 |
Drug Target Insights 2024; 18: 30-46 ISSN 1177-3928 | DOI: 10.33393/dti.2024.2707 REVIEW |
 |
Molecular targets and therapeutic potential of baicalein: a review
ABSTRACT
Aim: Researchers using herbs and natural products to find new drugs often prefer flavonoids because of their potential as antioxidants and anti-inflammatories. The planned review addressed baicalein research findings in detail. This manuscript provides a complete review of baicalein’s potential pharmacological effects along with several molecular targets for better understanding of its therapeutic activities.
Materials and methods: We targeted the review on in vitro and in vivo studies reported on baicalein. For this, the literature is gathered from the database available on search engines like PubMed, ScienceDirect, Scopus, and Google Scholar up to 21 December 2023. The keywords “Scutellaria baicalensis”, “Oroxylum indicum”, “Neuroprotective”, “Cardioprotective”, “Toxicity studies”, and “Baicalein” were used to fetch the content.
Results: Baicalein’s molecular receptor binding approach has shown anticancer, antidiabetic, antimicrobial, antiaging, neuroprotective, cardioprotective, respiratory protective, gastroprotective, hepatic protective, and renal protective effects. The synergistic effects of this drug with other selective herbs are also contributed towards significant therapeutic potential.
Conclusion: This systematic review article from a contemporary and scientific perspective offers fresh insight into S. baicalensis, O. indicum, and its bioactive component baicalein as a potential complementary medicine. Baicalein may be transformed into more efficacious and acceptable evidence-based medicine. However, we recommend more clinical and mechanistic approaches to confirm safety and efficacy of baicalein.
Keywords: Alzheimer’s, Baicalein, Cancer, Epilepsy, Heart failure, Hypertension, Parkinson, Stroke
Received: November 6, 2023
Accepted: January 11, 2024
Published online: June 6, 2024
Corresponding author:
Rohit Sharma
email: rohitsharma@bhu.ac.in
Hitesh Chopra
email: chopraontheride@gmail.com
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Baicalein, a flavone compound, is widely recognized in Chinese traditional medicine as Huang-qin or Chinese skullcap (1). Recent research has revealed the therapeutic significance and pharmacological findings of roots of Scutellaria baicalensis and Oroxylum indicum. S. baicalensis Georgi, a member of the Lamiaceae family, stands as one of the pharmacologically significant species of flowering plant. The herbaceous perennial under investigation exhibits distinct characteristics that contribute to its overall appeal. Its papery leaves, thick roots, and branching stems provide a sturdy foundation for its growth and development. Additionally, the vibrant purple-red to blue blooms add a touch of elegance and beauty to its appearance. The presence of black-brown ovoid nutlets further enhance its appearance. Traditional Chinese medicine has a rich history of utilization and continues to be widely practiced. The cultivation of this particular plant species has been observed in various regions, including eastern Russia, Japan, China, Siberia, Mongolia, and Korea. In addition to the well-known Chinese medicinal ingredients, various Scutellaria species including S. viscidula, S. rehderiana, S. amoena, S. likiangensis, and S. hypericifolia have also been utilized as Huang-qin in different regions (2). There are several ways available for extracting baicalein, as shown in Table 1. The auxiliary extraction methodology achieved the maximum yield while extracting from the roots of Scutellaria.
O. indicum, commonly referred to as the Indian trumpet flower, has received significant attention in the field of research. The plant in issue belongs to the Bignoniaceae family, which is well-known for its therapeutic qualities. The subject of investigation is a compact to moderate-sized deciduous tree, characterized by presence of light grayish brown bark adorned with cory lenticels. The vibrant reddish-purple blooms grow on its outer surface, while its inner core exhibited the presence of pinkish yellow flowers. Additionally, the tree bears distinctive woody, winged, large, and flat fruits, which add to its visual allure. Furthermore, this remarkable tree exhibits presence of pinnately compound evergreen leaves, enhancing its overall appeal. The geographical distribution of this particular phenomenon primarily encompasses the countries of India, China, Thailand, Sri Lanka, Cambodia, Bangladesh, and other nations within the South Asian region.
Baicalin, also known as baicalein-7-O-β-D-glucuronic acid, undergoes hydroxylation to yield a biologically active aglycone called baicalein (CID 64982), which is chemically known as 5,6,7-trihydroxyflavone (3). Baicalein, a naturally occurring flavonoid, exhibits a chemical structure derived from the fundamental framework of 2-phenyl chromen-4-one (also known as 2-phenyl-1-benzopyran-4-one). One intriguing aspect of bodily metabolism is the ability for certain substances to undergo a transformative process, allowing them to transition into different forms. Baicalin, a naturally occurring compound, exhibits a unique molecular structure, which contributes to the diverse biological activities and potential therapeutic applications. Understanding the structural features of baicalin is crucial for elucidating its mechanisms of action and exploring its potential in various fields of research (1,4).
This molecule demonstrates a wide range of biological actions, including significant domains such as reducing the risk of cancer, virus suppression, diabetic control, and mitigation of age-related deterioration. Additionally, it demonstrates notable protective effects on the neurological, cardiovascular, gastrointestinal (GI), hepatic, respiratory, and renal systems (5). This comprehensive review aims to consolidate a wealth of information on the diverse pharmacological effects associated with various diseases, encompassing multiple pathways, mechanisms of action, and in vitro studies (5). This review further highlight the attractive opportunities for baicalein-associated drug discovery and research across a range of therapeutic areas.
Chemistry of baicalein
Flavonoids, a class of polyphenolic compounds, have garnered significant attention in the realm of natural products due to their extensive research and exploration (11). One of the key structural components of this compound was produced using the acetate route, leading to the development of the phenolic ring chromophore (ring A) within its 15-carbon skeletal framework (11). One of the best examples of the most fundamental flavone molecule is chrysin, which has the unique property of retaining the necessary meta-substituted hydroxyl groups on the A-ring. The OH substitution at chrysin’s C-6 position causes it to change into the trihydroxy derivative baicalein (12) having chemical formula C15H10O5. More specifically, it is recognized as 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one, as Figure 1 illustrates. Using a multistep synthetic strategy, researchers have effectively recreated the biosynthetic transition of chrysin to baicalein in recent laboratory tests; 1-(2,4,6-trihydroxyphenyl)ethanone and 1-ethyl-3-phenyl-1,3-propanedione are combined in the first synthesis technique. Chen et al completed a pioneering investigation that revealed an amazing and novel method for extracting baicalein from 3,4,5-trimethoxyphenol. This approach, which consists of a brief fourstep procedure, showed promise for a more streamlined and effective manufacture of baicalein (13,14). One of the key structural characteristics that distinguishes baicalin and baicalein is the notable existence of a di-ortho OH on ring A. The remarkable characteristic exhibited by polyphenolic compounds lies in their ability to serve as efficient markers for metal chelation, free radical scavenging properties, and enzyme inhibition. The structural features of baicalein are believed to be responsible for its reported antioxidant effects and its ability to chelate divalent metal ions (14).
| S. no. | Species | Part of the plant | Extraction method | Analytical technique | Yield | Reference |
|---|---|---|---|---|---|---|
| 1. | Oroxylum indicum | Stem barks | Solvent (ethanol) extraction (Reflux method) | HPTLC | 26.498 mg/g | (6) |
| Solvent (acetone) extraction (Reflux method) | HPTLC | 8.631 mg/g | ||||
| DMSO extract (Reflux method) | HPTLC | 13.883 mg/g | ||||
| DMF extract (Reflux method) | HPTLC | 20.529 mg/g | ||||
| Seeds | Solvent (95% ethanol) extraction (maceration method) | HPLC | 0.72% ± 0.00% w/w | (7) | ||
| 2. | Scutellaria baicalensis Georgi | Roots | Solvent (methanol) extraction (maceration method) | HPLC | 0.5 mg/mL | (8) |
| Auxiliary extraction method | HPLC | 116.8 mg/g | (9) | |||
| Supercritical fluid extraction | HPLC-UV | 2.5-80 µg/mL | (10) |
DMF = dimethylformamide; DMSO = dimethyl sulfoxide; HPLC-UV = high-performance liquid chromatography ultraviolet; HPTLC = high-performance thin layer chromatography.
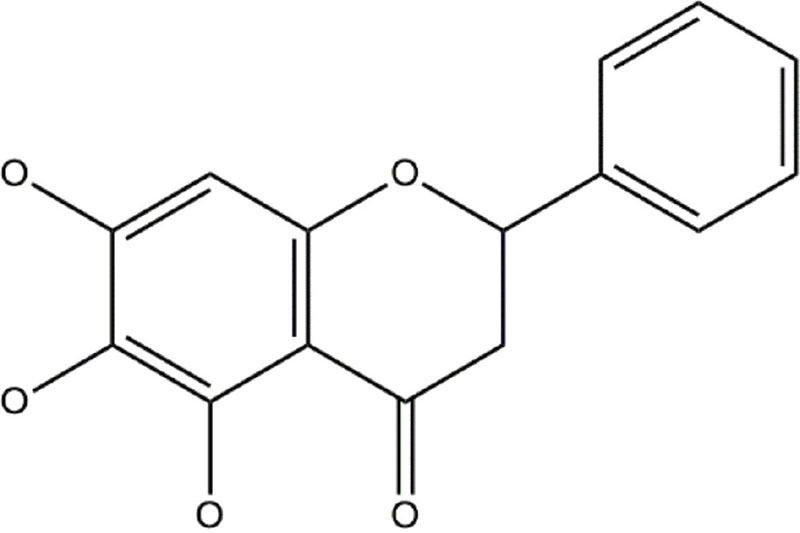
FIGURE 1 - Structure of baicalein.
Anticancer effects of baicalein
Cancer, uncontrolled cell proliferation, can show up phenotypically in a number of ways, from benign to fatal. There are numerous factors that can lead to the development of cancer, including deoxyribonucleic acid (DNA) damage, mutations in the DNA or any by-products of that DNA, and dysfunction of the regulatory and repressor mechanisms during the cell cycle. The four phases of the cell cycle in actively dividing eukaryotes occur in the sequence starting from gap 1, synthesis, gap 2 followed by mitosis. While entering the next phase of the cell cycle, there are a number of checkpoints available en route to ensure that the actions at each phase have been appropriately completed. Phosphorylated retinoblastoma protein (pRB), cyclin subunits, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CDKIs) are the components of these checkpoints (15). Cell cycle arrest occurs when cells are unable to pass cell cycle checkpoints. Cell death and growth inhibition may result from G1 arrest. G2/M arrest is linked to increased apoptosis and may increase the cytotoxicity of chemotherapy (16).
It is estimated that in 2024, around 2,001,140 new cancer cases and 611,720 cancer deaths are projected to occur in the United States. With around 350 cases of lung cancer fatalities every day, lung cancer is the most common cause of cancer-related mortality (17). Baicalein induces G1/S arrest in lung squamous carcinoma (CH27) cells by downregulating CDK4 and cyclin D1, as well as upregulating cyclin E expression, a crucial regulator of the G1/S checkpoint of the cell cycle (18).
Global breast cancer rates are rising, with 364,000 United States cases anticipated by 2040 (19). Baicalein inhibited 17- estradiol-induced transactivation of the estrogen recep-tor in MCF-7 human breast cancer cells in the S and G2/M stages (20).
Eastern Asia currently accounts for more than 60% of cases of gastric cancer. Currently, a major problem in Eastern Asia is how to manage gastric cancer in the older population. It is anticipated that the total number of gastric cancer cases and fatalities would reach its peak soon (21). The gastric cancer cell line SGC-7901 underwent a significant S-phase arrest as a result of baicalein. It resulted in SGC-7901 cells going into apoptosis. Analysis of protein expression levels in SGC-7901 cells showed that when baicalein was administered, Bcl-2 was downregulated and Bax was increased (22).
The third most common reason for cancer-related death worldwide is primary hepatic cancer. Most patients receive a bad prognosis at an advanced stage. In an in vitro investigation using hepatoma carcinoma cells (Hep G2 and J2), baicalein therapy resulted in S-phase arrest. Some of the underlying mechanisms include early-stage DNA damage, the inhibition of growth-stimulating substances, and the activation of cyclin- CDKIs (23).
The seventh most common cause of cancer-related fatalities worldwide is pancreatic cancer. However, many developed nations suffer from it more severely. Globally, there were 458,918 new cases of pancreatic cancer recorded in 2018, and 355,317 additional instances are expected to be reported before 2040 (24). Baicalein upregulates Bax while downregulating Bcl-2 and Mcl-1 to encourage apoptosis in pancreatic cancer cells (25). In PANC-1 cells, baicalein induced arrest of G0/G1 phase when studied in vitro (26).
Rising death rates from colorectal cancer (CRC) are a burden shared by developing Asian nations. Patients with CRC may be at danger if they are underweight or overweight (27). In HCT-116 CRC cells, baicalein causes a significant arrest of S phase and promotes apoptosis by activating caspase 3 and caspase 9 (28).
The most common form of male cancer in developed nations is prostate cancer (PC). In PC cells, baicalein caused G0/G1 arrest by lower down cyclins (D1 and D3) and pRB (29).
Bladder cancer (BC) ranks in the top 10 most common cancers globally. Baicalein caused G2/M arrest in TSGH8301 and BFTC905 BC cells by altering two essential proteins (cyclin B1 and phospho-Cdc2) required to initiate the final stage of cell cycle (30,31).
Ovarian cancer is the seventh most prevalent malignant tumor in women, gravely endangering the reproductive health of women. It has a poor prognosis, unknown pathophysiology, missed diagnoses, and a high recurrence rate (38). It only affects women and has a fatality rate of 46.2% after 5 years, making it unique to the female population (38). Baicalein inhibits the synthesis of vascular endothelial growth factor (VEGF), HIF-1, c-Myc, and nuclear factor kappa B (NF-κB) in the G1 and S phases of ovarian cancer cell lines (OVCAR-3 and CP-70). It inhibited the growth of ovarian cancer cells by lowering the expression of matrix metalloproteinase (MMP)-2 (32-35).
Osteosarcoma (OS) is the most prevalent primary bone cancer in children and adolescents. In its advanced stages, it has a low survival rate (36,37). Baicalein produced intracellular reactive oxygen species (ROS) and activated BNIP3 to slow down the development and hasten the apoptosis of MG-63,OS cells (38).
By reducing the levels of cyclin D1 and CDK4, which inhibits the advancement of the G1 phase, it slowed the growth of OS cells. By decreasing the levels of cyclin D1 and CDK4, it inhibited the growth of OS cells by inhibiting cell cycle progression at the G1 phase of division (Tab. 2) (39).
Baicalin for neuroprotection and cognitive enhancement
Alzheimer’s disease is responsible for the more than 25 million cases of dementia that exist today. Both in industrialized and developing nations, Alzheimer’s disease has a substantial impact on those who are affected, the caregivers, and society (40). One of the cellular disorders hypothesized to be responsible for the loss of neurons in Alzheimer’s disease is aggregation of A𝛽 (41). Baicalein exhibits neuroprotective qualities against amyloid (AN) functions by preventing AN from aggregating in PC12 neuronal cells to cause A𝛽-induced cytotoxicity (42). By activating GABA receptors, baicalein encourages non-amyloidogenic processing of APP, which lowers the generation of A𝛽 and enhances cognitive function (43). Baicalein demonstrated a potent inhibitory effect on A𝛽-induced cell death (43,44).
The prevalence of Parkinson’s disease looks to be rising with age, and it is a global concern. Males are affected by the disease 1.5-2 times more frequently than females worldwide. The prevalence of Parkinson’s disease appears to be higher in Western nations than in Asian nations (45). Amyloid-specific proteins aggregate to cause Parkinson’s disease. In 𝛽-sheet fibrillar aggregate, formed by several proteins including α-syn amyloid proteins are especially prevalent. The main constituent of Lewy bodies is abnormally folded aggregates of α-syn, which manifest as intracellular inclusions in nigral dopaminergic neurons in the brains of Parkinson’s disease patients (46). As a result, α-syn represents a viable therapeutic target for Parkinson’s disease treatment to stop the disease growth and spread (47). Baicalein functions by impeding the aggregation of disease-specific α-syn protein. Both baicalein and its oxidized counterpart prevent the production of α-syn fibrils at lower micromolar levels. Moreover, baicalein demonstrated the capacity to disintegrate the preexisting α-syn fibrils (48). Additionally, by eliminating iNOS protein, mRNA, and promoter activity in endotoxin/cytokine-induced microglia, baicalein effectively reduced NO generation and iNOS gene expression (49).
Stroke ranks second globally in terms of mortality and third globally in terms of disability, with ischemic heart disease coming in first in both categories (50). According to the Indian Global Burden of Disease Study, which was conducted between 1990 and 2019, stroke was India’s leading cause of both fatalities from neurological illnesses and disability-adjusted life years (DALYs) (51). In ischemic stroke, neuroprotection is achieved by inhibiting iNOS activity or by iNOS gene deletion. Neuroexcitotoxic and ischemic traumas cause the production of COX-2. Specific COX-2 inhibitors reduce brain damage brought on by localized ischemia. The optimal method for treating cerebral ischemia-reperfusion (I/R) injury involves targeting the NF-κB pathway, according to numerous experts. Baicalein therapy significantly decreased the expression of COX-2 and iNOS, as well as PGE2 and NF-κB, indicating a protective effect against cerebral I/R injury. Because baicalein blocks inflammatory mediators including LOX-1, COX-2, PGE2, and NF-κB, it may have anti-inflammatory and antioxidant effects that contribute to its capacity to prevent cerebral I/R (52). Baicalein therapy markedly elevated nuclear Nrf2 expression and AMPK phosphorylation in the ischemic cerebral cortex (53). Cell damage and a rise in oxidative stress are caused by an excess of ROS or by the malfunctioning of antioxidant enzymes. Basic leucine zipper transcription factor Nrf2 controls the expression of the gene that detoxifies ROS as well as the antioxidant gene (54,55).
| Plant compound | Name of cancers | Cell lines | Cell cycle phase arrest | Mechanism | References |
|---|---|---|---|---|---|
 |
Lung cancer | CH27 | G1/S | ↑sed cyclin E and ↓ sed levels of CDK4 and cyclin D1 | (18) |
| Breast cancer | MCF-7 | G2/S | Blocked 17-estradiol-induced transactivation of the estrogen receptor | (20) | |
| Gastric cancer | SGC-7901 | S | ↓sed Bcl-2 and ↑sed Bax | (22) | |
| Hepatic cancer | Hep G2 and Hep J2 | S | Activation of CDK inhibitors like p21 or p27 | (23) | |
| Pancreatic cancer | PANC-1 | G0/G1 | ↑sed Bax and ↓sed Bcl-2 and Mcl-1 | (26) | |
| Colorectal cancer | HCT-116 | S | Activation of Caspase 3 and 9 | (28) | |
| Prostate cancer | LNCaP | G1/S | ↓sed cyclin D1, cyclin D3, and pRB protein | (29) | |
| Bladder cancer | TSGH831 and BFTC905 | G2/M | Altering cyclin B1 and phospho-Cdc2 | (31) | |
| Ovarian cancer | OVCAR-3 and CP-70 | G1/S | ↓sed VEGF, HIF-1, c-Myc, NF-kB, and MMP-2 | (34) | |
| Osteosarcoma | MG-63 | G1 | Activating BNIP3 and ↓sed cyclin D1 and CDK4 | (39) |
Epilepsy is a chronic, noncommunicable brain illness that affects people of all ages. One of the most prevalent neurological conditions, epilepsy affects about 50 million people worldwide. More than 80% of people in low- and middle-income countries have epilepsy. FeCl3-induced post-traumatic epileptic seizures are a significant type of acquired epileptic seizures (56). Baicalein suppressed ferroptosis associated with 12/15-LOX, hence lessening the severity of post-traumatic epileptic episodes generated by FeCl3 (57). HT22 cells were damaged by ferroptosis, which is mitigated by baicalein may be due to its lipid peroxidation inhibitor (Fig. 2) (58).
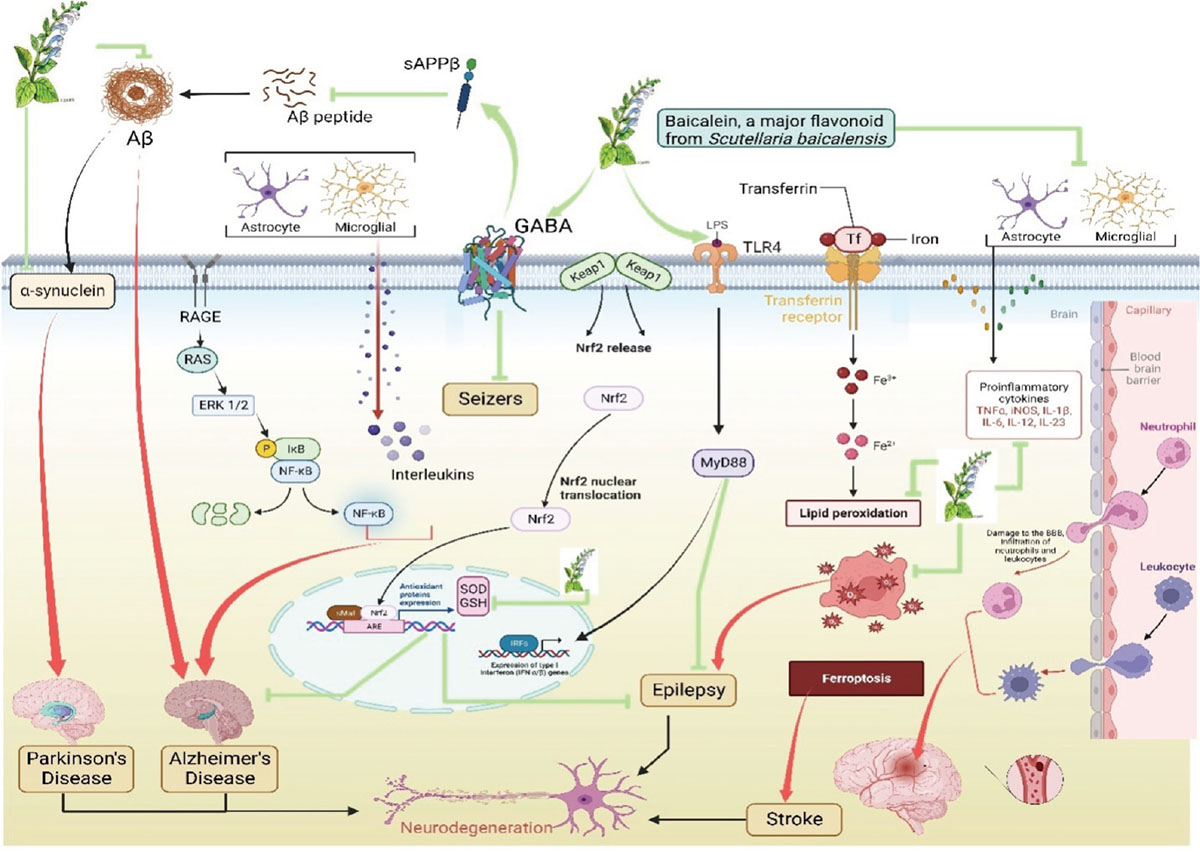
FIGURE 2 - Baicalein regulating a wide range of signaling pathways linked to the etiology of multiple degenerative conditions.
Respiratory protective action of baicalein
Chronic respiratory conditions are among the world’s leading causes of morbidity and mortality. They affect the lung’s airways and other structural components. Asthma is the most prevalent chronic respiratory illnesses. There are already approximately 300 million asthmatics worldwide, and by 2025, that figure may rise by an additional 100 million. Asthma is a long-term inflammatory illness of the airways characterized by thickening of the airway wall, hyper-responsiveness and remodeling of the airways, poor relaxation, and persistent blockage of airflow by the smooth muscle of the airways (59-61). Hypersecretion of airway mucus is one of the key features of asthma pathophysiology. Baicalein therapy showed dramatical decrease in MUC5AC and MUC5B mRNA expression levels in the submucosal glands and the epithelium, respectively, which were significantly greater in asthma. Baicalein also exhibits anti-asthmatic properties by inhibiting tumor necrosis factor (TNF)-α-induced NF-κB activation in normal bronchial epithelial (BEAS-2B) cells (62), which was mostly expressed by downstream iNOS production, IκBα phosphorylation and degradation, and nuclear translocation of p65 (63).
Another structural change associated with asthma has been described as increased extracellular matrix (ECM) deposition. The primary ECM producers in the lung are myofibroblasts and fibroblasts. During allergic airway inflammation, myofibroblasts deposit collagen types I and III. ECM’s constituents, especially collagens, are broken down and controlled by MMPs, which are secreted by fibroblasts. In allergic asthma, MMP-9, the main airway MMP, is upregulated, which results in airway remodeling (64). According to recent studies, baicalein administration reduces the ovalbumin (OVA)-induced expression of collagen I and MMP-9 (65).
Cardioprotective action of baicalein
Globally, cardiovascular disease (CVD) is one of the major causes of morbidity and motility. In past few decades, changes in lifestyle and socioeconomic conditions that have an impact on the progression of CVDs have been observed. Hypertension is a significant contributor to CVD and fatalities globally, particularly in low- and middle-income nations. As per the 2017 guidelines from the American College of Cardiology and American Heart Association, hypertension is defined as a systolic blood pressure over 130 mm Hg or a diastolic blood pressure above 80 mm Hg (66-70). Baicalin assisted in lowering high-sensitivity C-reactive protein (CRP), interleukin (IL)-6, and IL-1 levels in the serum, which served to forcibly lower blood pressure (71). Baicalin altered the expression of miR-145 and increased TNF-α levels in human aortic endothelial cells (HAECs), thereby reducing the inflammatory effects of TNF-α (72).
Angiotensin II (Ang II) may activate apoptosis-related proteins, encourage the production of ROS, and inhibit the synthesis of NO, which results in endothelial dysfunction that may lead to hypertension. Therefore, lowering cell apoptosis, suppressing oxidative stress, and preventing Ang II-induced endothelial dysfunction are effective ways to treat hypertension. Baicalin has been shown in a study utilizing the human umbilical vein endothelial cell (HUVEC) model of Ang II injury to significantly reduce oxidative stress and endothelial dysfunction caused by Ang II. The primary strategies employed to produce these beneficial effects include modulating the expression of Bax, Bcl-2, and cleaved caspase-3, activating the ACE2/Ang-(1-7)/mas axis, and upregulating the PI3K/AKT/eNOS pathway. They also found that baicalin raised NO level and total antioxidant capacity, and decreased markers of oxidative stress such as malondialdehyde (MDA) and ROS (73,74). It was also shown that the combination of baicalin and berberine relaxed blood arteries by acting on the voltage-dependent Ca2+ channel (VDCC) (75). By boosting endogenous NO synthesis, which is produced by the enzyme endothelial nitric oxide synthase (eNOS), baicalin may also lower blood pressure (76).
Heart failure is a complicated clinical condition that arises when the heart is unable to pump enough blood to meet the body’s needs. It arises from any ailment that impacts the ventricles’ ability to fill or the blood’s ability to be ejected into the systemic circulation (77). Baicalin seems to be very useful in treatment of CHF as it has an anti-fibrosis property. Baicalin may be able to treat myocardial fibrosis by inhibiting the expression of the fibrosis genes (type I and III collagen) and connective tissue growth factor (CTGF). Baicalin can efficiently induce endothelial cell migration and greatly boost the expression of VEGF when the concentration is between 10 and 50 g/mL, which aids in promoting angiogenesis. This outcome can be achieved by over-activating the ERR/PGC-1a pathway (78). Baicalin also decreased caspase-3 and the Bax/Bcl-2 ratio, which lowered apoptosis. This suggests that apoptosis inhibition could lessen adverse remodeling and the eventual development of heart failure (79).
Gastroprotective activity of baicalein
Peptic ulcer disease (PUD), as determined by hospitalization statistics and diagnosed by physicians, had an incidence rate of 0.03%-0.17% and 0.10%-0.19% per year, respectively. Although most research indicated that PUD incidence or prevalence had decreased over time, still it is a serious and life-threatening disease (80). Acetylsalicylic acid (ASA), Helicobacter pylori infection, and non-steroidal anti-inflammatory drugs (NSAIDs) are the major causes of PUD. While there has been a significant improvement in H. pylori infection therapy recently, ASA and NSAID prescriptions have grown within the same time frame (81). According to reports, inhibiting gastric acid output and exhibiting a reduction in gastric mucosal damage are both effects of activating α2-adrenergic receptors (82). The gastroprotective properties of baicalein may be mediated through these receptors. In contrast to the formation of free radicals, glutathione (GSH) protects the stomach mucosa from damage (82). Baicalein was found to exert protective effects via raising the tissue’s GSH concentration, signaling to an antioxidant mechanism (83). Baicalein exhibited cytoprotective effects by inducing the secretion of more gastric mucus, acting as an anti-secretory by suppressing hydrogen-potassium-ATPase activity, and acting as an antioxidant by raising GSH levels (83). By raising the SH compound, which naturally regulates mucus production, and the NO level, which is involved in maintenance of the integrity of the gastric mucosa, and regulates the secretions like acid, alkaline, mucus, and blood flow in gastric mucosa, baicalein demonstrated a gastroprotective effect against lesions (83). The stomach mucosa showed significant reductions in MDA, IL-8, and TNF-α contents, as well as increases in superoxide dismutase (SOD) activity and glutathione peroxidase (GPx) level due to inhibition of inflammation and ROS scavenging actions of the baicalein-Zn complex (84).
Hepatoprotective activity of baicalein
Worldwide, the prevalence and incidence of nonalcoholic fatty liver disease (NAFLD), the main cause of liver-related morbidity and mortality, have increased as a result of the obesity epidemic (85). Men and aging both increase the risk of NAFLD and fibrosis. Despite the fact that obesity is a major risk factor for NAFLD, current studies have revealed that slim individuals can also develop the condition (86). NAFLD is defined as the condition in which 5% or more hepatic cells develop macro-vesicular steatosis, when there is no secondary etiology like alcohol or drugs (86). An excessive buildup of lipids in the liver results from the increased hepatic lipogenesis and serum non-esterified fatty acids, which causes fatty liver, decreased liver function, and ultimately liver failure (87). Baicalein has several effects against FLD, suggesting that it may be used therapeutically. In order to improve lipid metabolism and reduce hepatic de novo lipogenesis, baicalein inhibits the Ca2+/CaM-dependent protein kinase/AMP-activated protein kinase/acetyl-CoA carboxylase (CaMKK/AMPK/ACC) pathway (88). Baicalein also has the capacity to reduce liver fibrosis, oxidative stress, and systemic inflammation in FLD. In order to reverse fibrosis, baicalein consequently inhibits the synthesis of collagen (type I and α-1) chain and transforming growth factor (TGF)-β1 (88,89). Additionally, it inhibits the TGF-1/Smad3 pathway in vitro to reverse the epithelial-mesenchymal transition and stop the progression of liver fibrosis (90). Baicalein also has an indirect antioxidant effect by decreasing MDA levels while increasing hepatic GSH and SOD (91). By inhibiting the TGF-β/Smad pathway, baicalein slowed down the epithelial-mesenchymal transition. Additionally, it decreased high levels of inflammatory factors such TNF-α, IL-1 and -6, inhibited the apoptotic proteins caspase-3 and -9, and B-cell lymphoma-2, and hindered the IκB kinase/IκB/NF-κB pathway. These all helped to lessen liver disorders (92). Because of its inhibitory influence on the production of p-p38, MAPK, p-CREB, FoxO1, PGC-1, PEPCK, and G6Pase, baicalein considerably reduces insulin concentrations brought on by a high-fat meal. Thus, because of its numerous pharmacological actions, baicalein may prevent FLD (93).
Baicalein action as a renal protective agent
Chronic kidney disease (CKD) prevalence is acknowledged as a major public health concern on a global scale. It is estimated that 13.4% (11.7%-15.1%) of the global population has KD and that 4.902-7.083 million have end-stage kidney disease (ESKD), requiring renal replacement therapy (94).
The incapacity of the kidneys to perform their excretory duties, which results in the retention of nitrogenous waste products in the blood, is known as renal failure. Kidney failure comes in two flavors: acute and chronic (95). One of the main causes of renal failure is diabetes mellitus (DM), which is why many diabetic individuals continue to struggle with diabetic nephropathy (DN), which is a serious condition (96). Patients with DN typically have elevated urine microalbumin, urine β2-MG, and Urinary albumin excretion rate in Diabetic neuropathy. Among these, an increase in urine microalbumin and urinary 2-MB indicates renal tubular injury, while an increase in UAER indicates glomerular injury (97). It was discovered that baicalin significantly reduced the patient’s urine 2-MG, UAER, and microalbumin levels. Additionally, it was discovered to considerably enhance the patient’s quality of life, reduce the rate at which DN progresses, and enhance kidney function (98-100).
Acute renal injury is a serious adverse effect of the anticancer drug cisplatin. Increased ROS generation from cisplatin damages endogenous intracellular targets like proteins, lipids, and DNA, resulting in cellular malfunction and death (101). Additionally, the toxic effects of cisplatin are made worse by the activation of various signaling pathways, such as MAPKs, NF-κB, and p53, by cisplatin-induced ROS (114). A key factor in the pathophysiology of cisplatin-induced kidney injury is the production of iNOS and strong cytotoxic peroxynitrite through the interaction of superoxide radical and NO (102). Baicalein has been demonstrated to have a significant antioxidant impact. Baicalein has been shown to adequately remove peroxynitrite anion radicals and inhibit peroxynitrite-induced cell death in LLC-PK1 cells. Pretreatment with baicalein significantly decreased these changes. Therefore, baicalein’s capacity to lessen cisplatin-induced nephrotoxicity is probably due, at least in part, to the attenuation of renal oxidative and/or nitrative stress (Fig. 3) (102).
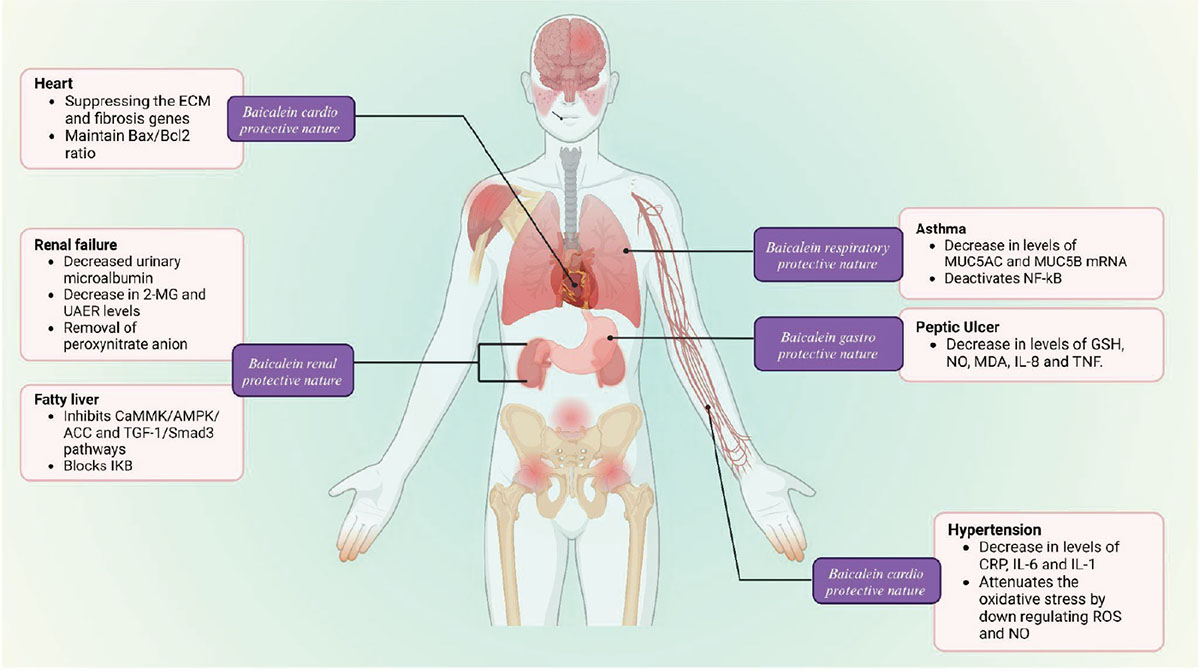
FIGURE 3 - The healing potential of baicalein by modulating several signaling pathways mitigating the symptoms of certain illnesses such as cardiac, renal, respiratory, and gastric.
Baicalein as an antimicrobial agent
Across the world, infectious diseases are a leading source of morbidity and mortality (103). The emergence of drug-resistant forms of bacteria has diminished the impact of antibiotics on germs and resulted in ongoing difficulties, despite notable advancements in the treatment of microbial infections (104). Combination therapy was developed in response to this problem, and as a result of the expanding prevalence of antibiotic resistance, it has improved treatment efficacy and partially reduced drug resistance. Furthermore, the bacterial resistance to antibiotics has motivated the creation of innovative antibacterial drugs for the treatment of infectious disorders. People from many cultures have used a variety of herbal remedies for many generations, and some of these natural medicines are crucial for the treatment and prevention of infectious diseases (105,106). Baicalein’s function as an antibacterial agent is crucial. Baicalin inhibits pro-inflammatory cytokine release, MAPK activation, NF-κB pathway activation, and NLRP3 to diminish inflammation in periodontal cells. By promoting the expression of interferon regulatory factor (IRF)4 and inhibiting the expression of IRF5, baicalin also lessens inflammation by regulating the transition of M1 to M2 macrophages. Baicalin inhibited NF-κB and p38 phosphorylation as well as mRNA expression, which decreases pro-inflammatory cytokines TNF-α, IL-β, and IL-6 levels (107).
Bacterial growth occurs naturally as biofilms, which are abundant in the environment (108). Biofilm production leads to an increase in resistance to antibiotics and antimicrobial agents. Quorum sensing (QS), a mechanism responsible for the intercellular exchanges of information, is essential to the production of biofilms (109). Baicalin suppresses the QS system, which inhibits inflammatory responses. Baicalein has been shown to have antimicrobial properties against Meningococcus, Staphylococcus aureus, Corynebacterium diphtheriae, Pseudomonas aeruginosa, and dysentery bacillus. Through a number of mechanisms, such as enhancing the host immune system, preventing the formation of biofilms, lowering antimicrobial medicine resistance, suppressing bacterial secretions, and upsetting microbial morphology, baicalin acts as a herbal antimicrobial agent against several microbial infections (110).
Baicalin has also been shown to suppress apoptosis, decrease FAS protein expression, block the caspase-8 pathway, and decrease Bax protein production. These actions are all thought to contribute to its antiviral efficacy (111). Baicalin lowers the raised levels of hepatic markers alanine transaminase (ALT), aspartate aminotransferase (AST), and total bilirubin along with hepatitis B virus (HBV)-DNA in patients with chronic hepatitis B. It can also be used in conjunction with other medications to treat viral infections in patients with an H1N1 influenza infection. Baicalin regulates the numbers of T-lymphocyte subsets and upregulates CD3+, CD4+/CD8+, and other positive lymphocyte subsets (112).
Invasive fungal infections increase morbidity and death of patients who have impaired immune systems (113). Currently, polyenes, echinocandins, and azoles are the three primary classes of frontline clinical antifungal agents used to treat such infections (114). However, each of these groups has drawbacks that may restrict their clinical use for the management of invasive fungal infections, and it is urgently necessary to identify new antifungal medicines that target novel targets. Baicalein has demonstrated potential antifungal activity. It significantly inhibits the growth of Aspergillus fumigatus, Cryptococcus neoformans, and Candida species. Because it targets Eno1, BE (baicalein) has anti-C. albicans action via preventing glycolysis. BE (baicalein) has a substantial inhibitory effect on CaEno1’s enolase activity (115). Baicalein inhibits TSLP/TSLPR pathway activity, which suppresses inflammatory response and protects against A. fumigatus keratitis by preventing fungal growth, biofilm formation, and adhesion (Tab. 3) (116,117).
Antidiabetic property of baicalein
DM is the most prevalent endocrine disorder in the 21st century, and is spreading all over the world. Diabetes increases blood sugar levels. Neglecting to manage it consistently and carefully increases the risk of significant adverse effects, including stroke and heart failure. Type 1, type 2, and gestational diabetes are the three most prevalent forms of the disease. By preventing islet cell death, baicalein reduces hyperglycemia, improves glucose intolerance, and encourages insulin production (118). Baicalein is used to treat diabetes, which lowers total cholesterol, plasma triglyceride plasma levels along with sugar lowering effect (119).
Baicalein additionally showed antihyperglycemic effects in both in vivo and in vitro models via blocking the α-glucosidase action in 3T3-L1 adipocyte cell line. The main function of the enzyme α-glucosidase is to catalyze the breakdown of starches and carbs in food to produce glucose that may be absorbed through the digestive tract (120). As a result, delaying the synthesis of glucose after meal digestion may minimize hyperglycemia. It was discovered that baicalein had α-glucosidase inhibitory effects comparable to those of the commonly prescribed, clinically effective α-glucosidase inhibitor, acarbose (120). The synergistic impact of baicalein and acarbose treatment is reported for showing reduced harmful hepatic side effects associated with using high dosages of the acarbose alone to treat diabetes by lowering the dosage from high (20 mg/kg) to low (4 mg/kg) levels (121).
Glycation of bovine serum albumin (BSA) is a complicated chain of events wherein reducing sugars like glucose alter the structure of the protein. Due to the excess glucose molecules in their serum, diabetics usually have greater glycation rates. This can lead to nonfunctional proteins and further activate several signaling pathways, increasing the risk of beta-cell damage, insulin resistance, and diabetes complications. Baicalein also has the potential to cure hyperglycemia by reducing BSA glycation (122).
Baicalein action as an antiaging agent
Skin aging is a natural process that is defined by the skin’s progressive physiologic and anatomical changes. The dermis experiences striking alterations including the huge deposition of irregular elastic fibers, collagen deterioration, and hyaluronic acid loss. Skin aging is caused by a variety of extrinsic (usually linked to aging) and natural (mostly genetically determined) factors, such as exposure to ultraviolet (UV) radiation, high intake of alcohol, and pollution. UV radiation is the main cause of photoaging in human skin (123).
Baicalein may find use in skin care products and as a photoprotective agent. UV radiation significantly raised the activities of MMP-1 mRNA (123), while baicalin treatment inhibited this UV radiation-induced action of MMP-1 and resulted in the formation of procollagen type 1. Rich baicalein inhibited collagen fiber loss, wrinkle formation, and skin thickness brought on by UV radiation.
UV causes high levels of ROS to be produced in the skin tissues. This can cause increase of oxidative stress to a variety of cellular macro and micro molecules, including proteins, nucleic acids, and cell membranes. ROS have a major contribution in both the onset and development of skin aging. MDA is a result of lipid peroxidation whose quantity reveals the extent of oxidative stress on cells. The antioxidant defense of the normal cell against ROS is carried out by SOD and GSH-Px enzymes (125). The results demonstrate that UV rays cause oxidative damage and slow down the dermal tissue capacity to eliminate ROS. It was also revealed that UVA radiation considerably elevated MDA levels in fibroblasts and significantly decreased SOD and GSH-Px activities. Additionally, baicalin therapy by itself dramatically reduced MDA levels in fibroblasts and increased SOD and GSH-Px activity (125).
Pharmacokinetics
Clinical and toxicological studies
It has been shown that baicalein is a unique, potentially effective medicinal agent for treating a range of illnesses. Therefore, clinical trials are required to ensure the therapeutic role of baicalein. Several studies are designed by various research groups for the purpose. Two phase I clinical trials were conducted on healthy adults in China to evaluate the safety and efficacy using chewable baicalein pills.
Seventy-two healthy Chinese adults participated in a phase I (2014) random, double-blind investigation to examine the pharmacokinetic (PK) characteristics of baicalein using a single-dose trial (100-2800 mg) (126). Blood, urine, and feces samples were obtained at fixed schedules for up to 48 hours post drug administration. After that, baicalein was investigated by the spectrometric analysis of samples through liquid chromatography with tandem mass spectrometry (LC/MS/MS). The PK profile of baicalein was found to be multiphasic, with a median Tmax of 0.75-3.5 hours and a t1/2 of 1.90-15.01 hours. The proportionality coefficient (90% CI) estimates for Cmax, AUC0-t, and AUC0 were 0.83 (0.70-0.96), 0.91 (0.81-1.00), and 0.92 (0.82-1.02), in that order. The predefined ranges of 0.89-1.11, 0.93-1.07, and 0.93-1.07, respectively, encompass all values. It was unclear what the dose proportionality was for a baicalein dose range of 100-2800 mg. Baicalein excreted in urine makes up 1% of the total. Baicalein was eliminated as a medication in approximately 27% of the feces. Baicalein’s Cmax and Cavg values at 800 mg single dose and 800 mg multiple dose, respectively, were higher than the in vitro effective concentration (0.1 μM) against SARS-CoV-2 (127). Baicalein was highly tolerable. Eleven adverse treatment-related occurrences were detected; all were considered “minor” and retreated on their own. There were no significant adverse occurrences. As a result, healthy volunteers tolerated single oral dosages of baicalein of 100-2800 mg without any adverse effects (127).
The study by Li M, Shi A, Pang H, et al. published in the Journal of Ethnopharmacology in 2014 investigated the safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. The research found that single oral doses of 100-2800 mg of baicalein were safe and well tolerated by healthy individuals, with no serious adverse events occurring. The study concluded that baicalein chewable tablets were generally safe and well-tolerated, supporting the further exploration of baicalein in clinical studies due to its favorable safety profile and pharmacokinetic properties (128). Baicalein suppressed the generation of mid-late mRNA, hence suppressing the H1N1 and H3N2 influenza viruses, A/FM1/1/47, and A/Beijing/32/92, respectively (163). Baicalein and its metabolites were found at higher concentrations, but not in a dose-proportionate way. In healthy Chinese volunteers, baicalein tablets within the studied dosage range were well-tolerated and safe, with no severe or potentially fatal side effects (128).
In order to assess the safety and efficacy of capecitabine (CAP) in conjunction with PHY906, a combination of four traditional Chinese herbs—Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fisch., Ziziphus jujuba Mill., and Paeonia lactiflora Pall.—in the treatment of advanced pancreatic carcinoma (APC), another study was carried out in 2006. Preclinical studies indicate that PHY906 is a potent inhibitor of NF-κB and that it works in concert with CAP to exhibit synergistic antitumor effects in PANC-1 cell lines. PHY906 does not alter the PK of CAP, as studies on several tumor types have shown, but it may reduce the GI toxicities associated with chemotherapy, especially diarrhea. These data have motivated the phase I/II study of the safety and tolerability of a weekly (7/7) dose-intense schedule for CAP plus PHY906. Patients with advanced solid tumors (STs) who did not respond to conventional therapy were recruited for the phase I research. On days 1-4, patients were given 800 mg of PHY906 twice a day, and for 14 days, they were given 1,500 mg/m2 of CAP every day. Patients with gemcitabine-refractory APC will be recruited for the phase II research. The study’s findings show that CAP and PHY906 together have tolerable toxicity in patients with ST (130).
In phase I/II clinical trials oral administration of baicalein has been shown to be safe for humans (130). However, more detailed research on baicalein’s therapeutic potential in patients with various diseases is required.
Nano-formulations of baicalein
Srivastava et al created baicalein-loaded mixed micelles to improve the solubility and bioavailability of baicalein oral preparation to treat breast cancer. Micelles encapsulating baicalein in pluronic F127 (F127) and D-tocopherol polyethylene glycol 1000 succinate (TPGS) were investigated for their anticancer properties. The micelles in the optimal formulation exhibited a zeta potential of 4.01 mV and a mean particle size of 25.04 nm. Baicalein was released from micelles in vitro with a sustained release profile at pH 7.4, and an 83.43% computed entrapment efficiency percentage was obtained. In vitro cell culture studies showed a significant increase in the absorption and cytotoxicity of baicalein formulation and performance evaluation of polymeric in-loaded micelles targeting MDAMB-231 cell lines. The cell cycle analysis findings demonstrated that cells were halted in the G0/G1 phase of the cell cycle and that baicalein micelles had a greater capacity to induce apoptosis than did baicalein in its free form. The results of the ROS and mitochondrial membrane potential experiment demonstrate that the novel formulation suppresses cell growth through the ROS-dependent mitochondrial-mediated apoptotic pathway (131).
Majumdar et al developed baicalein-loaded nanoliposome gel formulation, which was easy to apply to the skin’s surface because it has a high degree of homogeneity, a pH that is about equivalent to that of the skin, and an appropriate thixotropic characteristic. Baicalein’s release was greatly extended and concentration-independent after the development of nanoliposomal gel. In contrast to commercially available formulations, the nanoliposomes loaded with baicalein demonstrated remarkable anti-inflammatory effectiveness throughout testing. Accordingly, the engineered nanoliposomal gel filled with baicalein may be employed as an effective carrier for baicalein topical administration to suppress inflammatory reactions (132).
The efficacy of baicalein-loaded iron oxide nanoparticles (NPs) against the triple-negative breast cancer (TNBC) cell line MDA-MB-231 was examined by Kavithaa et al. Using an electron microscope to analyze a subset of cancer cells, it was revealed that the particles were absorbed by the various subcellular components of the cells. Furthermore, the assessment of mitochondrial membrane potential was conducted using flow cytometry employing JC-1 labeling. The results indicated the presence of significant aggregates in cells treated with iron oxide NPs loaded with baicalein, indicating a considerable reduction in mitochondrial membrane potential. Baicalein-loaded iron oxide NPs elevated apoptotic genes such as Bad, Bax, GADD45, and poly(ADP-ribose) polymerase (PARP) cleavage in a dose-dependent manner while downregulating anti-apoptotic genes. Comprehensive kit-based flow cytometric analysis confirms that nano conjugates may clearly induce apoptosis, DNA damage, and cell cycle arrest, as well as reduce the rate of cell proliferation in TNBC cells (133).
Li et al investigated the releasing patterns and loading efficiencies of baicalein (BE) and baicalin (BA) enclosed in mesoporous silica nanoparticles (MSNs) that were produced and modified with amines (Nano-BA and Nano-BE, respectively). Using primary human gingival epithelial cells (hGECs), the cytotoxicity of Nano-BA and Nano-BE was examined, and a transmission electron microscope was used to observe the cells’ uptake of the compounds. Their anti-inflammatory effects in IL-1-treated hGECs were measured using the cytokine array and the enzyme-linked immunosorbent test. This study shows that amine-modified MSNs are capable of encapsulating BA and BE, and that this nano-encapsulation greatly increases the rate of drug delivery and prolongs the release of BA and BE for up to 216 hours. Furthermore, when exposed to a solution devoid of NPs, hGECs were able to internalize Nano-BA and Nano-BE and maintain them inside the cells for at least 24 hours. It is noteworthy that IL-1-induced expression of IL-6 and IL-8 in hGECs is effectively suppressed by pretreatment with Nano-BE. To summarize, BE encapsulated in a NP may efficiently release its contents and be taken up by cells, which has major anti-inflammatory effects (134).
Researchers created a pH-responsive drug delivery system (DDS) encapsulating baicalein zeolite imidazole framework-8 (ZIF-8). The synthesized nanocomposite exhibited a drug-loading capacity of 40.32%. The in vitro drug release kinetics from the nanocomposite showed excellent stimuli-responsive drug release capabilities in an acidic environment. The growth of E. coli, S. aureus, P. aeruginosa, and Staphylococcus epidermidis was significantly inhibited by the nanocomposite. The synergistic effect of zinc (II) ions and baicalein molecules was demonstrated to be the antibacterial mechanism of the BA@ZIF-8 nanocomposite. According to in vitro biocompatibility tests, BA@ZIF-8 did not cause any cytotoxicity in L929 fibroblast cells. The created nanocomposite dramatically enhanced the number of proliferating and migrating cells in the wounded area, according to tests conducted on a scratch wound. According to the findings of the pH-responsive drug release mechanism, the synthesized nanocomposite exhibited greater stability at a neutral pH. The nanocomposite demonstrated rapid baicalein release (65.8%) in acidic conditions, indicating that it is a great choice for pH-responsive drug delivery (135).
This investigation of the anticancer properties of BSA-baicalein @Zn-Glu nanostructure-mediated GluRs was conducted using human glioblastoma U87 cells. The transport of baicalein active component was studied with hybrid NPs of BSA-Ba@Zn-Glu. BSA-Ba@Zn-Glu NPs were effectively synthesized in a single reduction process. The cytotoxic efficacy and apoptotic rate of the nanostructures on U87 glioblastoma cells were assessed using 3-(4,5-dimethylthiazol-a-yl)-2,5 diphenyltetrazolium bromide (MTT) assays and flow cytometry, respectively. The synthesized BSA-Ba@Zn-Glu nanostructures, with diameters ranging from 142.40 to 177.10 nm and zeta potentials between 10.57 and 35.77 mV, have the potential to extravasate into cancer cells. The drug release of BSA-Ba@Zn NPs was both pH-dependent and pH-controlled. In vitro, it was demonstrated that BSA-Ba@Zn-Glu NPs significantly reduced cell viability and increased apoptosis in U87 cancer cells. A green manufacturing approach was used to create Zn NPs, and it was shown that these particles had a deadly effect in addition to increasing the uptake of NPs by cells via Glu receptors. Glu conjugation and drug delivery of baicalein were achieved through the use of BSA NPs as a nano-platform. BSA-Ba@Zn-Glu NPs can cause dose-dependent cytotoxicity and death in human brain cancer cells (U87). Finally, future research on targeted medication delivery in vivo may make use of this nanostructure. It may also be paired with other treatments, such as x-ray irradiation (Table 3) (136).
Patents on baicalein
The patents related to pharmacological activity of baicalein have been tabulated in Table 4.
Conclusion
This systematic review provides a new scientific and modern perspective on the traditional Chinese medicine baicalein as a possible supplementary therapy. Baicalein contains anticancer, antimicrobial, antidiabetic, and antiaging properties in addition to protective effects on the brain, heart, lungs, stomach, hepatic, and renal systems, based on the published data reviewed. Repeatedly administering baicalein orally, at dosages ranging from 200 to 600 mg, was shown to be safe and well-tolerated by healthy individuals. There was no impairment seen in liver and renal function; however, it may affect the metabolism of triglycerides. Baicalein is rapidly and extensively metabolized in the body, resulting in the production of several metabolites. Out of the seven metabolites, 7-BS and BGG exhibited a significantly larger amount in the plasma. The scientific literature has shown the antiviral activity of baicalein and baicalin (7-BG). There are direct and indirect consequences among them. It is critical to know each mode of action in order to maximize this flavonoid’s efficacy in treating diseases. There are direct and indirect consequences among them. Clinical research on this natural substance’s efficacy, however, did not provide enough information. Consequently, additional evidence-based clinical trials are required to verify the safety and effectiveness of baicalein as a potential therapeutic agent for a range of human illnesses and for the good of humanity.
| S. no. | Disorder | Formulation | Combination | Application | Outcome | Reference |
|---|---|---|---|---|---|---|
| 1. | Diabetes | NLC | No | In vitro and in vivo | B-NLCs possess favorable physical stability and enhanced capability for drug retention along with improved antidiabetic effectiveness | (137) |
| Selenium NPs | Naringenin + baicalin | In vitro and in vivo | Maintain optimal blood glucose levels by enhancing insulin sensitivity, production, and mitigating the dysfunction of pancreatic β-cells in T2DM | (138) | ||
| 2 | Breast cancer | Iron oxide NPs | No | In vitro | At a very low dose, it inhibits antiapoptotic protein and increases apoptotic proteins | (139) |
| Nanoemulsion | Paclitaxel + baicalein | In vitro and in vivo | Improved permeability and retention effects | (140) | ||
| 3. | Human lung cancer cells | Nanoparticles (prodrug) | Paclitaxel + baicalein | In vitro and in vivo | Exhibited synergism and anticancer potential and reduction of multidrug resistance of paclitaxel occurs | (142) |
| 4. | Cervical tumor | Nanoliposomes | No | In vivo | This formulation demonstrated significant antitumor efficacy | (142) |
| 5. | Cerebral ischemia | SLN | OX26 antibody + baicalin | In vivo | It regulates amino acid levels, which are responsible for the therapeutic effect of treating excitotoxic neuronal damage | (143) |
NLC = nanostructured lipid carriers; NP = nanoparticle; SLN = solid lipid nanoparticles; T2DM = type 2 diabetes mellitus.
| S. no. | Patent number | Year of publishing/grant | Details of patent |
|---|---|---|---|
| 1. | CN1411815A | 2003 | The current innovation relates to the preparation method for baicalin eye drops. The root of the natural Chinese medicinal plant Scutellaria is the source of baicalin, the main active element in the product. It exhibits a robust therapeutic effect in the management of pathogen-induced ophthalmopathy without causing ocular discomfort or deleterious side effects. |
| 2. | CN103720650A | 2014 | The innovation is an injection of baicalin that has anti-influenza viral properties. Baicalin, 2.16 g of disodium hydrogen phosphate, 0.6 g of sodium dihydrogen phosphate, and 100 mL of injection water make up this mixture. The injection is sterilized by applying high pressure while keeping the sodium hydroxide pH at 7.0. The medication blocks neuraminidase action, which prevents the virus from releasing its infection. It is highly bioavailable and rarely causes negative side effects. |
| 3. | CN101642426B | 2009 | The innovation is a technique for making nanoscale eye drops of the baicalein adhesion type. Baicalein (w/w) = 0.001%-3%, lipid (w/w) = 0.1%-20%, surfactant (w/w) = 0.1%-10%, preservative (w/w) = 0.001%-3%, isotonic regulator (w/w) = 0.1%-10%, penetration enhancer (w/w) = 0.005%-10%. The remaining volume of deionized water is used to create the baicalin adhesion type nanoscale eye drops, with the pH being kept between 5 and 9. |
| 4. | CN101856350A | 2010 | The use of baicalein in the preparation of medications for the treatment and prevention of PD is disclosed in the invention. The formulation has the ability to improve and treat PD symptoms while also lowering the trembling frequencies and amplitudes. By preventing damage to dopaminergic neurons, the formulation can effectively prevent and treat pathological changes and the development of PD. Baicalein, on the other hand, inhibits Parkinson’s symptoms by acting on nervous systems and protects dopaminergic neurons through apoptosis resistance, inflammatory resistance, and antioxidation. |
| 5. | CN101701245A | 2010 | The innovation offers a way to extract the major proteinase inhibitor of the SARS coronavirus from a traditional Chinese remedy. The steps in the procedure are as follows: (1) Determining the exosomatic suppressive activities of different extractives from a single traditional Chinese medicine that are main proteinase inhibitors of the SARS coronavirus; (2) choosing the extractive that has the highest exosomatic suppressive activity; and (3) sorting and choosing the extractives chosen in step (2) at least once. The substance that the procedure separated has exosomatic inhibitory actions on the major proteinase of the SARS coronavirus, making it a potentially ideal medication or a viable prodrug for commercialization. |
| 6. | CN102068452A | 2011 | The invention relates to a pharmaceutical composition with a synergistic antiviral ratio of 20:1 to 1:10 for baicalein and ribavirin. It lessens the minimum medicine tolerance as well as the adverse effects and side effects. Moreover, the antiviral component works by inhibiting viral replication. It has great advantages and looks to have a bright future in treating and preventing influenza viruses. |
| 7. | CN105560177A | 2016 | The invention discloses the veterinary mixed suspension containing amoxicillin and baicalein, as well as the manufacturing process. The suspension is made by dispersing the active components, amoxicillin and baicalein, 1:1, in a dispersion medium. The problems of amoxicillin being easily broken down in an aqueous solution and the medication being slowly released in an oily mixed suspension are both solved for time-dependent antibiotics. T>MIC is prolonged, effective acting time is prolonged, and a user only needs to take the medication three times consecutively at intervals of 12 hours. |
| 8. | CN112007023A | 2020 | According to the invention, baicalein alpha, beta, or (beta 0+ beta 1) mixed crystal form can be used for preventing and/or treating obesity and its related conditions. More precisely, to the administration of a drug in the range of 0.001-2000 mg/kg/day/po as an active element in the management of obesity and its by-products, including hyperlipidemia, insulin resistance, and blood sugar. |
| 9. | CN108653206B | 2020 | The innovation, which falls under the category of pharmaceutical preparations, describes how to prepare a baicalein nanosuspension. The following elements comprise the baicalein nanosuspension prescription, expressed as a percentage by mass: phospholipid (0.05%-10%), F-68 (0.1-3%), glycerol (1%-10%), and the rest water. 0.1%-2% of the phospholipid compound is called baicalein. The invention offers a low cost and energy consumption, excellent safety, enhanced stability, better medication loading rate, and a straightforward and convenient operation. |
| 10. | CN112691102A | 2021 | The invention describes using baicalein to make a medication that treats and prevents PD as well as the depressive symptoms of Parkinson’s syndrome. The invention finds that baicalein can clearly increase the levels of neurotransmitters like DA, NE, and 5-HT in the brain and metabolites thereof, decrease the level of neuroinflammation factors in blood plasma, and improve the symptoms of a rotenone-induced depression mood model of a PD/Parkinson syndrome mouse. Consequently, baicalein can be utilized to make medications that prevent and treat PD as well as the symptoms of depression associated with the condition. |
| 11. | CN113244216A | 2021 | The innovation reveals the use of baicalein in the development of a medication to suppress a novel coronavirus and reveals that baicalein and SARS-CoV-2Mpro can be coupled, also preventing SARS-CoV-2Mpro from functioning as an enzyme in vitro. The protease activity, which impedes the new coronavirus’s ability to replicate, has promising applications in the field of creating new coronavirus drugs. |
| 12. | WO2021159570A1 | 2021 | This invention involves using baicalein to make a drug that will either treat or prevent a disease caused by a novel coronavirus infection. The invention in question pertains specifically to the use of baicalein and a pharmaceutical composition containing baicalein in the preparation of a drug intended to prevent and/or treat diseases caused by novel coronavirus infections (SARS-CoV-2). These infections can range in severity from mild to severe, and they include novel coronavirus pneumonia. |
| 13. | CN113925861A | 2022 | The invention describes the use of a Scutellaria flavone active ingredient and its preparation in the manufacturing of a medication intended to cure or prevent inflammatory storm. Wogonin, oroxylin A, and baicalein are the components from which the active ingredient of baicalein is chosen. Reducing the frequency of inflammatory storms, particularly in patients who are severely ill, helps to lessen organ damage and halt the disease’s course. The innovation finds that wogonin, baicalein, and oroxylin A all operate to varying degrees to block the mouse cytokine storm. Lung damage and inflammatory cell infiltration brought on by an inflammatory storm can be lessened by baicalein. Thus, the medication for both preventing and treating the inflammatory storm can be made using the active ingredient of baicalein. |
DA = dopamine; MIC = minimum inhibitory concentration; NE = norepinephrine; PD = Parkinson’s disease; SARS-CoV = severe acute respiratory syndrome coronavirus.
Acknowledgment
The images were created using Biorender software.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: Nil
Author contributions: Kavita Munjal, YashGoel, Vinod Kumar Gauttam: conceptualization, methodology, investigation, data collection, and writing—original manuscript. Hitesh Chopra, Madhav Singla, Smriti, Saurabh Gupta, Rohit Sharma: editing and proofreading. Hitesh Chopra, Rohit Sharma: supervision. All authors approved the submission of the final manuscript.
References
- 1. Liang W, Huang X, Chen W. The effects of baicalin and baicalein on cerebral ischemia: a review. Aging Dis. 2017;8(6):850-867. CrossRef PubMed
- 2. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57-68. CrossRef PubMed
- 3. Nik Salleh NNH, Othman FA, Kamarudin NA, Tan SC. The biological activities and therapeutic potentials of baicalein extracted from Oroxylum indicum: a systematic review. Molecules. 2020;25(23):5677. CrossRef PubMed
- 4. Rossi M, Meyer R, Constantinou P, et al. Molecular structure and activity toward DNA of baicalein, a flavone constituent of the Asian herbal medicine “Sho-saiko-to”. J Nat Prod. 2001;64(1):26-31. CrossRef PubMed
- 5. Gao Y, Snyder SA, Smith JN, Chen YC. Anticancer properties of baicalein: a review. Med Chem Res. 2016;25(8):1515-1523. CrossRef PubMed
- 6. Adin SN, Gupta I, Ahad A, Aqil M, Mujeeb M. A developed high-performance thin-layer chromatography method for the determination of baicalin in Oroxylum indicum L. and its antioxidant activity. J Planar Chromatogr Mod TLC. 2022;35(4):383-393. CrossRef
- 7. Rojsanga P, Bunsupa S, Sithisarn P. Flavones contents in extracts from Oroxylum indicum seeds and plant tissue cultures. Molecules. 2020;25(7):1545. CrossRef PubMed
- 8. Yu H, Han Y, Liu C, et al. Preparation of baicalein from baicalin using a baicalin-β-D-glucuronidase from Aspergillus niger b.48 strain. Process Biochem. 2020;97:168-175. CrossRef
- 9. Wang H, Ma X, Cheng Q, Wang L, Zhang L. Deep eutectic solvent-based ultrahigh pressure extraction of baicalin from Scutellaria baicalensis Georgi. Molecules. 2018;23(12):3233. CrossRef PubMed
- 10. Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1-2):277-290. CrossRef PubMed
- 11. de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol Res. 2015;100:296-308. CrossRef PubMed
- 12. Jelić D, Lower-Nedza AD, Brantner AH, et al. Baicalein and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J Chem. 2016;2510621. CrossRef
- 13. Li L, Liu WY, Feng F, Wu CY, Xie N. Synthesis and in vitro cytotoxicity evaluation of baicalein amino acid derivatives. Chin J Nat Med. 2013;11(3):284-288. CrossRef PubMed
- 14. Huang WH, Lee AR, Chien PY, Chou TC. Synthesis of baicalein derivatives as potential anti-aggregatory and anti-inflammatory agents. J Pharm Pharmacol. 2005;57(2):219-225. CrossRef PubMed
- 15. Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759-778. CrossRef PubMed
- 16. Cheng YH, Li LA, Lin P, et al. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol Appl Pharmacol. 2012;263(3):360-367. CrossRef PubMed
- 17. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. CrossRef PubMed
- 18. Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25(2A):959-964. PubMed
- 19. Arzanova E, Mayrovitz HN. The Epidemiology of Breast Cancer. In: Mayrovitz HN, ed. Breast Cancer [Internet Brisbane (AU): Exon Publications. 2022 Aug 6:chap 1. CrossRef
- 20. Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17(1):63-68. CrossRef PubMed
- 21. Sekiguchi M, Oda I, Matsuda T, Saito Y. Epidemiological trends and future perspectives of gastric cancer in Eastern Asia. Digestion. 2022;103(1):22-28. CrossRef PubMed
- 22. Mu J, Liu T, Jiang L, et al. The Traditional Chinese Medicine Baicalein Potently Inhibits Gastric Cancer Cells. J Cancer. 2016;7(4):453-461. CrossRef PubMed
- 23. Zheng, YH, Yin, LH, Grahn TH, Ye AF, Zhao YR, & Zhang QY. Anticancer effects of baicalein on hepatocellular carcinoma cells. Phytother Res. 2014;28(9),1 342-1348. CrossRef
- 24. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27. CrossRef PubMed
- 25. Takahashi H, Chen MC, Pham H, et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011;1813(8):1465-1474. CrossRef PubMed
- 26. Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2002;1(11):929-935. PubMed
- 27. Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20(8):517-535. CrossRef PubMed
- 28. Phan T, Nguyen VH, A’lincourt Salazar M, et al. Inhibition of Autophagy Amplifies Baicalein-Induced Apoptosis in Human Colorectal Cancer. Mol Ther Oncolytics. 2020;19:1-7. CrossRef PubMed
- 29. Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62(9):2721-2727. PubMed
- 30. Dianatinasab M, Forozani E, Akbari A, et al. Dietary patterns and risk of bladder cancer: a systematic review and meta-analysis. BMC Public Health. 2022;22(1):73. CrossRef PubMed
- 31. Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6(11):3039-3048. CrossRef PubMed
- 32. Wu J, Zhou T, Wang Y, Jiang Y, Wang Y. Mechanisms and advances in anti-ovarian cancer with natural plants component. Molecules. 2021;26(19):5949. CrossRef PubMed
- 33. Liu H, Dong Y, Gao Y, et al. The fascinating effects of baicalein on cancer: a review. Int J Mol Sci. 2016;17(10):1681. CrossRef PubMed
- 34. Chen J, Li Z, Chen AY, et al. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int J Mol Sci. 2013;14(3):6012-6025. CrossRef PubMed
- 35. Yan H, Xin S, Wang H, Ma J, Zhang H, Wei H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-κB signaling pathway. Anticancer Drugs. 2015;26(6):649-656. CrossRef PubMed
- 36. Sanapour N, Malakoti F, Shanebandi D, et al. Thymoquinone augments methotrexate-induced apoptosis on osteosarcoma cells. Drug Res (Stuttg). 2022;72(4):220-225. CrossRef PubMed
- 37. Ye F, Wang H, Zhang L, Zou Y, Han H, Huang J. Baicalein induces human osteosarcoma cell line MG-63 apoptosis via ROS-induced BNIP3 expression. Tumour Biol. 2015;36(6):4731-4740. CrossRef PubMed
- 38. Verma S, Gupta S, Das R, et al. Unravelling the approaches to treat osteoarthritis: a focus on the potential of medicinal plants. Pharmacognosy Res. 2023;15(1):13-25. CrossRef
- 39. Zhang Y, Song L, Cai L, Wei R, Hu H, Jin W. Effects of baicalein on apoptosis, cell cycle arrest, migration and invasion of osteosarcoma cells. Food Chem Toxicol. 2013;53:325-333. CrossRef PubMed
- 40. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111-128. CrossRef PubMed
- 41. Malik J, Munjal K, Deshmukh R. Attenuating effect of standardized lyophilized Cinnamomum zeylanicum bark extract against streptozotocin-induced experimental dementia of Alzheimer’s type. J Basic Clin Physiol Pharmacol. 2015;26(3):275-285. CrossRef PubMed
- 42. Choi RC, Zhu JT, Yung AW, et al. Synergistic action of flavonoids, baicalein, and daidzein in estrogenic and neuroprotective effects: a development of potential health products and therapeutic drugs against Alzheimer’s disease. Evid Based Complement Alternat Med. 2013;2013:635694. CrossRef PubMed
- 43. Zhang S-Q, Obregon D, Ehrhart J, et al. Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer’s disease transgenic mouse model. J Neurosci Res. 2013;91(9):1239-1246. CrossRef PubMed
- 44. Zhu JT, Choi RC, Chu GK, et al. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death. J Agric Food Chem. 2007;55(6):2438-2445. CrossRef PubMed
- 45. Patil RR. Epidemiology of Parkinson’s disease—current understanding of causation and risk factors. In: Arjunan SP, Kumar DK, eds. Techniques for assessment of parkinsonism for diagnosis and rehabilitation. Springer Singapore 2022; 31-48.
- 46. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. CrossRef PubMed
- 47. Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66(2)(suppl 1):S74-S78. CrossRef PubMed
- 48. Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279(26):26846-26857. CrossRef PubMed
- 49. Suk K, Lee H, Kang SS, Cho GJ, Choi WS. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. J Pharmacol Exp Ther. 2003;305(2):638-645. CrossRef PubMed
- 50. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke. 2022;17(1):18-29. CrossRef PubMed
- 51. Singh G, Sharma M, Kumar GA, et al; India State-Level Disease Burden Initiative Neurological Disorders Collaborators. The burden of neurological disorders across the states of India: the Global Burden of Disease Study 1990-2019. Lancet Glob Health. 2021;9(8):e1129-e1144. CrossRef PubMed
- 52. Zhou P, Iadecola C. iNOS and COX-2 in ischemic stroke. In: Lajtha A, Chan PH, eds. Handbook of neurochemistry and molecular neurobiology: acute ischemic injury and repair in the nervous system. New York, NY: Springer US 2007; 33-45.
- 53. Yuan Y, Men W, Shan X, et al. Baicalein exerts neuroprotective effect against ischaemic/reperfusion injury via alteration of NF-kB and LOX and AMPK/Nrf2 pathway. Inflammopharmacology. 2020;28(5):1327-1341. CrossRef PubMed
- 54. Chen W, Teng X, Ding H, et al. Nrf2 protects against cerebral ischemia-reperfusion injury by suppressing programmed necrosis and inflammatory signaling pathways. Ann Transl Med. 2022;10(6):285. CrossRef PubMed
- 55. Vaibhav K, Shrivastava P, Javed H, et al. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol Cell Biochem. 2012;367(1-2):73-84. CrossRef PubMed
- 56. Pitkänen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23(2):241-261. CrossRef PubMed
- 57. Li Q, Li QQ, Jia JN, et al. Baicalein exerts neuroprotective effects in FeCl3-induced posttraumatic epileptic seizures via suppressing ferroptosis. Front Pharmacol. 2019;10:638. CrossRef PubMed
- 58. Li Y, Chen Q, Ran D, et al. Changes in the levels of 12/15-lipoxygenase, apoptosis-related proteins and inflammatory factors in the cortex of diabetic rats and the neuroprotection of baicalein. Free Radic Biol Med. 2019;134:239-247. CrossRef PubMed
- 59. The Lancet. GBD 2017: a fragile world. Lancet. 2018;392(10159):1683. CrossRef PubMed
- 60. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. CrossRef PubMed
- 61. Quirt J, Hildebrand KJ, Mazza J, Noya F, Kim H. Asthma. Allergy Asthma Clin Immunol. 2018;14(S2)(suppl 2):50. CrossRef PubMed
- 62. Alsharairi NA. Scutellaria baicalensis and their natural flavone compounds as potential medicinal drugs for the treatment of nicotine-induced non-small-cell lung cancer and asthma. Int J Environ Res Public Health. 2021;18(10):5243. CrossRef PubMed
- 63. Xu T, Ge X, Lu C, et al. Baicalein attenuates OVA-induced allergic airway inflammation through the inhibition of the NF-κB signaling pathway. Aging (Albany NY). 2019;11(21):9310-9327. CrossRef PubMed
- 64. Westergren-Thorsson G, Larsen K, Nihlberg K, et al. Pathological airway remodelling in inflammation. Clin Respir J. 2010;4(s1)(suppl 1):1-8. CrossRef PubMed
- 65. Sampsonas F, Kaparianos A, Lykouras D, Karkoulias K, Spiropoulos K. DNA sequence variations of metalloproteinases: their role in asthma and COPD. Postgrad Med J. 2007;83(978):244-250. CrossRef PubMed
- 66. Bakhtiyari M, Kazemian E, Kabir K, et al. Contribution of obesity and cardiometabolic risk factors in developing cardiovascular disease: a population-based cohort study. Sci Rep. 2022;12(1):1544. CrossRef PubMed
- 67. Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35(2):72-115. CrossRef PubMed
- 68. Sharma A, et al. Combination effect of Spirulina fusiformis with rutin or chlorogenic acid in lipopolysaccharide-induced septic cardiac inflammation in experimental diabetic rat model. Pharmacogn Mag. 2021;17(6):257-267. CrossRef
- 69. Satyavert GS, Gupta S, Choudhury H, et al. Pharmacokinetics and tissue distribution of hydrazinocurcumin in rats. Pharmacol Rep. 2021;73(6):1734-1743. CrossRef PubMed
- 70. Al-Makki A, DiPette D, Whelton PK, et al. Hypertension pharmacological treatment in adults: A World Health Organization guideline executive summary. Hypertension. 2022;79(1):293-301. CrossRef PubMed
- 71. Wu D, Ding L, Tang X, Wang W, Chen Y, Zhang T. Baicalin protects against hypertension-associated intestinal barrier impairment in part through enhanced microbial production of short-chain fatty acids. Front Pharmacol. 2019;10:1271. CrossRef PubMed
- 72. Li M, Ren C. Exploring the protective mechanism of baicalin in treatment of atherosclerosis using endothelial cells deregulation model and network pharmacology. BMC Complement Med Ther. 2022 Oct 3;22(1):257. CrossRef PubMed
- 73. Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185-192. CrossRef PubMed
- 74. Alshehri B, Vijayakumar R, Senthilkumar S, et al. Therapeutic potential of nitric oxide synthase inhibitor from natural sources for the treatment of ischemic stroke. Saudi J Biol Sci. 2022;29(2):984-991. CrossRef PubMed
- 75. Huang P, Gao JW, Shi Z, et al. A novel UPLC-MS/MS method for simultaneous quantification of rhein, emodin, berberine and baicalin in rat plasma and its application in a pharmacokinetic study. Bioanalysis. 2012;4(10):1205-1213. CrossRef PubMed
- 76. Xin L, Gao J, Lin H, Qu Y, Shang C, Wang Y, Lu Y, Cui X. Regulatory Mechanisms of Baicalin in Cardiovascular Diseases: A Review. Frontiers in Pharmacology. 2020; 2;11:583200. CrossRef
- 77. Wencker D, Chandra M, Nguyen K, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111(10):1497-1504. CrossRef PubMed
- 78. Zhang K, Lu J, Mori T, et al. Baicalin increases VEGF expression and angiogenesis by activating the ERR{α}/PGC-1{α} pathway. Cardiovasc Res. 2011;89(2):426-435. CrossRef PubMed
- 79. Pang H, Wu T, Peng Z, Tan Q, Peng X, Zhan Z, Song L, Wei B. Baicalin induces apoptosis and autophagy in human osteosarcoma cells by increasing ROS to inhibit PI3K/Akt/mTOR, ERK1/2 and β-catenin signaling pathways. Journal of Bone Oncology. 2022; 1;33:100415. CrossRef PubMed
- 80. Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29(9):938-946. CrossRef PubMed
- 81. Cheng HC, Gleason EM, Nathan BA, Lachmann PJ, Woodward JK. Effects of clonidine on gastric acid secretion in the rat. J Pharmacol Exp Ther. 1981;217(1):121-126. PubMed
- 82. DiJoseph JF, Eash JR, Mir GN. Gastric antisecretory and antiulcer effects of WHR1582A, a compound exerting alpha-2 adrenoceptor agonist activity. J Pharmacol Exp Ther. 1987;241(1):97-102. PubMed
- 83. Ribeiro ARS, do Nascimento Valença JD, da Silva Santos J, et al. The effects of baicalein on gastric mucosal ulcerations in mice: protective pathways and anti-secretory mechanisms. Chem Biol Interact. 2016;260:33-41. CrossRef PubMed
- 84. Yang H, Lu Y, Zeng XF, et al. Antichronic gastric ulcer effect of zinc-baicalin complex on the acetic acid-induced chronic gastric ulcer rat model. Gastroenterol Res Pract. 2018;2018:1275486. CrossRef PubMed
- 85. Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851-861. CrossRef PubMed
- 86. Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022;31(1):17-27. CrossRef PubMed
- 87. Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med (Lond). 2018;18(3):245-250. CrossRef PubMed
- 88. Yang JY, Li M, Zhang CL, Liu D. Pharmacological properties of baicalin on liver diseases: a narrative review. Pharmacol Rep. 2021;73(5):1230-1239. CrossRef PubMed
- 89. Ma Y, Yang F, Wang Y, et al. CaMKKβ is involved in AMP-activated protein kinase activation by baicalin in LKB1 deficient cell lines. PLoS One. 2012;7(10):e47900. CrossRef PubMed
- 90. Guo HX, Liu DH, Ma Y, et al. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. 2009;30(11):1505-1512. CrossRef PubMed
- 91. Zhong X, Liu H. Baicalin attenuates diet induced nonalcoholic steatohepatitis by inhibiting inflammation and oxidative stress via suppressing JNK signaling pathways. Biomed Pharmacother. 2018;98:111-117. CrossRef PubMed
- 92. Wu T, Liu T, Xing L, Ji G. Baicalin and puerarin reverse epithelial-mesenchymal transition via the TGF-β1/Smad3 pathway in vitro. Exp Ther Med. 2018;16(3):1968-1974. CrossRef PubMed
- 93. Fang P, Sun Y, Gu X, et al. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1α pathway. Phytomedicine. 2019;64:153074. CrossRef PubMed
- 94. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3-15. CrossRef PubMed
- 95. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303-1353. CrossRef PubMed
- 96. Gauttam V, Munjal K, Mujwar S, Sawale J, Rohilla M, Gupta S. Comparative study of developed formulation and market formulation for antidiabetic potential in alloxan-induced diabetic Wistar rats. J Young Pharm. 2022;14(4):387-393. CrossRef
- 97. Ahad A, Mujeeb M, Ahsan H, Siddiqui WA. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie. 2014;106:101-110. CrossRef PubMed
- 98. Yang M, Kan L, Wu L, Zhu Y, Wang Q. Effect of baicalin on renal function in patients with diabetic nephropathy and its therapeutic mechanism. Exp Ther Med. 2019;17(3):2071-2076. CrossRef PubMed
- 99. Haye A, Ansari MA, Saini A, et al. Polyherbal formulation improves glucose-lipid metabolism and prevent hepatotoxicity in streptozotocin-induced diabetic rats: plausible role of IRS-PI3K-Akt-GLUT2 signaling. Pharmacogn Mag. 2022;18(77):52. CrossRef
- 100. Hu Z, Guan Y, Hu W, Xu Z, Ishfaq M. An overview of pharmacological activities of baicalin and its aglycone baicalein: new insights into molecular mechanisms and signaling pathways. Iran J Basic Med Sci. 2022;25(1):14-26. PubMed
- 101. Piao XL, Cho EJ, Jang MH. Cytoprotective effect of baicalein against peroxynitrite-induced toxicity in LLC-PK(1) cells. Food Chem Toxicol. 2008;46(5):1576-1581. CrossRef PubMed
- 102. Sahu BD, Mahesh Kumar J, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One. 2015;10(7):e0134139. CrossRef PubMed
- 103. Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G. Nanotechnology approaches for global infectious diseases. Nat Nanotechnol. 2021;16(4):369-384. CrossRef PubMed
- 104. Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25-64. CrossRef PubMed
- 105. Aghazadeh M, Zahedi Bialvaei A, Aghazadeh M, et al. Survey of the antibiofilm and antimicrobial effects of zingiber officinale (in vitro study). Jundishapur J Microbiol. 2016;9(2):e30167. CrossRef PubMed
- 106. Li C, Huang P, Wong K, et al. Coptisine-induced inhibition of Helicobacter pylori: elucidation of specific mechanisms by probing urease active site and its maturation process. J Enzyme Inhib Med Chem. 2018;33(1):1362-1375. CrossRef PubMed
- 107. Guo M, Zhang N, Li D, et al. Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int Immunopharmacol. 2013;16(2):125-130. CrossRef PubMed
- 108. Ozma MA, Khodadadi E, Pakdel F, et al. Baicalin, a natural antimicrobial and anti-biofilm agent. J Herb Med. 2021;27:100432. CrossRef
- 109. Luo J, Dong B, Wang K, et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One. 2017;12(4):e0176883. CrossRef PubMed
- 110. Chen Y, Liu T, Wang K, et al. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One. 2016;11(4):e0153468. CrossRef PubMed
- 111. Li K, Liang Y, Cheng A, et al. Antiviral properties of baicalin: a concise review. Rev Bras Farmacogn. 2021;31(4):408-419. CrossRef PubMed
- 112. Su Y, Xiang Y. Clinical efficacy of baicalin combined with antivirals in the treatment of severe influenza A HIN1 influenza. Chin Hosp Pharm J. 2014;34:306-308.
- 113. Jenks JD, Cornely OA, Chen SC, Thompson GR III, Hoenigl M. Breakthrough invasive fungal infections: who is at risk? Mycoses. 2020;63(10):1021-1032. CrossRef PubMed
- 114. Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017;133:86-96. CrossRef PubMed
- 115. Li L, Lu H, Zhang X, et al. Baicalein acts against Candida albicans by targeting Eno1 and inhibiting glycolysis. Microbiol Spectr. 2022;10(4):e0208522. CrossRef PubMed
- 116. Zhu Y, Peng X, Zhang Y, Lin J, Zhao G. Baicalein protects against Aspergillus fumigatus keratitis by reducing fungal load and inhibiting TSLP-induced inflammatory response. Invest Ophthalmol Vis Sci. 2021;62(6):26. CrossRef PubMed
- 117. Wang T, Shi G, Shao J, et al. In vitro antifungal activity of baicalin against Candida albicans biofilms via apoptotic induction. Microb Pathog. 2015;87:21-29. CrossRef PubMed
- 118. Singh J, Kakkar P. Modulation of liver function, antioxidant responses, insulin resistance and glucose transport by Oroxylum indicum stem bark in STZ induced diabetic rats. Food Chem Toxicol. 2013;62:722-731. CrossRef PubMed
- 119. Fu Y, Luo J, Jia Z, et al. Baicalein protects against type 2 diabetes via promoting islet β-cell function in obese diabetic mice. Int J Endocrinol. 2014;2014:846742. CrossRef PubMed
- 120. Alqahtani AS, Hidayathulla S, Rehman MT, et al. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules. 2019;10(1):61. CrossRef PubMed
- 121. Sun W, Sang Y, Zhang B, et al. Synergistic effects of acarbose and an Oroxylum indicum seed extract in streptozotocin and high-fat-diet induced prediabetic mice. Biomed Pharmacother. 2017;87:160-170. CrossRef PubMed
- 122. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453. CrossRef PubMed
- 123. Min W, Liu X, Qian Q, et al. Effects of baicalin against UVA-induced photoaging in skin fibroblasts. Am J Chin Med. 2014;42(3):709-727. CrossRef PubMed
- 124. Yun M-Y, Won E-Y, Lee J-H, Jung J-I, Choi H-J. Bioconversion from Scutellaria baicalensis (baicalin) fermented with Leatiporus sulphureus into enriched-baicalein and anti-wrinkle effects. Pharmacogn Mag. 2018;14(57):S453-457. Online
- 125. Bossi O, Gartsbein M, Leitges M, Kuroki T, Grossman S, Tennenbaum T. UV irradiation increases ROS production via PKCdelta signaling in primary murine fibroblasts. J Cell Biochem. 2008;105(1):194-207. CrossRef PubMed
- 126. Dong R, Li L, Gao H, et al. Safety, tolerability, pharmacokinetics, and food effect of baicalein tablets in healthy Chinese subjects: A single-center, randomized, double-blind, placebo-controlled, single-dose phase I study. J Ethnopharmacol. 2021;274:114052. CrossRef PubMed
- 127. Song J, Zhang L, Xu Y, et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem Pharmacol. 2021;183:114302. CrossRef PubMed
- 128. Li M, Shi A, Pang H, et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J Ethnopharmacol. 2014;156:210-215. CrossRef PubMed
- 129. Tyagi S, Raghvendra, Sing U, et al. Applications of metabolomics—a systematic study of the unique chemical fingerprints: an overview. International Journal of Pharmaceutical Sciences Review and Research 2010;3:83-86 Online (Accessed October 2023)
- 130. Hoimes CJ, Lamb L, Ruta S, et al. A phase I/II study of PHY906 plus capecitabine (CAP) in patients (pts) with advanced pancreatic cancer (APC). J Clin Oncol. 2008;26(15_suppl):15538. CrossRef
- 131. Srivastava S, Kumar A, Yadav PK, et al. Formulation and performance evaluation of polymeric mixed micelles encapsulated with baicalein for breast cancer treatment. Drug Dev Ind Pharm. 2021;47(9):1512-1522. CrossRef PubMed
- 132. Majumdar S, Dey S, Ganguly D, Mazumder R. Enhanced topical permeability of natural flavonoid baicalein through nano liposomal gel: in vitro and in vivo investigation. J Drug Deliv Sci Technol. 2020;57:101666. CrossRef
- 133. Kavithaa K, Sumathi S, Padma P. Intracellular uptake of PEG-functionalized baicalein loaded iron oxide nanoparticles regulates apoptotic genes in triple negative breast cancer cells: mitochondrial pathway targeted therapy for breast cancer. J Cluster Sci. 2017;28(4):2057-2073. CrossRef
- 134. Li X, Luo W, Ng TW, et al. Nanoparticle-encapsulated baicalein markedly modulates pro-inflammatory response in gingival epithelial cells. Nanoscale. 2017;9(35):12897-12907. CrossRef PubMed
- 135. Yang J, Chen A, He X, Lu S. Fabrication of baicalein-encapsulated zeolitic imidazole framework as a novel nanocomposited wound closure material to persuade pH-responsive healing efficacy in post-caesarean section wound care. Int Wound J. 2023;20(6):1921-1933. CrossRef PubMed
- 136. Gharbavi M, Johari B, Ghorbani R, Madanchi H, Sharafi A. Green synthesis of Zn nanoparticles and in situ hybridized with BSA nanoparticles for Baicalein targeted delivery mediated with glutamate receptors to U87‐MG cancer cell lines. Appl Organomet Chem. 2023;37(1):e6926. CrossRef
- 137. Shi F, Wei Z, Zhao Y, Xu X. Nanostructured lipid carriers loaded with baicalin: an efficient carrier for enhanced antidiabetic effects. Pharmacogn Mag. 2016;12(47):198-202. CrossRef PubMed
- 138. Abdel Maksoud HA, Abou Zaid OAR, Elharrif MG, Omnia MA, Alaa EA. Selenium cleome droserifolia nanoparticles (Se-CNPs) and it's ameliorative effects in experimentally induced diabetes mellitus. Clin Nutr ESPEN. 2020 Dec;40:383-391. CrossRef PubMed
- 139. Kavithaa K, Paulpandi M, Padma PR, Sumathi S. Induction of intrinsic apoptotic pathway and cell cycle arrest via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. RSC Adv. 2016;6(69):64531-64543. CrossRef
- 140. Yadav PK, Saklani R, Tiwari AK, et al. Ratiometric codelivery of Paclitaxel and Baicalein loaded nanoemulsion for enhancement of breast cancer treatment. Int J Pharm. 2023;643:123209. CrossRef PubMed
- 141. Wang W, Xi M, Duan X, Wang Y, Kong F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. Int J Nanomedicine. 2015;10:3737-3750. PubMed
- 142. Li K, Zhang H, Gao L, et al. Preparation and characterization of baicalein-loaded nanoliposomes for antitumor therapy. J Nanomater. 2016;2861915. CrossRef
- 143. Liu Z, Zhang L, He Q, et al. Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia-reperfusion in rats. Int J Pharm. 2015;489(1-2):131-138. CrossRef PubMed