss heatmap diagram shows synergistic, antagonistic, and additive scores and between the dose points of the combination of SBE extract and DOX (B), combination of SBW extract and DOX (D), and combination of VER and DOX (F) on HCT15 cells. DOX = doxorubicin; SBE = Sclerocarya birrea ethanol; SBW = Sclerocarya birrea water; VER = verapamil.
From the combinations analysis, cytotoxicity was only evaluated on the 3T3 cells using the strongest synergistic dose points. Only combinations of VER and DOX as well as SBE extract and DOX yielded synergistic dose points. Therefore, the control inhibited the cell growth of 3T3 cells with IC50 value of 58.18 ± 0.98 µg/mL while SBE and DOX combinations yielded IC50 values of 55.54 ± 0.11 µg/mL. Compound selectivity is an essential criterion for chemotherapeutic evaluation.
| Extract | DOX (µg/mL) | VER (µg/mL) | SBW (µg/mL) | SBE (µg/mL) |
|---|---|---|---|---|
| IC50 | <1 | 0.14 ± 0.04 | 290.62 ± 48.37 | 80.38 ± 4.09 |
DOX = doxorubicin; IC = inhibitory concentration; SBE = Sclerocarya birrea ethanol; SBW = Sclerocarya birrea water; VER = verapamil.
Discussion
The herbal plant extracts acquired increased attention as potential agents that can be used to potentiate the efficacy of conventional chemotherapy drugs when used in combination for the management of cancer (20). S. birrea possesses antitumour effects (21), which was investigated for its combined effect with DOX against HT29 and HCT15 cell lines in this study.
According to the National Cancer Institute, an extract is considered strongly active when the IC50 value is ˂20 µg/mL, moderately active when it is between 20 and 100 µg/mL, and inactive when the IC50 is greater than 100 µg/mL based on their cytotoxicity criteria (22). Based on the NCI criterion, findings from this study showed that the SBE and SBW extract showed no activity against HT29 cells while growth inhibition was demonstrated in a dose-dependent manner. Moreover, the SBE extract showed moderate inhibition of HCT15 cells while the SBW extract demonstrated no activity. Both SBE and SBW extracts of S. birrea have been reported in the literature to contain high levels of bioactive compounds (e.g., polyphenols) with potential medicinal properties (11). The polyphenols are known to exhibit antioxidant activity, which is considered crucial in preventing cancer development (23,24). The scavenging of free radicals by polyphenols may help prevent DNA damage, cell membrane damage, and oxidative stress, all of which can contribute to cancer development. Hence the presence of these compounds might be responsible for the remarkable anticancer activity observed for these extracts against the investigated CRC cells (25).
In addition, the extracts’ cytotoxic activity was assessed in normal 3T3 cells. It was observed that DOX and VER were toxic while the extracts displayed no toxicity towards the normal cells, particularly the SBW extract (IC50 value of 290.62 ± 48.37 µg/mL). According to a study by Russo et al (25), S. birrea extracts exhibited toxic effects at high concentrations, potentially due to the concentrated compounds found in the extracts, which results in cellular morphological changes considered as the key evidence of cytotoxicity to natural compounds or plant extracts, along with metabolic dysfunctions, differentiation processes, and apoptosis.
We also investigated the combination of the intercalating agent DOX with VER and plant extracts in drug-sensitive and drug-resistant colon cancer cells. The study focused on the CI method (based on the multiple drug effects equation), the DRI, and the synergistic dose scores to evaluate the combinatory effects in the cells. It is noteworthy to understand that one of the major objectives of synergistic drug combination is to reduce the dose of the cytotoxic drug, thereby reducing the toxicity while maintaining efficacy. The CI provides a quantitative definition of an additive effect or interaction, for example, a CI value of 1, <1, and >1 indicates additivity, synergism, and antagonism, respectively. The DRI is a measure of how many folds a combination treatment reduces cytotoxicity dose (26).
The co-treatment of VER combined with DOX (control) moderately inhibited HT29 cells’ growth and decreased the IC50 of VER and DOX from 3.04 and 0.45 µg/mL to 1.31 and 0.26 µg/mL, respectively, thus indicating decreased sensitivity of DOX. Interestingly, IC75 to IC95 resulted in a synergistic effect ascribed by an increase in the DRI, which was also observed in both drugs indicating that sensitivity at higher concentrations is required to exhibit good combinatory effect. Moreover, the combination of VER and DOX also showed three synergistic scores of 0.99, 2.56, and 6.84, whereby the strongest synergy score of 6.84 was achieved with a significantly higher concentration of VER (4.76 µg/mL) and lower concentration of DOX (0.11 µg/mL).
In HCT15, this combination showed moderate inhibition of the cells and decreased the IC50 of VER and DOX from 6.63 and 0.38 µg/mL to 2.99 and 0.33 µg/mL, respectively. Similarly, synergistic interactions were also depicted at IC75 and IC95 ascribed by ten synergistic dose points ranging from 1.3 to 17.21.
In HT29 cells, combinations of DOX with the SBE extract resulted in an antagonistic effect at IC50 with an increase in IC50 values from 0.35 and 140.09 µg/mL to 1.70 and 669.89 µg/mL, respectively. However, only the SBW extract combinations seemed to have depicted synergistic interactions from IC75 to IC95 in comparison to the SBE extract combinations that demonstrated antagonistic interactions. These results suggest the potentiation effects of each extract at IC50 when used in combination with DOX compared to the control as they were coupled with significantly lower DRI ratios.
A similar behavioural pattern was demonstrated by combinations involving DOX and the SBE extract where strong synergism was observed from IC75 to IC90. This suggests that the SBE extract might potentially yield similar adverse effects to VER and thus cannot be considered as a resistant reversal agent seeing that extremely higher concentrations are required to yield strong synergistic interactions.
The SBW extract did not show any resistance reversal activity based on its antagonistic effects recorded at all the IC ranges compared to combinations that include ethanol and VER. It was also observed that the DRI of the SBE extract and DOX increased when they were used in combination but the DRI of the SBW extract decreased drastically. According to the bliss heatmap diagram, two synergistic dose points were found from the combination of the SBE extract (40.84 µg/mL) and DOX (0.625 µg/mL) with a synergy score of 6.63, while the combination of the SBE extract (40.84 µg/mL) and DOX (0.312 µg/mL) synergy score was found to be 4.7. The dosage for DOX was decreased twice in these synergistic dose points and according to Poofery et al a reduction in the dosage of the drug in combination treatment potentially lessens toxicity and adverse side effects and subsequently reverses drug resistance (27).
Conclusion
In this study, the combined effects of S. birrea extracts and DOX were examined on CRC cell lines, with a particular focus on sensitive and resistant cell types. Various compounds were identified within the plant extracts, with gallic acid being the most abundant and known for its anticancer properties. It was found that both SBE and SBW extracts were non-toxic to normal 3T3 cells at the concentrations tested. The efficacy of SBW and SBE extracts against sensitive HT29 and resistant HCT15 cells revealed that the SBE extract exhibited a more substantial anticancer effect compared to SBW. Furthermore, a synergistic effect was observed when combining SBE or SBW extract and DOX at higher concentrations of each extract, implying that concomitant use of S. birrea and DOX should be closely monitored, and DOX dosage adjusted as needed to achieve maximum therapeutic benefits while minimizing potential side effects. These findings indicate the need for further research on the SBE extract to investigate its potential as an anticancer agent in vivo, as well as to elucidate its underlying mechanisms of action. While some combinations of S. birrea extracts with doxorubicin showed synergistic or additive effects, other combinations exhibited an antagonistic effect, meaning that the extracts actually decreased the efficacy of the standard anticancer drug. The antagonistic effect could be due to various factors, such as interactions between the components of the extracts and doxorubicin, which could alter the pharmacokinetics or pharmacodynamics of the drug.
Acknowledgements
The African Medicines Innovation and Technology Development Centre (AMITD), Department of Pharmacology, Faculty of Health Sciences, University of the Free State, AvaCare Health Honours Bursary, and Centre for Scientific and Industrial Research (CSIR) Inter-bursary are hereby acknowledged for their support and assistance, and Ms. Recardia Schoeman for her assistance with the culturing of the cells.
Disclosures
Financial support: This research was supported by Technology Innovation Agency (TIA), South Africa, by seed funding Grant no. 1047L0521.
Conflict of interest: The authors declare no conflict of interest.
Author’s contribution: Conceptualization: MGM; Data Curation: THN, IMM,PMM, APM; Formal Analysis: THN, IMM, APM; Investigation: THN, IMM; Methodology: IMM, PMM; Project Administration: IMM, MGM; Supervision: IMM, MGM; Writing Original Draft: THN; Writing – Review & Editing: APM, IMM, MGM.
References
- 1. Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. CrossRef PubMed
- 2. McCabe M, Perner Y, Magobo R, Mirza S, Penny C. Descriptive epidemiological study of South African colorectal cancer patients at a Johannesburg Hospital Academic institution. JGH Open. 2019;4(3):360-367. CrossRef PubMed
- 3. Yun UJ, Lee JH, Shim J, et al. Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab Invest. 2019;99(8):1157-1172. CrossRef PubMed
- 4. Pilotto Heming C, Muriithi W, Wanjiku Macharia L, Niemeyer Filho P, Moura-Neto V, Aran V. P-glycoprotein and cancer: what do we currently know? Heliyon. 2022;8(10):e11171. CrossRef PubMed
- 5. Zhang T, Ma K, Huang J, et al. CDKN2B is critical for verapamil-mediated reversal of doxorubicin resistance in hepatocellular carcinoma. Oncotarget. 2017;8(66):110052-110063. CrossRef PubMed
- 6. Aiello P, Sharghi M, Mansourkhani SM, et al. Medicinal plants in the prevention and treatment of colon cancer. Oxid Med Cell Longev. 2019;2019:2075614. CrossRef PubMed
- 7. Esmeeta A, Adhikary S, Dharshnaa V, et al. Plant-derived bioactive compounds in colon cancer treatment: an updated review. Biomed Pharmacother. 2022;153:113384. CrossRef PubMed
- 8. Huang XM, Yang ZJ, Xie Q, Zhang ZK, Zhang H, Ma JY. Natural products for treating colorectal cancer: a mechanistic review. Biomed Pharmacother. 2019;117:109142. CrossRef PubMed
- 9. Yang C, Mai Z, Liu C, Yin S, Cai Y, Xia C. Natural products in preventing tumor drug resistance and related signaling pathways. Molecules. 2022;27(11):3513. CrossRef PubMed
- 10. Kamanula M, Munthali CY, Kamanula JF. Nutritional and phytochemical variation of Marula (Sclerocarya birrea) (Subspecies caffra and birrea) fruit among nine international provenances tested in Malawi. Int J Food Sci. 2022;2022:4686368. CrossRef PubMed
- 11. Cádiz-Gurrea ML, Lozano-Sánchez J, Fernández-Ochoa Á, Segura-Carretero A. Enhancing the yield of bioactive compounds from Sclerocarya birrea bark by green extraction approaches. Molecules. 2019;24(5):966. CrossRef PubMed
- 12. Masoko P, Mmushi TJ, Mogashoa MM, Mokgotho MP, Mampuru LJ, Howard RL. In vitro evaluation of the antifungal activity of Sclerocarya birrea extracts against pathogenic yeasts. Afr J Biotechnol. 2008;7(20):3521-3526.
- 13. Daniel JA, Mathiyazhagan J, Anbazhagan M, Jayaraj R, Gothandam KM. Phytochemistry and pharmacology of Sclerocarya birrea (A. Rich.) Hochst.: a review. In: Pullaiah T, ed. Bioactives and pharmacology of medicinal plants. Apple Academic Press; 2022:139-147. CrossRef.
- 14. Mohammed M, Adamu H, Magashi L, Sarkinnoma A, Daniel S, Zakiyya R, Aisha S. Purification and characterization of extracts from the stem bark of Sclerocarya birrea (Marula). International Journal of Modelling and Applied Science Research. 2023;28(9):141-152. Online (Accessed July 2024)
- 15. Nyoni S, Muzenda E, Mukaratirwa-Muchanyereyi N. Characterization and evaluation of antibacterial activity of silver nanoparticles prepared from Sclerocarva birrea stem bark and leaf extracts. Nano Biomed Eng. 2019;11(1):28-34. CrossRef
- 16. Abdulhamid A, Sani I, Fakai IM, Kankiya IH, Shinkafi TS. Antibacterial potentials of compounds isolated from the stem bark extract of Sclerocarya birrea (A. Rich, Hochst). J Appl Sci. 2019;19(3):210-216. CrossRef
- 17. Indrayanto G, Putra GS, Suhud F. Validation of in-vitro bioassay methods: application in herbal drug research. In: Al-Majed AA. Profiles of drug substances, excipients and related methodology. Vol 46. Academic Press; 2021:273-307. CrossRef
- 18. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440-6. CrossRef. PubMed.
- 19. Aftab A, Yousaf Z, Rashid M, et al. Vegetative part of Nigella sativa L. potential antineoplastic sources against Hep2 and MCF7 human cancer cell lines. J Taibah Univ Sci. 2023;17(1):2161294. CrossRef
- 20. Lin SR, Chang CH, Hsu CF, et al. Natural compounds as potential adjuvants to cancer therapy: preclinical evidence. Br J Pharmacol. 2020;177(6):1409-1423. CrossRef PubMed
- 21. Tanih NF, Ndip RN. The acetone extract of Sclerocarya birrea (Anacardiaceae) possesses antiproliferative and apoptotic potential against human breast cancer cell lines (MCF-7). Sci World J. 2013;2013(1):956206. CrossRef PubMed
- 22. Al-Rajhi AMH, Yahya R, Abdelghany TM, et al. Anticancer, anticoagulant, antioxidant and antimicrobial activities of Thevetia peruviana Latex with molecular docking of antimicrobial and anticancer activities. Molecules. 2022;27(10):3165. CrossRef PubMed
- 23. Luo M, Zhou L, Huang Z, et al. Antioxidant therapy in cancer: rationale and progress. Antioxidants. 2022;11(6):1128. CrossRef PubMed
- 24. Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527(7577):186-191. CrossRef PubMed
- 25. Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037-1043. CrossRef PubMed
- 26. Eid SY, Althubiti MA, Abdallah ME, Wink M, El-Readi MZ. The carotenoid fucoxanthin can sensitize multidrug resistant cancer cells to doxorubicin via induction of apoptosis, inhibition of multidrug resistance proteins and metabolic enzymes. Phytomedicine. 2020;77:153280. CrossRef PubMed
- 27. Poofery J, Khaw-On P, Subhawa S, et al. Potential of Thai herbal extracts on lung cancer treatment by inducing apoptosis and synergizing chemotherapy. Molecules. 2020;25(1):231. CrossRef PubMed