 |
Drug Target Insights 2024; 18: 105-111 ISSN 1177-3928 | DOI: 10.33393/dti.2024.3213 ORIGINAL RESEARCH ARTICLE |
 |
Faricimab versus the standard of care for neovascular age-related macular degeneration in Italy: an indirect treatment comparison
ABSTRACT
Objectives: To assess through an indirect treatment comparison (ITC) the potential benefit of faricimab over the anti-vascular endothelial growth factor (VEGF) real-life scenario, hereby defined standard of care (SoC), in Italy, that is, aflibercept, bevacizumab, and ranibizumab, in patients with neovascular age-related macular degeneration (nAMD) naïve to any anti-VEGF treatment.
Methods: Individual patient-level data from the phase III clinical trials TENAYA and LUCERNE (faricimab cohort) and the real-world study RADIANCE (RADIANCE cohort) were used. Efficacy was evaluated with changes in best corrected visual acuity (BCVA) and central subfield thickness (CST) from baseline to 1 year (week 52 in the RADIANCE and week 48 in the faricimab cohorts, respectively). Propensity score-based inverse probability of treatment weighting was utilized to balance cohorts and mitigate bias due to potential confounding. Sensitivity analyses were performed to evaluate treatment differences adjusted for the number of injections.
Results: The ITC included 513 patients treated with faricimab and 263 patients treated with SoC. At 1 year, faricimab showed a greater mean BCVA gain (treatment difference +5.4 letters, p<0.001) and CST reduction (treatment difference −71.8 μm, p<0.001) compared to SoC. Sensitivity analyses confirmed the robustness of results, showing a BCVA improvement of +4.0 letters and a CST reduction of −71.5 μm in favor of faricimab.
Conclusions: Despite the limitations due to the use of ITC and the comparison between clinical trials and real-world cohorts, the present analysis suggests potential benefits in terms of vision gain and CST reduction in naïve nAMD patients treated with faricimab compared with SoC in a real-world setting.
Keywords: Faricimab, Indirect treatment comparison, nAMD, Vascular endothelial growth factor A
Received: July 18, 2024
Accepted: November 13, 2024
Published online: December 12, 2024
Corresponding author:
Carlotta Galeone
email: carlotta.galeone@unimib.it
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness and visual impairment in elderly subjects (1-3). It is a chronic, multifactorial degenerative pathology affecting the macula, typically occurring after age 55 (1).
Neovascular AMD (nAMD) is an advanced form of AMD characterized by the development of subretinal new vessels that may lead to leakage, accumulation of fluid intra- and subretinally, macular edema, hemorrhage, and serous detachments of the retinal pigment epithelium. Angiogenesis and increased vascular permeability are mainly caused by an abnormally high expression of vascular endothelial growth factor (VEGF) (4-8). Over the last couple of decades, the treatment of nAMD has evolved, gradually shifting from laser therapy to the use of anti-VEGF intravitreal injections. Anti-VEGF agents have proven to be effective for the management of patients with nAMD, removing exudative fluid from the retina, suppressing the formation of leaking new blood vessels, and improving or maintaining visual acuity (VA) over time (9-17). In Italy, the prevalence of nAMD in individuals aged ≥55 was estimated at 4.6 per 1,000 inhabitants (18).
Currently, there is no single approach for anti-VEGF administration. The most common approaches are the reactive pro-re-nata (PRN), the proactive treat-and-extend (T&E), and the fixed bimonthly regimen. In the reactive PRN approach, three-monthly loading doses are followed by adaptable dosing based on monthly monitoring of VA and/or macular morphology. In clinical practice, this approach has been associated with suboptimal outcomes and risk of undertreatment (19). The proactive fixed bimonthly dosing consists of bimonthly injections for at least 1 year (13,15) and has been shown to improve outcomes compared to PRN (20), although it is associated with a considerable treatment burden (21).
In the proactive T&E regimen, anti-VEGF is administered at every visit. Following the loading phase, the treatment interval can be gradually extended or reduced based on anatomic and VA status. This approach allows the extension of treatment intervals, reducing the overall number of visits and improving VA outcomes (20-26).
In 2023, faricimab, a novel bispecific antibody that simultaneously binds and neutralizes Ang-2 and VEGF-A, received approval by the European Medicines Agency (EMA) and reimbursement by Italian Medicines Agency (Agenzia Italiana del Farmaco—AIFA) for the treatment of adult patients with nAMD (27). In Italy, the currently available anti-VEGFs include aflibercept, ranibizumab, bevacizumab, and brolucizumab (28). Aflibercept, ranibizumab, and brolucizumab have received marketing authorization from EMA for the treatment of nAMD, while bevacizumab can be administered on an off-label regimen according to the Law 648/96 (Law 648/96 provides for the reimbursement of the medicinal product by the National Health Service when there is no valid therapeutic alternative for innovative medicines authorized in other states but not in Italy, for medicines not yet authorized but undergoing clinical trials, and for medicines already authorized in Italy, for indications other than authorized one) (29).
Efficacy and safety of faricimab were evaluated in the randomized, double-masked, phase III, non-inferiority trials TENAYA and LUCERNE (30) that randomized patients to receive faricimab 6.0 mg up to every 16 weeks (TENAYA n = 334, LUCERNE n = 331) or aflibercept 2.0 mg every 8 weeks (TENAYA n = 337, LUCERNE n = 327). In both studies, faricimab was non-inferior to aflibercept in terms of change in best corrected VA (BCVA) from baseline averaged over weeks 40, 44, and 48, with mean changes of 5.8 letters (95% confidence interval, CI: 4.6 to 7.1) and 6.6 letters (95% CI: 5.23 to 7.8) in TENAYA and LUCERNE, respectively. The treatment difference was equal to 0.7 letters (95% CI: −1.1 to 2.5) in TENAYA and 0.0 letters (95% CI: −1.7 to 1.8) in LUCERNE.
RADIANCE is an Italian, retrospective, observational, multicenter cohort study (31) that aimed to describe the real-world treatment patterns of available intravitreal anti-VEGF in Italy and the associated effectiveness. The study enrolled all consecutive anti-VEGF treatment-naïve subjects with a diagnosis of nAMD who initiated therapy with one of the available agents (i.e., aflibercept, bevacizumab, and ranibizumab) between January 2017 and November 2018. The primary objective of the study was to evaluate the change in VA 52 weeks after starting treatment with any of the three agents. All VA measurements were converted to the approximate ETDRS letter score. Overall, after 52 weeks of treatment, the patients showed a median change in VA of 1.0 letter (25th-75th percentile: −5; +1.9). By stratifying VA changes by the number of injections per year, patients treated with >6 injections in the first year of treatment showed a median VA improvement of 3 letters (25th-75th percentile: −1; 11), while no improvement was observed in patients treated with <2 (median 0 letters, 25th-75th percentile: −2; 0) and 3-5 injections (median 0 letters; 25th-75th percentile: −5.0; +9.0).
In this study an indirect treatment comparison using individual patient-level data (IPD) was performed to evaluate the potential benefit of faricimab vs. the real-life scenario of intravitreal anti-VEGF monotherapies available at the time of analysis (namely aflibercept 2 mg, bevacizumab, and ranibizumab, hereby defined standard of care—SoC) for nAMD patients in Italy.
Methods
An indirect comparison of the efficacy at 1 year of faricimab in two phase III clinical trials and the corresponding effectiveness of anti-VEGF in a real-world study was performed on patients with nAMD matched through propensity score (PS) weighting. Efficacy was assessed in terms of change from baseline to 1 year (week 52 in the RADIANCE and week 48 in the faricimab cohorts) in BCVA, as assessed by ETDRS letter score, and central subfield thickness (CST), as determined by Spectral Domain-Optical Coherence Tomography.
Data sources
IPD for the faricimab cohort was taken from the pivotal trials TENAYA (ClinicalTrials.gov identifier NCT03823287) and LUCERNE (ClinicalTrials.gov identifier NCT03823300) (30).
IPD for the RADIANCE cohort was taken from the Italian, real-world study RADIANCE (31).
In the TENAYA and LUCERNE studies bilateral treatment was not allowed and for patients with bilateral nAMD, the eye with the worst BCVA at diagnosis was included (study eye). In the RADIANCE study, in case of bilateral treatment during the index period, the eye with the worst condition at treatment start was selected as the study eye.
Population
This analysis included all the patients enrolled in the RADIANCE study (31) with at least two VA measurements after the one at baseline, of which at least one was at week 52 ± 10 (RADIANCE cohort). A cohort of patients treated with faricimab was created by combining data from the active arms in the LUCERNE and TENAYA trials (faricimab cohort). Since the pivotal trials (30) included patients worldwide and the RADIANCE study included Italian patients only, to ensure consistency across the cohorts, only Caucasian patients from LUCERNE and TENAYA were considered; in addition, patients with no VA and CST data at week 48 or with polypoidal choroidal vasculopathy (PCV) lesions were excluded from the faricimab cohort. We excluded PCV lesions from the faricimab cohort because there were no patients with PCV in the RADIANCE cohort.
Statistical analyses
PS-based inverse probability of treatment weighting (IPTW) was utilized to balance the two cohorts and mitigate bias due to potential confounding. In IPTW, weights are calculated for each individual as 1/PS for the treated group and 1/(1 − PS) for the control group, where the PS is defined as the inverse probability of receiving the treatment based on the baseline characteristics. This creates pseudo-populations to achieve a balanced distribution of baseline covariates between groups (32). In this analysis, the PS was estimated by logistic regression including the following baseline covariates, selected according to both expert opinion and evaluation of covariates’ imbalance between cohorts:
- VA or CST at baseline, respectively, when analyzing BCVA and CST change;
- Age;
- Sex;
- Type of lesion (type 1, 2, and 3);
- Presence of intraretinal fluid (IRF) at baseline;
- Presence of subretinal fluid (SRF) at baseline.
Missing values in type of lesion, and baseline IRF and SRF were handled by inclusion of categories for missing values in the PS models.
Standardized mean differences (SMDs) and appropriate statistical tests (i.e., chi-square tests for categorical variables, and t-tests or non-parametric Mann-Whitney tests, respectively, for normally and not normally distributed continuous variables) were used to compare the distribution of baseline covariates between the RADIANCE and faricimab cohorts before and after IPTW.
A weighted one-way analysis of variance (ANOVA) was applied to compare mean changes in BCVA and CST between the two cohorts.
The frequency of injections may differ in randomized controlled trial (RCT) and clinical practice and, as already reported in the RADIANCE study, patients with a higher number of injections showed more favorable clinical outcomes (31). To account for the difference in treatment regime and protocol between the pivotal and real-world studies, in a sensitivity analysis an analysis of covariance (ANCOVA) model incorporating the number of injections as covariate and weighting for IPTW was used to obtain adjusted mean treatment differences.
All tests were two-sided, and a p value <0.05 was considered statistically significant.
Statistical analyses were performed with SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Patients and baseline characteristics
The RADIANCE study enrolled 405 patients. Of those, 17 were excluded because they did not satisfy the inclusion criteria (i.e., patients with diagnosis of nAMD, naïve to any intraocular anti-VEGF treatment, with age ≥50 years on the date of the first anti-VEGF injection), 1 because of the lack of hospital charts/clinical records at anti-VEGF treatment start, 43 because of the lack of at least two VA measurements after the baseline, and 81 because of the lack of a VA measurement at week 52 ± 10. The TENAYA and LUCERNE studies included a total of 665 patients treated with faricimab; of those, 152 patients were not eligible for this analysis (85 were non-Caucasian, 65 had missing BCVA and CST data at week 48, and, among the remaining, 2 were diagnosed as having PCV at baseline by the centralized reading center). Thus, finally, a total of 513 patients treated with faricimab from TENAYA and LUCERNE trials and 263 patients treated with aflibercept (n = 101), bevacizumab (n = 53), or ranibizumab (n = 109) from the RADIANCE study were included for comparison. All patients from both the faricimab and the RADIANCE cohorts had BCVA data at 1 year (i.e., week 48 and 52, respectively) and were therefore included in the analysis of BCVA change from baseline. CST data at week 48-52 were missing in 97 patients from the RADIANCE cohort and 4 patients from the faricimab cohort; therefore, the analysis of CST changes from baseline at 1 year was based on 509 patients treated with faricimab and 166 patients treated with the anti-VEGF SoC.
Before IPTW, significant differences in baseline patients’ characteristics between the two groups were observed. Specifically, in the RADIANCE cohort, compared to the faricimab cohort, patients were older, had lower BCVA and higher CST, and there was a higher proportion of patients with IRF. After IPTW, the cohorts were well-balanced (Tabs. 1 and 2).
| Before IPTW | After IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| TENAYA+LUCERNE
(faricimab) |
RADIANCE
(SoC) |
SMD | p value for comparison* | TENAYA+LUCERNE
(faricimab) |
RADIANCE
(SoC) |
SMD | p value for comparison* | |
| N | 513 | 263 | 772.4 | 297.2 | ||||
| Age, median (IQR) | 76 (70-81) | 78 (73-82) | −0.254 | 0.0007 | 77 (71-82) | 76 (71-81) | 0.040 | 0.601 |
| Sex, n (%) | ||||||||
| Men | 187 (36.5) | 115 (43.7) | −0.149 | 0.049 | 315.2 (40.8) | 319.7 (40.1) | 0.014 | 0.778 |
| Women | 326 (63.6) | 148 (56.3) | 457.2 (59.2) | 477.6 (59.9) | ||||
| Lesion type, n (%) | ||||||||
| Occult (type 1) | 275 (54.6) | 103 (40.9) | 0.292 | <0.001 | 395.7 (52.3) | 406.4 (52.1) | 0.005 | 0.951 |
| Classic (type 2)† | 210 (41.7) | 121 (48.0) | −0.102 | 316.6 (41.8) | 323.6 (41.5) | 0.008 | ||
| Type 3 | 19 (3.8) | 28 (11.1) | −0.271 | 45.1 (6.0) | 49.4 (6.3) | −0.014 | ||
| Missing | 9 | 11 | 14.9 | 17.8 | ||||
| IRF at baseline, n (%) | ||||||||
| No | 276 (54.9) | 84 (42.6) | 0.026 | 0.004 | 359.2 (51.5) | 371.8 (51.5) | −0.003 | 0.998 |
| Yes | 227 (45.1) | 113 (57.4) | 337.9 (48.5) | 349.9 (48.5) | ||||
| Missing | 10 | 66 | 75.3 | 75.4 | ||||
| SRF at baseline, n (%) | ||||||||
| No | 172 (34.0) | 58 (29.2) | 0.236 | 0.217 | 233.4 (33.3) | 264.5 (36.4) | 0.055 | 0.211 |
| Yes | 334 (66.0) | 141 (70.8) | 468.2 (66.7) | 461.7 (63.6) | ||||
| Missing | 7 | 64 | 70.8 | 71.0 | ||||
| VA (ETDRS Letter) at baseline, mean ± SD | 60.5 ± 13.1 | 57.1 ± 21.1 | 0.219 | 0.004 | 59.6 ± 16.5 | 61.1 ± 34.3 | −0.088 | 0.244 |
*p values were nominal.
†Including “minimally” and “predominantly” classic lesions.
IPTW = inverse probability of treatment weighting; IQR = interquartile range; IRF = intraretinal fluid; SD = standard deviation; SMD = standardized mean difference; SoC = standard of care (aflibercept, ranibizumab, and bevacizumab); SRF = subretinal fluid; VA = visual acuity.
| Before IPTW | After IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| TENAYA+LUCERNE
(Faricimab) |
RADIANCE
(SoC) |
SMD | p value for comparison* | TENAYA+LUCERNE
(faricimab) |
RADIANCE
(SoC) |
SMD | p value for comparison* | |
| N | 509 | 166 | 673.3 | 683.3 | ||||
| Age, median (IQR) | 76 (70-81) | 77 (72-82) | −0.155 | 0.077 | 76 (70-81) | 76 (70-80) | 0.030 | 0.739 |
| Sex, n (%) | ||||||||
| Men | 184 (36.2) | 80 (48.2) | −0.246 | 0.006 | 365.4 (39.4) | 275.7 (40.4) | −0.019 | 0.727 |
| Women | 325 (63.8) | 86 (51.8) | 407.8 (60.6) | 407.6 (59.7) | ||||
| Lesion type, n (%) | ||||||||
| Occult (type 1) | 274 (54.8) | 76 (47.2) | 0.161 | <0.001 | 350.5 (53.1) | 363.1 (54.2) | −0.022 | 0.916 |
| Classic (type 2)† | 207 (41.4) | 63 (39.1) | 0.056 | 268.4 (40.7) | 265.4 (39.6) | 0.022 | ||
| Type 3 | 19 (3.8) | 22 (13.7) | −0.346 | 40.8 (6.2) | 41.9 (6.5) | −0.002 | ||
| Missing | 9 | 5 | 13.3 | 12.8 | ||||
| IRF at baseline, n (%) | ||||||||
| No | 276 (55.2) | 68 (43.9) | −0.168 | 0.004 | 343.4 (52.4) | 342.6 (51.6) | −0.016 | 0.755 |
| Yes | 224 (44.8) | 87 (56.1) | 311.8 (47.6) | 321.8 (48.4) | ||||
| Missing | 9 | 11 | 18.0 | 18.8 | ||||
| SRF at baseline, n (%) | ||||||||
| No | 170 (33.9) | 46 (29.5) | −0.022 | 0.309 | 219.3 (33.3) | 242.6 (36.4) | 0.065 | 0.244 |
| Yes | 332 (66.1) | 110 (70.5) | 438.8 (66.7) | 424.4 (63.6) | ||||
| Missing | 7 | 10 | 15.1 | 16.2 | ||||
| CST (μm) at baseline, mean ± SD | 359.8 ± 121.3 | 392.0 ± 163.6 | −0.223 | 0.021 | 366.3 ± 144.3 | 362.0 ± 287.6 | 0.030 | 0.725 |
*P values were nominal.
†Including “minimally” and “predominantly” classic lesions.
CST = central subfield thickness; IPTW = inverse probability of treatment weighting; IQR = interquartile range; IRF = intraretinal fluid; SD = standard deviation; SMD = standardized mean difference; SoC = standard of care (aflibercept, ranibizumab, and bevacizumab); SRF = subretinal fluid.
Change from baseline in BCVA
After IPTW, mean vision gains in BCVA after 1 year of treatment were 6.4 letters (95% CI: 5.0 to 7.9) in the faricimab cohort and 1.0 letter (95% CI: −0.4 to 2.5) in the RADIANCE cohort, with a corresponding treatment difference of 5.4 letters (95% CI: 3.4 to 7.5, p<0.001) in favor of faricimab (Fig. 1).
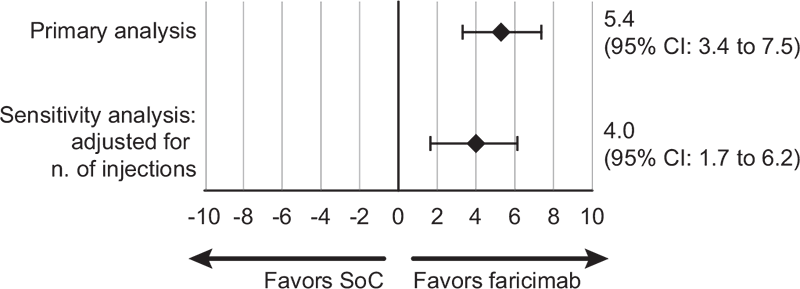
FIGURE 1 - Difference in BCVA mean change (95% CI) from baseline to 1 year after IPTW. BCVA = best corrected visual acuity; CI = confidential interval; IPTW = inverse probability of treatment weighting; SoC = standard of care (aflibercept, ranibizumab, and bevacizumab).
Change from baseline in CST
After IPTW, the mean CST change from baseline was −143.2 μm (95% CI: −156.2 to −130.1) in the faricimab cohort and −71.4 μm (95% CI: −84.3 to −58.4) in the RADIANCE cohort, with a corresponding treatment difference of −71.8 μm (95% CI: −90.2 to −53.4, p<0.001) in favor of faricimab (Fig. 2).
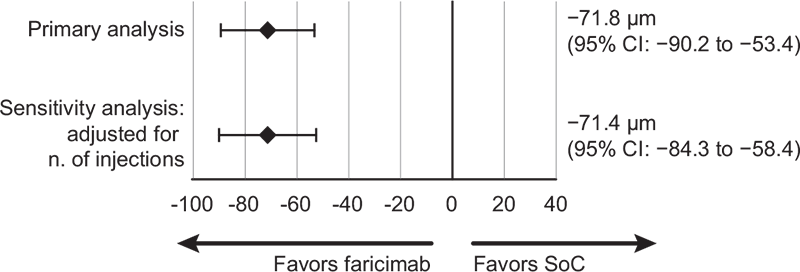
FIGURE 2 - Difference in CST mean change (95% CI) from baseline to 1 year after IPTW. CI = confidential interval; CST = central subfield thickness; IPTW = inverse probability of treatment weighting; SoC = standard of care (aflibercept, ranibizumab, and bevacizumab).
Sensitivity analysis
After adjusting for the number of injections, results were consistent with the main scenarios. There was a slight reduction in the weighted BCVA change from baseline in the faricimab cohort (+5.9 letters [95% CI: 4.4 to 7.4]) and a slight increase in the RADIANCE cohort (+1.9 letters [95% CI: 0.4 to 3.5]). The weighted treatment difference remained statistically significant and was consistent with that observed in the main scenario (+4.0 letters in favor of faricimab [95% CI: 1.7 to 6.2; p = 0.0005]). Similarly, after adjusting for the number of injections, the estimated treatment difference in CST change from baseline remained in favor of faricimab (−71.5 μm [95% CI: −91 to −52; p<0.001]) (Figs. 1 and 2).
Discussion
This indirect treatment comparison was conducted to evaluate the effectiveness of the novel anti-VEGF/Ang2 inhibitor faricimab compared to commonly used anti-VEGF agents in Italy. The analysis was performed using IPD from the real-world study RADIANCE (31) and a subset of faricimab-treated patients from the randomized clinical trials TENAYA and LUCERNE (30).
Despite the initial significant differences of baseline characteristics between the two cohorts, the IPTW allowed to balance the cohorts and mitigate the effect of potential confounding variables (i.e., age, sex, type of lesion, IRF, SRF, and baseline VA).
The base case analyses showed a favorable effect for faricimab with a treatment difference compared with SoC equal to a BCVA gain of 5.4 letters (95% CI: 3.4 to 7.5, p<0.001) and a CST reduction of −71.8 μm (95% CI: −90.2 to −53.4, p<0.001). The weighted BCVA change from baseline in the faricimab cohort (+6.5 letters [95% CI: 5.0 to 7.9]) was in line with the results of the TENAYA and LUCERNE trials (+6.2 letters, average of weeks 40-48) (30), and the weighted BCVA change from baseline in the RADIANCE cohort (+1.0 letter [95% CI: −0.4 to 2.5]) was in line with that observed in the RADIANCE study (+1.0 letter [SD 19.3]) (31).
Similarly, the weighted CST changes observed in this analysis (−143 μm [95% CI: −156 to −130] for faricimab and −71 μm [95% CI: −84 to 58]) for SoC) were comparable to those observed in the TENAYA and LUCERNE trials (−137 μm, average of weeks 40-48) and in the RADIANCE study (−58 μm [95% CI: −161 to 15]), respectively.
In the RADIANCE study, after stratification by the number of anti-VEGF injections per year, the median VA improvement was better in patients treated with >6 injections, whereas no improvement was observed in patients treated with fewer injections (31). The association observed in the RADIANCE study between the higher number of anti-VEGF injections and better outcomes was in line with what has been reported by other real-world studies (11,14,16,33). Since the injection frequency in the controlled setting of a clinical trial is often different to that observed in the real world (9,10,13,15,17,34,35), a sensitivity analysis was undertaken to adjust for the number of injections between the RADIANCE and faricimab cohorts. The BCVA gains and CST reductions observed in this sensitivity analysis were consistent with the main scenarios, with the weighted treatment difference in BCVA and CST equal to +4.0 letters and −71.5 μm, respectively, in favor of faricimab. Despite the adjustment for the number of injections, residual confounding by the different regime and protocol between the pivotal and real-world studies cannot be excluded, which may limit the interpretation and generalizability of the results.
To the best of our knowledge, this is the first study that compared faricimab with SoC for patients with nAMD in Italy. First-line treatment with faricimab was associated with greater visual gain and CST reduction, compared to current intravitreal anti-VEGF SoC, showing potential to redefine the treatment landscape for nAMD. Clinicians may consider faricimab as an effective option, particularly given its favorable outcomes in comparison to employed anti-VEGF agents.
The limitations of the study include the size of the real-world cohort, in particular for the analysis on CST, which was too small to allow subgroup analysis by anti-VEGF agent, along with all limitations associated with the comparison of clinical trial and real-world cohorts. In particular, the treatment regimens in the clinical trial setting are more controlled than in routine clinical practice. Although we used PS weighting to balance potential confounding factors, residual confounding as well as an impact of unmeasured confounders cannot be ruled out. In addition, in the RADIANCE cohort we included patients with at least two post-baseline endpoint measurements, including one around 52 weeks, and this could have introduced some selection bias if these patients were different from those with only one post-baseline measurement.
Conclusions
Despite limitations of indirect treatment comparisons, which call for specific post-marketing real-world studies, and the possibility that the difference may be due to frequency and time of treatment rather than to type of agents used, the present analysis supports potential benefits in terms of VA and CST reduction in naïve nAMD patients treated with faricimab compared with intravitreal anti-VEGF SoC (aflibercept, ranibizumab, and bevacizumab). Additional evidence from the real-world setting is required to confirm our finding.
Acknowledgments
The authors acknowledge Ombretta Bandi from SEEd Medical Publishers who provided writing assistance and journal styling services.
Disclosures
Financial support: This study was sponsored and financially supported by Roche S.p.A.
The study was presented as a poster at ISPOR Europe 2023 (Copenhagen, November 12-15, 2023).
Conflict of interest: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
CG Roche; FT None; MP Abbvie, Novartis, Bayer, Roche, Zeiss; FV Bayer, Novartis, Abbvie, Roche; MN Roche; SV Abbvie, Alimera, Apellis, Bayer. B&I, Novartis, Roche, Zeiss; LB is an employee at Roche; ES is an employee at Roche; GV is an employee at Roche; PL Aerie, Allergan, Annexon, Apellis, Bausch & Lomb, Bayer, Biogen, Boehringer Ingelheim, Eyepoint Pharmaceuticals, I-Care, Genentech, Novartis, Ocular Therapeutix, Outlook Therapeutics, and Roche
Authors’ contributions: All authors contributed to conceptualization, writing—review and editing.
CG and FT performed data management and statistical analysis.
LB, ES, and GV contributed to funding acquisition, project administration, resources, and supervision.
All authors have read and agreed to the published version of the manuscript.
References
- 1. Colijn JM, Buitendijk GHS, Prokofyeva E, et al; EYE-RISK consortium; European Eye Epidemiology (E3) consortium. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017;124(12):1753-1763. CrossRef PubMed
- 2. Flaxman SR, Bourne RRA, Resnikoff S, et al; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1234. CrossRef PubMed
- 3. Steinmetz JD, Bourne RRA, Briant PS, et al; GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-e160. CrossRef PubMed
- 4. Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26-39. CrossRef PubMed
- 5. Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy. 2013;33(8):838-855. CrossRef PubMed
- 6. Ferris FL III, Wilkinson CP, Bird A, et al; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. CrossRef PubMed
- 7. Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59(2):74-77. PubMed
- 8. Patel P, Sheth V. New and innovative treatments for neovascular age-related macular degeneration (nAMD). J Clin Med. 2021;10(11):2436. CrossRef PubMed
- 9. Brown DM, Kaiser PK, Michels M, et al; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. CrossRef PubMed
- 10. Busbee BG, Ho AC, Brown DM, et al; HARBOR Study Group. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056. CrossRef PubMed
- 11. Cohen SY, Mimoun G, Oubraham H, et al; LUMIERE Study Group. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33(3):474-481. CrossRef PubMed
- 12. Dugel PU, Koh A, Ogura Y, et al; HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. CrossRef PubMed
- 13. Heier JS, Brown DM, Chong V, et al; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. CrossRef PubMed
- 14. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220-226. CrossRef PubMed
- 15. Rosenfeld PJ, Brown DM, Heier JS, et al; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. CrossRef PubMed
- 16. Staurenghi G, Bandello F, Viola F, et al. Effectiveness of anti-vascular endothelial growth factors in neovascular age-related macular degeneration and variables associated with visual acuity outcomes: results from the EAGLE study. Khetan V, ed. PLoS One. 2021;16(9):e0256461. CrossRef PubMed
- 17. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. CrossRef PubMed
- 18. Calabria S, Ronconi G, Dondi L, et al. La popolazione con degenerazione maculare neovascolare correlata all’età trattata con anti-Vegf attraverso i dati amministrativi sanitari. Recenti Prog Med. 2023;114(7):447-461. PubMed
- 19. Kodjikian L, Mehanna CJ, Cohen SY, et al. The role of future treatments in the management of neovascular age-related macular degeneration in Europe. Eur J Ophthalmol. 2021;31(5):2179-2188. CrossRef PubMed
- 20. Lanzetta P. Anti-VEGF therapies for age-related macular degeneration: a powerful tactical gear or a blunt weapon? The choice is ours. Graefes Arch Clin Exp Ophthalmol. 2021;259(12):3561-3567. CrossRef PubMed
- 21. Daien V, Finger RP, Talks JS, et al. Evolution of treatment paradigms in neovascular age-related macular degeneration: a review of real-world evidence. Br J Ophthalmol. 2021;105(11):1475-1479. CrossRef PubMed
- 22. Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244-250. CrossRef PubMed
- 23. Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418-1431. CrossRef PubMed
- 24. Okada M, Kandasamy R, Chong EW, McGuiness M, Guymer RH. The treat-and-extend injection regimen versus alternate dosing strategies in age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2018;192:184-197. CrossRef PubMed
- 25. Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J; TREND Study Group. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57-65. CrossRef PubMed
- 26. Wykoff CC, Clark WL, Nielsen JS, Brill JV, Greene LS, Heggen CL. Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. JMCP. 2018;24(2-a Suppl):S3-S15. CrossRef PubMed
- 27. AIFA. Determina 2 Ottobre 2023 Riclassificazione Del Medicinale per Uso Umano «Vabysmo», Ai Sensi Dell’articolo 8, Comma 10, Della Legge 24 Dicembre 1993, n. 537. (Determina n. 602/2023). (23A05528) (GU n.235 Del 7-10-2023). 2023. Online. (Accessed July 2024)
- 28. AIFA. Determina 28 Dicembre 2020 Istituzione Della Nota AIFA 98 Relativa Alla Prescrizione e Alla Somministrazione Intravitreale Di Anti-VEGF Nella AMD e DME. (Determina n. DG/1379/2020). (20A07338) (GU n.323 Del 31-12-2020). 2020. Online. (Accessed July 2024)
- 29. AIFA. Determina 30 Gennaio 2015 Inserimento Di Una Indicazione Terapeutica Del Medicinale per Uso Umano «Bevacizumab – Avastin» Nell’elenco Ex Lege n. 648/1996 – Parziale Modifica Alla Determina n. 622 DG/2014 Del 23 Giugno 2014 e Sostituzione Della Stessa. (Determina n. 79/2015). (15A01013) (GU n.38 Del 16-2-2015). 2015. Online. (Accessed July 2024)
- 30. Heier JS, Khanani AM, Quezada Ruiz C, et al; TENAYA and LUCERNE Investigators. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729-740. CrossRef PubMed
- 31. Parravano MC, Viola F, Nicolo M, et al. Real-world evidence of anti-VEGF therapies in neovascular age-related macular degeneration in Italy: the RADIANCE study. Eur J Ophthalmol. (In Press)
- 32. Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021;15(1):14-20. CrossRef PubMed
- 33. Holz FG, Tadayoni R, Beatty S, et al. Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti-VEGF agents: an instrumental variable analysis of the AURA study. Eye (Lond). 2016;30(8):1063-1071. CrossRef PubMed
- 34. Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br J Ophthalmol. 2020;104(8):1077-1084. CrossRef PubMed
- 35. Pina Marín B, Gajate Paniagua NM, Gómez-Baldó L, Gallego-Pinazo R. Burden of disease assessment in patients with neovascular age-related macular degeneration in Spain: results of the AMD-MANAGE study. Eur J Ophthalmol. 2022;32(1):385-394. CrossRef PubMed