 |
Drug Target Insights 2024; 18: 47-53 ISSN 1177-3928 | DOI: 10.33393/dti.2024.2745 ORIGINAL RESEARCH ARTICLE |
 |
In vivo analgesic, anti-inflammatory, sedative, muscle relaxant activities, and docking studies of 3’,4’,7,8-tetrahydroxy-3-methoxyflavone isolated from Pistacia chinensis
ABSTRACT
Background: Pistacia chinensis is extensively employed in traditional medicine. This study aimed to isolate and evaluate the therapeutic effects of 3’4’78-tetrahydroxy-3-methoxyflavone from P. chinensis crude extract.
Materials and Methods: The study utilized column chromatography for isolation. The plant extract and its isolated compound were assessed for in vivo analgesic (hot plate model), anti-inflammatory (carrageenan-induced paw edema), sedative (open field model), and muscle relaxing properties (inclined plane and traction test).
Results: In the thermal-induced analgesic model, a significant analgesic effect was observed for the extract (25, 50, and 100 mg/kg) and the isolated compound (2.5, 5, 10, and 15 mg/kg) at higher doses. The extract (100 mg/kg) significantly prolonged latency time (21.98 seconds) after 120 minutes of administration. The isolated compound elevated the latency time (20.03 seconds) after 30 minutes, remaining significant up to 120 minutes with a latency time of 24.11 seconds. The anti-inflammatory effect showed a reduction in inflammatory reactions by 50.23% (extract) and 67.09% (compound) after the fifth hour of treatment. Both samples demonstrated significant sedative effects, with the extract hindering movement by 54.11 lines crossed compared to the negative control (180.99 lines). The isolated compound reduced the number of lines crossed to 15.23±SEM compared to the negative control. Both samples were also significant muscle relaxants. Docking studies indicated that the compound’s therapeutic effect is due to inhibiting COX and nociceptive pathways.
Conclusion: The isolated compound from Pistacia chinensis exhibits significant analgesic, anti-inflammatory, sedative, and muscle relaxing properties, with potential therapeutic applications by inhibiting COX and nociceptive pathways.
Keywords: Anacardiaceae, Analgesic, Anti-inflammatory, In vivo, Muscle relaxant, Pistacia chinensis, Sedative
Received: November 28, 2023
Accepted: May 6, 2024
Published online: June 18, 2024
Corresponding authors:
Abdur Rauf and Marcello Iriti
email: mashaljcs@yahoo.com and marcello.iriti@unimi.it
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Plants have been acknowledged for millennia as having direct therapeutic effects for treating common disorders (1,2). The etymology of the term pistachio can be traced back to its Avestan origin, precisely the word pstk, which translates to pistag. The term pistachio can also be traced back to the word pista in many languages, such as Persian and the early classical tongues of Central Asia (3,4). The Anacardiaceae (R.Br.) Lindl. family (order Sapindales) originally comprised 82 genera and 700 species of pantropical (tropical and subtropical) trees, shrubs, and lianas that secrete gums and resins (5). Recently included are 83 genera and 860 tree species (6).
The kakra shringi is the Chinese Pistacia chinensis variety integerrima. Located in the Himalayas from the Indus to Kumaon, this moderate-sized deciduous tree reaches heights of 18 m (7). The leaves and petioles of plants infested by the Pemphigus species of bug develop hard, horn-shaped, rugose, hollow galls (8). The crushed and dried galls possess a distinct aroma and flavor reminiscent of terebinth. The flavor profile of these items exhibits a sharp and slightly spicy taste reminiscent of terebinthine notes. Additionally, there is a subtle balsamic aroma present (9).
P. chinensis is a widespread Pistacia species found in Asia. Traditional applications of P. chinensis include wood, seed oil, ornamental uses, and folk remedies for detoxification, sore pharynx, and diarrhea (10). According to De Pooter et al (11), the primary chemical components of P. chinensis leaf essential oils grown in Egypt were trans-8-ocimene limonene. Two 3-3ʹ-dimeric 4-phenyl dihydro coumarin compounds with estrogen-like activity were isolated from the twig extract of P. chinensis (12). The galls of P. chinensis are used in Ayurvedic medicine to treat a wide range of ailments, including but not limited to hiccups, asthma, chronic bronchitis, fever, vomiting in infants, skin diseases, psoriasis, snake bites, scorpion stings, and increased hunger (13). The plant has considerable therapeutic potential under its pharmacological activity and active constituents. The P. integerrima plant has captured the interest of researchers, and a wealth of literature is already available (14). Few studies explored particular aspects of P. chinensis. In Pakistan, hepatitis and liver disorders are treated with P. chinensis galls. There have been reports of its leishmanicidal (15), depressant (16), analgesic and anti-inflammatory (17), spasmolytic (18), and hyperuricemic (10) properties. Noureen et al (19) concluded that bioactive antioxidants found in the bark of P. chinensis could be a suitable source for isolating potent antioxidant compounds.
In addition to its therapeutic uses, P. chinensis has numerous applications in ecology and energy production, among others (19). P. chinensis oil, mostly fatty acids with 16 to 18 carbon chain lengths, is chemically identical to fossil diesel (C15 C19) (20). P. chinensis biodiesel meets EU, US, and Chinese light diesel standards (21). A compound with a chemical structure of 2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-3-methoxy-4H-chromen-4-one compound derived from P. chinensis exhibits properties that can help regulate blood sugar levels and prevent glycation (22). P. chinensis isolated flavonoids have been shown to exhibit ENPP1 inhibition, which is a therapeutic intervention (23).
To date, only a limited number of flavones have been successfully commercialized, despite their potential as analgesics, sedatives, muscle relaxants, and anti-inflammatory agents. Considerable evidence exists regarding the potential toxicity and adverse effects associated with nearly all commercially available flavones. Hence, the present study was designed to investigate the potential in vivo analgesic, sedative, muscle relaxant, and anti-inflammatory properties of the compound 3’,4’,7,8-tetrahydroxy-3-methoxyflavone derived from P. chinensis. The aim was to determine if these effects could be achieved with minimal adverse effects, taking into consideration the traditional use of the plant.
Materials and methods
Plant collection
P. chinensis (Bunge) seeds were acquired from the University of Peshawar’s botanical garden. Dr. Muhammad Ilyas, Department of Botany, University of Swabi, KP, Pakistan, identified the plant species delivered to the department. The specimen with the voucher number Pistacia chinensis was stored in the department’s herbarium.
The obtained seeds were shade-dried for 20 days and washed with water to remove grit. After that, the desiccated grain was ground into a powder using a grinder. One kilogram of powdered plant material was immersed in methanol for 10 days to extract the most significant number of polar secondary metabolites. The obtained extract was concentrated at low temperature and pressure using a rotary evaporator, yielding 18.9 g of extract (2.11%).
Extraction and isolation
The seeds of P. chinensis were rinsed with water to remove dust. The washed seeds (8.32 kg) were pulverized using a machine to produce the powdered plant materials. The plant material was subjected to a 20-day cold extraction with methanol (10 mL). The extract was concentrated using a rotary evaporator under reduced temperature and pressure, yielding 112 g of crude extract. To remove the less polar compounds from the crude extract, it was defatted with n-hexane. The 22.2 g defatted crude extract was subjected to column chromatography, yielding compound 1 (Figure 1). Physical and spectroscopic data were compared to previously reported data to determine the isolated compound chemical structure (20,21,24).
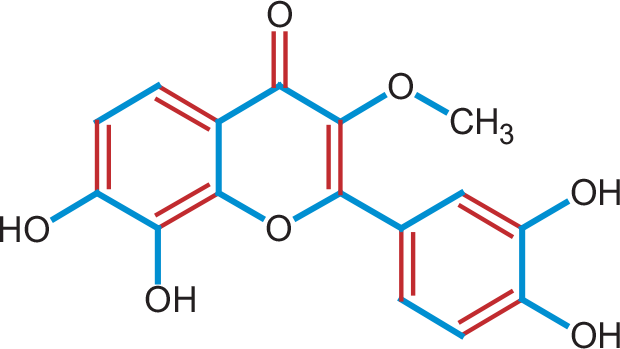
FIGURE 1 - The chemical structure of the isolated compound 1 from Pistacia chinensis.
Classification of animals
The BLAB/c mice of either sex weighing 23-27 g were obtained from the National Institute of Heath Islamabad, Pakistan. The animals were classified for the in vivo experiments as negative control (treated with normal saline, 10 mL/kg), positive control (treated with standard drug), and extract/compounds were administered to the tested groups. This study was approved by the ethical committee (SOU/Pharm-23), Department of Pharmacy, University of Swabi, KP, Pakistan, on January 15, 2023.
Analgesic screening
A hot plate analgesia meter was used to evolve the analgesic effect. Animals were classified as above. All the animals were tested on hot plates for positive responses. The animals were treated with extract or compound, and after 30 minutes of treatment, each animal was placed at the center of a hot plate, and the time of stay on the hot plate was counted in seconds (latency time). The animal that stayed more than 25 seconds (cut-off time) was excluded from the study. The latency time was periodically observed at 30, 60, 90, and 120 minutes posttreatment (25). Tramadol was used as a standard drug.
Anti-inflammatory activity
After correctly classifying animals into the abovementioned categories, the negative control group was administered normal saline, and the positive control group was administered diclofenac sodium. The rest of the groups were treated with extract and isolated compound 1. After 30 minutes of these treatments, carrageenan (1%, 0.05 mL) was administered subcutaneously to the right hind paw of each animal. The induced inflammation was determined in the form of paw edema. This paw volume was measured regularly, and the anti-inflammatory effect percentage was calculated using the following formula:
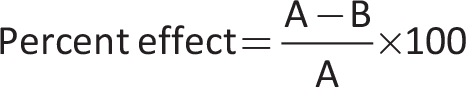
where A and B represent the paw edema of the negative and positive control groups, respectively (26).
Sedative screening
Using our previously published paradigm, the open-field method was used to evaluate the sedative effect of the extract and the compound. The experimental room’s soundproofing consisted of a wooden cage with equal space. The animals were categorized as described earlier, and the positive control group was administered diazepam (0.5 mg/kg). After 30 minutes of treatment with normal saline (negative control), diazepam (positive control), and extract/compound (tested groups), the sedative effect of each animal in the special wooden box was evaluated. The animal was deposited in the center of the box, and for 10 minutes, the number of lines it crossed was recorded. The greater the number of crossed lines, the less sedative the drug, and vice versa (27).
Muscle relaxant activity
Inclined plane and traction tests were used to determine muscle coordination potential. In the inclined plane test, a plane was designed with an angle of 65° on which an animal will slide. A metallic wire coated with rubber was used in case of traction test on which the animal will hang. After correctly classifying animals into various groups as above, the groups were appropriately treated with normal saline, diazepam, and extract/compound. After 30 minutes of these treatments, the animal’s muscle coordination effect was checked on an inclined plan for 5 seconds and the hanging duration for 5 seconds. The sliding of animals within 5 seconds meant animals with relaxed muscles, and the passing of animals to hang with wire for 5 seconds reflects no muscle relaxation effect.
Docking
Interaction analysis via docking between receptors and ligands is essential for finding ligand inhibition patterns. We performed docking studies using MOE software version MOE 2016.0802 (Chemical Computing Group, Canada). Interactions of the new ligand and the superimposed co-crystallized ligand have been found.
Results
The extract and isolated compound were significantly analgesic in thermally induced algesia, as shown in Table 1. The analgesic effect of the extract (25, 50, and 100 mg/kg) and the isolated compound (2.5, 5, 10, and 15 mg/kg) was dose dependent. The extract (100 mg/kg) exhibited a significant (p<0.01) prolonged latency time (16.76 seconds) as compared to negative control after 30 minutes of administration, and this effect remained significant (p<0.001) up to 120 minutes (21.98 seconds latency time) after administration. The isolated compound elevated the latency time (20.03 seconds) after 30 minutes where the effect remained significant up to 120 minutes with a latency time of 24.11 seconds.
| Group | Dose (mg/kg) | Time (minutes) | |||
|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | ||
| NS | 10 mL | 9.18 ± 0.06 | 9.19 ± 0.09 | 9.17 ± 0.10 | 9.20 ± 0.08 |
| Tramadol | 10 | 25.23 ± 0.07*** | 25.30 ± 0.13*** | 26.00 ± 0.15*** | 26.43 ± 0.12*** |
| Extract | 25 | 9.65 ± 0.90 | 13.98 ± 0.43 | 14.00 ± 0.27 | 13.90 ± 0.87 |
| 50 | 12.43 ± 0.79 | 16.09 ± 0.66** | 16.98 ± 0.64** | 17.00 ± 0.54** | |
| 100 | 14.76 ± 0.66 | 19.49 ± 0.65** | 17.07 ± 0.43** | 16.90 ± 0.23** | |
| 250 | 16.76 ± 0.76** | 21.65 ± 0.54*** | 22.24 ± 0.21*** | 21.98 ± 0.11*** | |
| Compound 1 | 2.5 | 11.76 ± 0.45 | 14.98 ± 0.54 | 15.00 ± 0.34 | 14.87 ± 0.39 |
| 5 | 13.09 ± 0.66 | 17.34 ± 0.28** | 17.98 ± 0.54** | 17.32 ± 0.40** | |
| 10 | 17.32 ± 0.55 | 20.87 ± 0.51*** | 20.98 ± 0.28*** | 20.07 ± 0.45*** | |
| 15 | 20.03 ± 0.45*** | 24.08 ± 0.55 | 24.65 ± 0.54*** | 24.11 ± 0.43*** | |
ANOVA = analysis of variance; NS = normal saline, SEM = standard error of the mean.
***p<0.001, **p<0.01.
Data are presented as mean ± SEM. The level of statistical significance was calculated through GraphPad Prism using an ANOVA analysis test.
The anti-inflammatory effect of the extract and compound is exhibited in Figure 2. The extract reduced the edema by 20.23% after 3 hours of administration and significantly by 50.23% after 5 hours of experimental duration. The isolated compound attenuated the inflammatory reactions up to 86.32% after 3 hours, and this anti-inflammatory process was improved by 67.09% after 5 hours of compound administration.
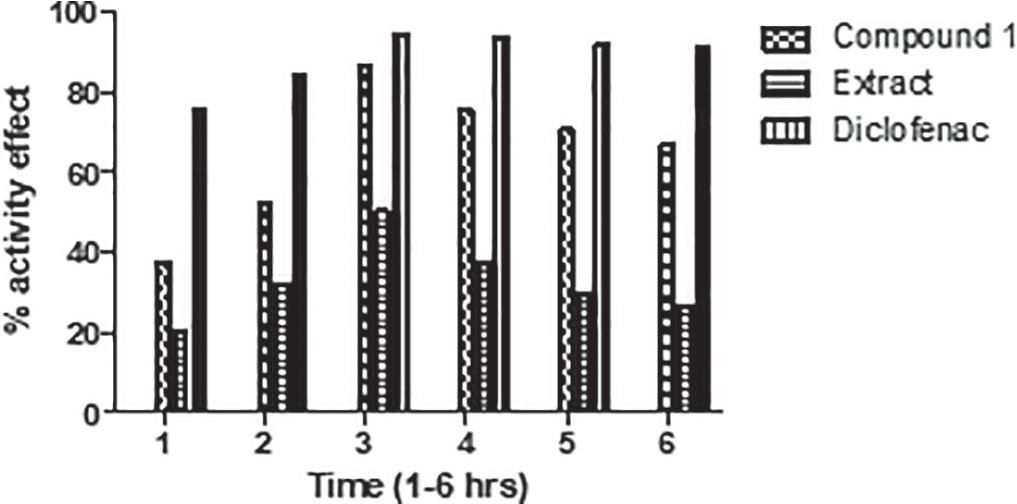
FIGURE 2 - Percentage of the anti-inflammatory effect of crude extract and isolated compound 1 of Pistacia chinensis on carrageenan paw in mice.
The crude extract and isolated compound from P. chinensis were found to be significant sedatives in the open-field model, as shown in Table 2. No sedative effect was observed in lower doses (25 and 50 mg/kg), while the higher doses (100 and 250 mg/kg) demonstrated significant (p<0.001) hindrance in the movement of animals. The isolated compound resulted in a more sedative effect as compared to the extract. The movement of animals was significantly (p<0.01) hindered (54.11 lines crossed as compared to the negative control – 180.99 lines crossed by animals). The isolated compound reduced the number of lines crossed up to 15.23 compared to the negative control.
| Samples | Dose (mg/kg) | Number of lines crossed |
|---|---|---|
| NS | 5 mL | 180.99 ± 3.54 |
| Diazepam | 0.5 | 2.32 ± 0.43*** |
| Extract | 25 | 85.29 ± 3.33 |
| 50 | 76.76 ± 3.09 | |
| 100 | 67.09 ± 2.64* | |
| 250 | 54.11 ± 2.65** | |
| Compound 1 | 2.5 | 45.24 ± 2.01** |
| 5 | 36.23 ± 1.77*** | |
| 10 | 25.09 ± 1.50*** | |
| 15 | 15.23 ± 1.32*** |
ANOVA = analysis of variance; NS = normal saline, SEM = standard error of the mean.
***p<0.001, **p<0.01.
Data are presented as mean ± SEM. The level of statistical significance was calculated through GraphPad Prism using the ANOVA analysis test.
The effect on muscle coordination of crude extract and isolated compound from P. chinensis is presented in Table 3. A significant muscle relaxant effect was noticed in both experimental models. Uniform muscle relaxation was noted dose-dependently and time-dependently. The extract (250 mg/kg) demonstrated a 42.88% and 43.56% effect in the inclined plane and traction model, respectively. The isolated compound exhibited up to 70% muscle relaxation at 15 mg/kg.
We also performed docking studies by using Molecular Operating Environment (MOE) software. Compound 3’,4’,7,8-tetrahydroxy-3-methoxyflavone isolated from P. chinensis (Anacardiaceae) docked in the binding sites of pain modulating and inflammatory receptors having PDB accession codes 1EQG, 1CX2, 4EJ4, 4DJH, 4DKL for COX-1, COX-2, DOR, KOR, and MOR (28-31).
| Group | Dose (mg/kg) | Inclined plane model (%) | Traction model (%) | ||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 30 | 60 | 90 | ||
| NS | 10 mL/kg | – | – | – | – | – | – |
| Diazepam | 0.5 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| Crude extract | 25 | 14.43 ± 3.02 | 20.43 ± 2.76 | 21.98 ± 2.00 | 15.23 ± 2.54 | 21.43 ± 2.87 | 22.06 ± 2.41 |
| 50 | 20.98 ± 2.87 | 26.98 ± 2.80 | 27.55 ± 2.04 | 21.64 ± 2.89 | 27.98 ± 2.98 | 28.54 ± 2.47 | |
| 100 | 27.43 ± 2.88 | 33.98 ± 2.82 | 34.80 ± 2.76 | 28.09 ± 2.54 | 34.09 ± 2.93 | 34.87 ± 2.44 | |
| 250 | 34.54 ± 2.90 | 41.09 ± 2.66 | 42.88 ± 2.54 | 35.88 ± 2.34 | 43.09 ± 2.65 | 43.56 ± 2.56 | |
| Compound 1 | 2.5 | 41.67 ± 2.20 | 47.54 ± 2.12 | 48.54 ± 2.10 | 42.01 ± 2.11 | 47.99 ± 2.04 | 48.39 ± 3.01 |
| 5 | 48.09 ± 2.80 | 55.87 ± 2.00 | 55.32 ± 2.11 | 49.32 ± 1.80 | 56.43 ± 2.13 | 56.91 ± 2.98 | |
| 10 | 56.43 ± 2.32 | 63.98 ± 2.34 | 63.98 ± 2.65 | 57.13 ± 1.90 | 64.36 ± 2.06 | 65.08 ± 2.662 | |
| 15 | 62.09 ± 2.77 | 68.33 ± 2.66 | 69.03 ± 2.90 | 62.78 ± 1.66 | 70.02 ± 2.08 | 70.98 ± 2.14 | |
ANOVA = analysis of variance; NS = normal saline, SEM = standard error of the mean.
***p<0.001, **p<0.01.
Data are presented as mean ± SEM. The level of statistical significance was calculated through GraphPad Prism using the ANOVA analysis test.
Two-dimensional interaction plots of native ligand ibuprofen and isolated flavon in the binding sites of cyclooxygenase (COX)-1 are shown in Figure 3. Ibuprofen showed hydrogen bonding interaction with the receptor COX-1 (Fig. 3A). Isolated flavone interacts with COX-1 residues (Gly526, Ser530, Tyr355) and showed good interactions for inhibiting pain and inflammation modulation that includes one conventional hydrogen bond with residue Ser530, π-π interaction with residue Gly526, and amide-π stacking with residue Tyr355 (Fig. 3B).
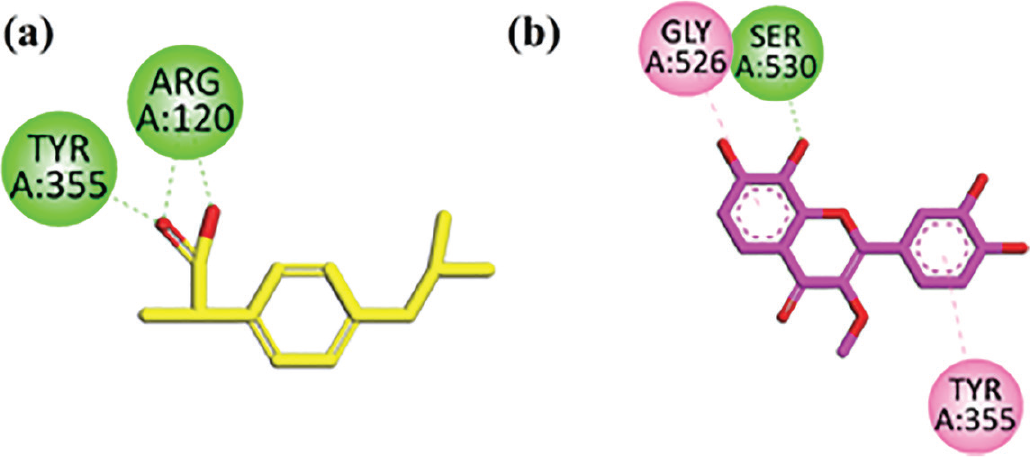
FIGURE 3 - A) Ibuprofen (co-crystalized native ligand) two-dimensional interaction plot in the cyclooxygenase-1 (COX-1) receptor binding site. B) Isolated compound 3’,4’,7,8-tetrahydroxy-3-methoxyflavone two-dimensional interaction plot in the COX-1 receptor.
Two-dimensional interaction plots of native ligand SC-558 and isolated compound in the binding site of COX-2 are shown in Figure 4. SC-558 inhibitor has good hydrogen bonding interaction with the receptor COX-2 (Fig. 4A). Isolated flavone interacts with the COX-2 receptor and showed five conventional hydrogen bond interactions with residues Tyr385, Leu352, His90, Arg120, and Tyr355, which enhance the ligand-inhibiting efficacy (Fig. 4B).
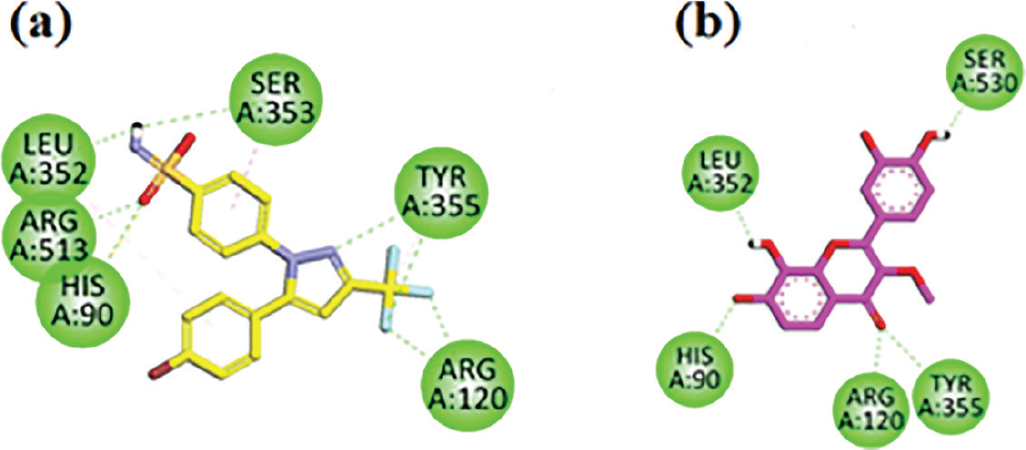
FIGURE 4 - A) SC-558 (co-crystalized native ligand) two-dimensional interaction plot in the cyclooxygenase-2 (COX-2) receptor binding site. B) Isolated flavone two-dimensional interaction plot in the COX-2 receptor binding site.
Two-dimensional interaction plots of native ligand naltrindole and isolated compound in the binding site of delta opioid receptor (DOR) are shown in Figure 5. Naltrindole has good interactions such as conventional hydrogen bonding interaction and π-sulfur with the DOR (Figure 5A). Isolated compound flavone interacts with the DOR by residues Met132, His278, Asp128, Ile304 and exhibited three conventional hydrogen bonds with residues His278, Asp128, Ile304 and a π-sulfur interaction with residue Met132 (Figure 5B).
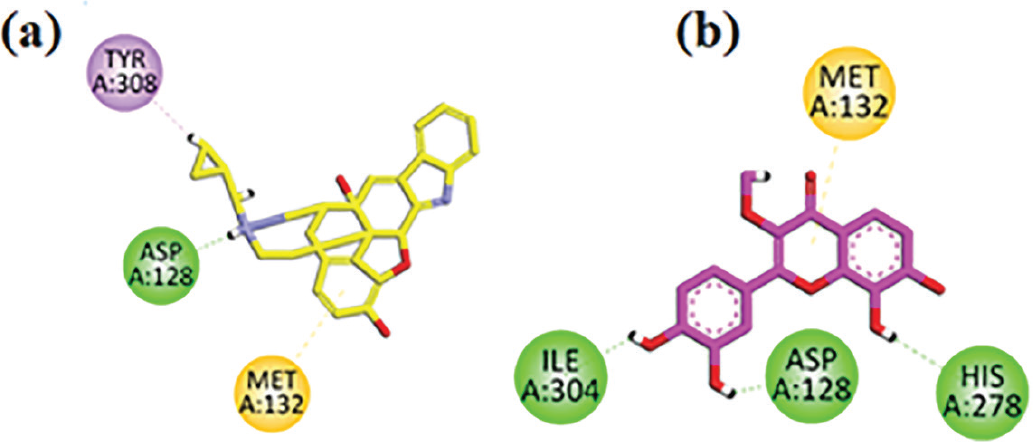
FIGURE 5 - A) Naltrindole (co-crystalized native ligand) two-dimensional interaction plot in the delta opioid receptor (DOR) receptor binding site. B) Isolated flavone two-dimensional interaction plot in the DOR binding site.
Two-dimensional interaction plots of native ligand JDTic and isolated compound in the binding site of kappa opioid receptor (KOR) are shown in Figure 6. JDTic inhibitor has good interactions such as conventional hydrogen bonding, π-π stacking, and π-sulfur interaction with KOR (Figure 6A). Isolated flavone interacted with KOR residues (Met142, His291, Tyr312, Tyr139) and showed three conventional hydrogen bonds with residues His291, Tyr312, Tyr139 and π-sulfur interaction with residue Met142 (Figure 6B).
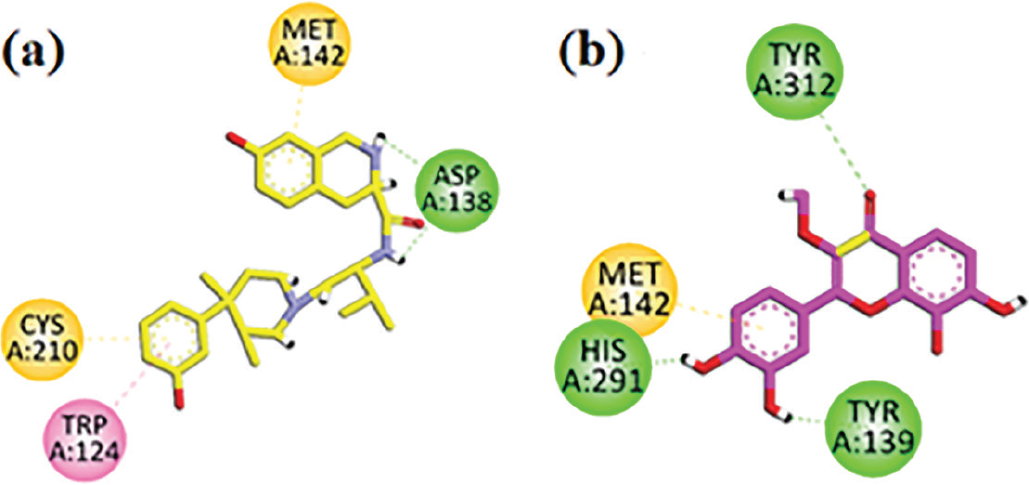
FIGURE 6 - A) JDTic (co-crystallized native ligand) two-dimensional interaction plot in the kappa opioid receptor (KOR) receptor binding site. B) Isolated flavone two-dimensional interaction plot in the KOR binding site.
Two-dimensional interaction plots of native ligand (b-FNA) and isolated compound in the binding site of mu opioid receptor (MOR) are shown in Figure 7. The co-crystalized ligand has good interactions, such as conventional hydrogen bonding and π-sulfur interaction with the MOR (Figure 7A). Isolated compound interacts with MOR binding site residues Val300, Asp147, Tyr326, Asn150, and His297 and showed good interactions for inhibiting pain, euphoria, sedation, and respiratory depression receptors, which included three conventional hydrogen bonds with residues Asp147, Asn150, His297, π-π stacking with residue Tyr326, and π-sigma interaction with residue Val300 (Figure 7B).
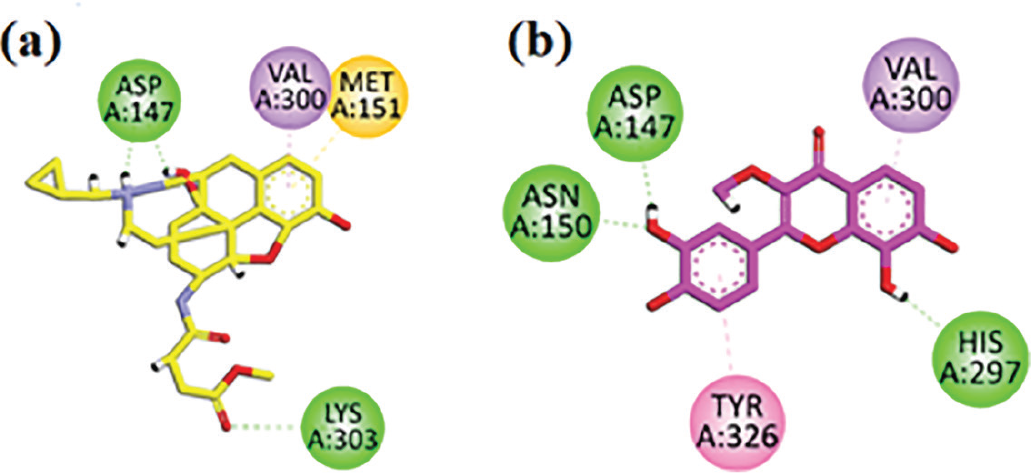
FIGURE 7 - A) b-FNA (co-crystalized native ligand) two-dimensional interaction plot in the mu opioid receptor (MOR) receptor binding site. B) Isolated compound two-dimensional interaction plot in the MOR binding site.
Discussion
The search for new, safe and effective economical drug candidates is challenging for medicinal chemists in the modern era. The pain, inflammation, and insomnia are treated with various drug regimens, and each of these analgesic, anti-inflammatory, and sedative drugs has mild or significant side effects. Due to these side effects, patient compliance is declining, and ultimately, the patient seeks an alternative therapeutic agent. This switching of therapeutic options leads to therapy failure. Therefore, searching for a safe and effective drug molecule is essential. The present study was conducted to find a suitable drug molecule from P. chinensis. This medicinal plant is used locally to treat pain, inflammation (31), fever, and depression (10). To provide a scientific background to this folklore, the crude extract and isolated compound of P. chinensis were tested for analgesic, anti-inflammatory, and muscle coordination effects. The extract and isolated constituent demonstrated a significant analgesic effect in the thermal-induced pain model. This pain model is used to find central analgesic potential (32). The prolongation of latency time by extracting and isolating molecules reflects the central analgesic potential. The central analgesic pathway is attributed to the stimulation of opioid receptors (25). Once these inhibitor receptors link with inhibitor proteins and are stimulated with ligand, the neurotransmitters’ (substance p) release is blocked, and pain sensation is diminished. The results of the hot plate indicate that the extract or compound might be agnostic for opioid receptors. The samples to be tested also attenuated the induced paw edema in the inflammatory model. This attenuation of induced paw edema by inflammatory mediators (carrageenan) indicates the COX inhibitory potential. The extract and compound also proved to be a sedative following previous studies (33). The muscle relaxation effect is also a promising adjuvant with analgesic and anti-inflammatory effects.
Docking studies were performed for the isolated compound against COX-1,2, DOR, KOR, and MOR, and their interactions were also compared with native co-crystalized ligands of each receptor. Analgesic drugs help to reduce the pain and are also called painkillers. In our current study, we performed analgesic and anti-inflammatory in vivo activity by keeping the tramadol and diclofenac as standard drugs. Results showed that isolated compounds have good analgesic and anti-inflammatory effects. COX receptors synthesize prostaglandins, prostanoids, and thromboxane, which cause inflammation and pain. COX receptor inhibition helps to get relief from pain and inflammation. COX-1 and COX-2 are the two COX receptors. Opioid receptors belong to the seven transmembrane G protein-coupled receptors (GPCRs). Opioid receptors are known for mediating the hormones and neurotransmitters. Opioid receptors are also the primary target for anti-analgesics, anti-inflammatory drugs, antidepressants, sedatives, and muscle relaxant medications. Opioid receptors are extensively dispersed in the central and peripheral nervous systems (CNS and PNS). Opioid receptors are classified as delta, kappa, and mu opioid receptors.
In our current study, the experimental results showed that the isolated compound has a significant sedative and muscle relaxant effect. We performed docking studies on two forms of COX (COX-1, COX-2) and three opioid receptors (delta, kappa, and mu). Isolated flavone from P. chinensis (Anacardiaceae) showed strong interactions with COXs. In the binding site of COX-1, the compound exhibited one hydrogen bond formed with residue Ser530, π-π interaction with Gly526, and amide-π stacking with residue Tyr355. In contrast, the isolated flavone interacted with two critical residues (His90 and Leu352) in the COX-2-specific binding site. The computed crucial energy values for isolated flavone in the binding sites of COX-1 and COX-2 are −6.374 and −7.019 kcal/mol, respectively. These values showed that it predominantly inhibited COX-2. However, it may be classified as a nonselective COX inhibitor. Isolated flavone also exhibited strong interactions with all studied opioid receptors. The calculated binding energy values for DOR, KOR, and MOR are −6.2942, −6.7136, and −6.6148 kcal/mol, respectively. Docking studies on COX isoforms and opioid receptors showed that the compound exerts its therapeutic effect by inhibiting COX and nociceptive pathways.
P. chinensis extract and isolated compound exhibited potent analgesic, anti-inflammatory, sedative, and muscle relaxant properties. Consequently, our findings justify using P. chinensis extract and isolated compounds to treat numerous diseases. The discovery of novel pharmaceutical products will result from the detailed mechanism studies conducted for drug discovery. Docking studies on COX isoforms and opioid receptors showed that the compound exerts its therapeutic effect by inhibiting COX and nociceptive pathways.
Acknowledgment
The authors thank Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2024R18), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Disclosures
Financial support: The financial contributors had no role in the design, analysis, or writing of this article.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors contributions: Conceptualization, AR, UR, ZA; Methodology, AS, NM, NA, Formal analysis, TSA, SM; Writing original draft preparation, Supervision, AR, MI; Writing-Editing, MI. All authors read this article and approved it for submission.
References
- 1. Sharma V, Gautam DNS, Radu AF, Behl T, Bungau SG, Vesa CM. Reviewing the traditional/modern uses, phytochemistry, essential oils/extracts and pharmacology of Embelia ribes Burm. Antioxidants. 2022;11(7):1359. CrossRef PubMed
- 2. Dai W, Long L, Wang X, Li S, Xu H. Phytochemicals targeting Toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin Med. 2022;17(1):53. CrossRef PubMed
- 3. Kashaninejad M, Tabil LG. Pistachio (Pistacia vera L.). In Yahia EM, ed. Postharvest biology and technology of tropical and subtropical fruits. Cambridge, UK: Woodhead Publishing; 2011: 218-247e.
- 4. Mir-Makhamad B, Bjørn R, Stark S, Spengler RN III. Pistachio (Pistaciavera L.) domestication and dispersal out of Central Asia. Agronomy (Basel). 2022;12(8):1758. CrossRef
- 5. Glimn-Lacy J, Kaufman PB. Botany illustrated: introduction to plants, major groups, flowering plant families. Springer; 2006.
- 6. Christenhusz MJ, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261(3):201-217. CrossRef
- 7. Anonymous. The wealth of India. A dictionary of Indian raw materials and industrial products publication and information directorate, vol 8. New Delhi: CSIR; 1998:120.
- 8. Chopra RN, Chopra IC, Handa KL, Kapur LD. Chopra’s indigenous drugs of India. Academic Publishers; 1994.
- 9. Keys JD. Chinese herbs. Tuttle Publishing; 2011 Dec 6.
- 10. Huang CY, Chang YY, Chang ST, Chang HT. Xanthine oxidase inhibitory activity and chemical composition of Pistacia chinensis leaf essential oil. Pharmaceutics. 2022;14(10):1982. CrossRef PubMed
- 11. De Pooter HL, Schamp NM, Aboutabl EA, El Tohamy SF, Doss SL. Essential oils from the leaves of three Pistacia species grown in Egypt. Flavour Fragrance J. 1991;6(3):229-232. CrossRef
- 12. Nishimuta S. Taki M, Takaishi S, Iijima Y, Akiyama T. Structures of 4-aryl-coumarin (neoflavone) dimers isolated from Pistacia chinensis Bunge and their estrogen-like activity. Chem Pharm Bull (Tokyo). 2000;48(4):505-508. CrossRef PubMed
- 13. Chopra RN, Badhwar RK, Ghosh S. Poisonous plants of India. Indian Council of Agricultural Research; 1965:270.
- 14. Bibi Y, Zia M, Qayyum A. Review – an overview of Pistacia integerrima a medicinal plant species: ethnobotany, biological activities and phytochemistry. Pak J Pharm Sci. 2015;28(3):1009-1013. PubMed
- 15. Rauf A, Rashid U, Shbeer AM, et al. Flavonoids from Pistacia chinensis subsp. integerrima with leishmanicidal activity: computational and experimental evidence. Nat Prod Res. 2023;20:1-6. CrossRef PubMed
- 16. Ansari SH, Ali M, Qadry JS. Essential oils of Pistacia integerrima Galls and their effect on the central nervous system. In J Pharmacog 1993; 31(2):89-95.
- 17. Ahmad NS, Waheed A, Farman M, Qayyum A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J Ethnopharmacol. 2010;129(2):250-253. CrossRef PubMed
- 18. Shirole RL, Shirole NL, Saraf MN. In vitro relaxant and spasmolytic effects of essential oil of Pistacia integerrima Stewart ex Brandis Galls. J Ethnopharmacol. 2015;168:61-65. CrossRef PubMed
- 19. Noureen F, Khan MR, Shah NA, Khan RA, Naz K, Sattar S. Pistacia chinensis: strong antioxidant and potent testicular toxicity amelioration agent. Asian Pac J Trop Med. 2017;10(4):380-389. CrossRef PubMed
- 20. Uddin G, Ismail, Rauf A, et al. Urease inhibitory profile of extracts and chemical constituents of Pistacia atlantica ssp. cabulica Stocks. Nat Prod Res. 2016;30(12):1411-1416. CrossRef PubMed
- 21. Wu J-H, Tung Y-T, Chien S-C, et al. Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J Agric Food Chem. 2008;56(5):1567-1573. CrossRef PubMed
- 22. Abu-Izneid T, Rauf A, Akram Z, et al. Discovery of new α-glucosides, antiglycation agent, and in silico study of 2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-3-methoxy-4H-chromen-4-one isolated from Pistacia chinensis. Heliyon. 2024;10(5):e27298. CrossRef PubMed
- 23. Rauf A, Akram Z, Naveed M, et al. Studies on the inhibition of ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) by 2-(3, 4-Dihydroxyphenyl)-7, 8-dihydroxy-3-methoxychromen-4-one, a flavonoid from Pistacia chinensis. Chemistry (Basel). 2023;5(4):2094-2103. CrossRef
- 24. Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med. 2012;12(1):59. CrossRef PubMed
- 25. Law P-Y, Loh HH. Regulation of opioid receptor activities. J Pharmacol Exp Ther. 1999;289(2):607-624. PubMed
- 26. Sadiq A, Mahnashi MH, Alyami BA, Alqahtani YS, Alqarni AO, Rashid U. Tailoring the substitution pattern of pyrrolidine-2,5-dione for discovery of new structural template for dual COX/LOX inhibition. Bioorg Chem. 2021;112:104969. CrossRef PubMed
- 27. Muhammad N, Saeed M, Khan H, Haq I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J Nat Med. 2013;67(1):1-8. CrossRef PubMed
- 28. Javed MA, Ashraf N, Saeed Jan M, et al. Structural modification, in vitro, in vivo, ex vivo, and in silico exploration of pyrimidine and pyrrolidine cores for targeting enzymes associated with neuroinflammation and cholinergic deficit in Alzheimer’s disease. ACS Chem Neurosci. 2021;12(21):4123-4143. CrossRef PubMed
- 29. Mahnashi MH, Alyami BA, Alqahtani YS, et al. Phytochemical profiling of bioactive compounds, anti-inflammatory and analgesic potentials of Habenaria digitata Lindl.: molecular docking based synergistic effect of the identified compounds. J Ethnopharmacol. 2021 Jun 12;273:113976. CrossRef PubMed
- 30. Ullah Q, Ali Z, Rashid U, et al. Involvement of the opioidergic mechanism in the analgesic potential of a novel indazolone derivative: efficacy in the management of pain, neuropathy, and inflammation using in vivo and in silico approaches. ACS Omega. 2023;8(25):22809-22819. CrossRef PubMed
- 31. Jan MS, Ahmad S, Hussain F, et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2,5-dione derivatives as multitarget anti-inflammatory agents. Eur J Med Chem. 2020;186:111863. CrossRef PubMed
- 32. Sattar S, Khan MR, Shah NA, Noureen F, Naz K. Nephroprotective potential of Pistacia chinensis bark extract against induced toxicity in rats. Nus Biosci. 2016;8(2):192-200. CrossRef
- 33. Muhammad N. In-vivo models for management of pain. Pharmacol Pharm. 2014;5(1):92-96. CrossRef