 |
Drug Target Insights 2024; 18: 1-3 ISSN 1177-3928 | DOI: 10.33393/dti.2024.2670 ORIGINAL RESEARCH ARTICLE |
 |
Evaluation of non-conformities in the drafting of bulletins for urine cytobacteriological examinations at Sikasso Hospital (Mali)
ABSTRACT
Background: Non-compliance in the drafting of examination bulletins makes it difficult to perform them and interpret the results. With the aim of continuously improving laboratory services and guaranteeing the quality of urine cytobacteriological examination (ECBU) results, we initiated this study to evaluate non-compliance in the drafting of ECBU reports.
Materials and methods: This was a retrospective descriptive cross-sectional study which focused on non-compliance in the drafting of ECBU reports analysed in the laboratory from January to December 2022.
Results: During the study period, we collected 383 non-compliant ECBU reports out of 672, with a frequency of 56.99%. Non-compliances were related to age (2.68%), profession (24.40%), clinical information (6.70%) and residence (52.08%). The majority of non-compliant reports came from the medicine (35.51%) and urology (25.85%) departments.
Conclusion: The high frequency of non-compliance is a cause for concern and is of concern to all prescribers in this hospital.
Keywords: Bulletins, ECBU, Mali, Non-compliance, Sikasso
Received: September 25, 2023
Accepted: November 30, 2023
Published online: January 16, 2024
Corresponding author:
Luka Diarra
lukadiarra@yahoo.fr
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
A biological test report is a medical prescription for diagnostic purposes, specifying the parameter to be measured, in relation to the diagnostic hypothesis envisaged, and all the information needed for the laboratory to carry out the test correctly (1). Non-compliance in the prescription of medical biology tests makes it difficult to carry them out and interpret the results (2). A number of researchers have concluded that non-compliance has a number of possible consequences for patients, such as misdiagnosis, mismanagement and treatment, as well as therapeutic abstention (3). In Burkina Faso, 59.4% of non-compliances concerning clinical information were reported in a study in 2021 (2). In Niger, Djobo et al recorded 53.99% of non-compliances concerning failure to notify the name of the service on biological examination forms (4). In Mali, 41% of non-compliances concerning clinical information were found (5). With a view to continuous quality improvement and guaranteeing the quality of urine cytobacteriological examination (ECBU) results, we initiated this study with the aim of evaluating non-compliance in the drafting of ECBU reports analysed at the Sikasso Hospital laboratory.
Materials and methods
Study design and population
This was a retrospective descriptive cross-sectional study of non-compliance in the completion of ECBU reports at Sikasso Hospital from January to December 2022. The study population consisted of reports containing the ECBU analysed in the laboratory during the study period. Data were collected from examination request forms and the laboratory register.
Inclusion criteria
All bulletins with ECBU analysed at the hospital laboratory were included in this study.
Non-inclusion criteria
All reports of ECBU analysed in other laboratories, as well as reports of biological examinations other than ECBU were not included.
Study variables
The study variables were age, sex, occupation, clinical information and place of residence.
Statistical analysis
Excel 2013 and Epi Info 7.2.1.0 were used for statistical analysis of the data.
Ethical considerations
Anonymity was preserved before each inclusion.
Results
During the study period, we identified 383 non-compliant reports out of 672, with a frequency of 56.99%. The non-compliances identified were related to age, profession, clinical information and residence (Tab. I).
| Variables | Number | Frequency (%) |
|---|---|---|
| Non-compliance by age | ||
| Yes | 18 | 2.68 |
| No | 654 | 97.32 |
| Non-compliance by profession | ||
| Yes | 164 | 24.40 |
| No | 508 | 75.60 |
| Non-compliance by clinical information | ||
| Yes | 45 | 6.70 |
| No | 627 | 93.30 |
| Non-compliance by residence | ||
| Yes | 350 | 52.08 |
| No | 322 | 47.92 |
The majority of non-compliance bulletins came from the medicine (35.51%) and urology (25.85%) departments (Fig. 1).
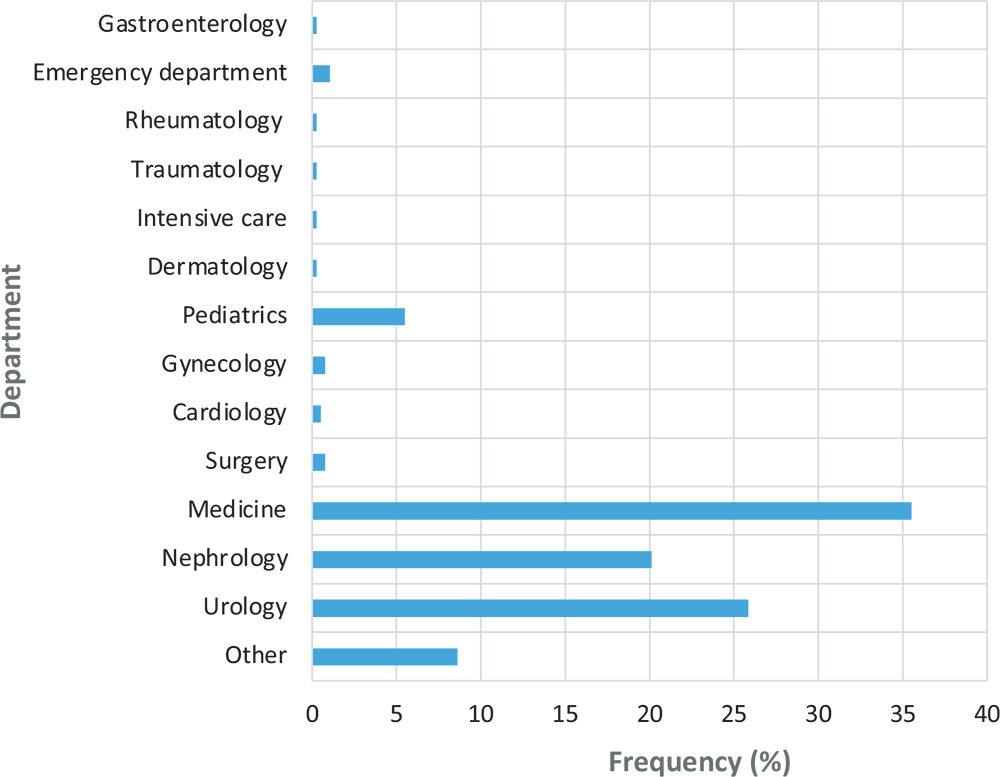
Fig. 1 - Breakdown of non-compliant forms by department.
The non-compliances concerning age were 35.29% for the medicine department and 29.41% for the urology department. The lack of information on prescriptions in the urology department was 41.98%, and 21.60% in the nephrology department. The absence of clinical information was frequent on the urology department forms (44.19%). Non-compliances concerning residence accounted for 39.08% for medicine and 22.70% for urology.
Study limitations
The non-conformities concerned only ECBU bulletins, which justifies their high frequency during the course of this work.
Discussion
Prescription is the result of careful consideration, taking into account the epidemiological and clinical context on the one hand, and precise knowledge of what can be expected from the examination on the other (6). ECBU reports received during the course of this study were 56.99% non-compliant. Yacouba et al in Burkina Faso reported a non-compliant rate of 5.2% (2). Our result could be explained by the size of our sample, which was not large enough, and by the fact that the study was limited to ECBU reports. The majority of non-compliant reports came from medicine (35.51%) and urology (25.85%) departments. Djobo et al found 3.64% and 1.33%, respectively, for the medicine and urology departments (4). This could be explained by automatic prescribing, which is often based on personal habits rather than established protocols (6). In our study, non-compliance relating to clinical information accounted for 6.70%. This proportion is lower than that reported by Djobo et al, who found the rate to be 46.63%. This situation could be explained by the lack of awareness of the importance of this parameter in the interpretation of ECBU by several of our prescribers (4). Including clinical and therapeutic information can help the biologist in choosing the technique to be used, or lead him/her to advise against a costly investigation and suggest a more appropriate one (2). Age was missing from 2.68% of the forms in our study. This low rate confirms our prescribers’ mastery of the impact of this parameter in interpreting the results (2). Those with no occupation or residence accounted for 24.40% and 52.08%, respectively. These socio-demographic parameters are one of the factors that will add value to our knowledge of the factors that contribute to urinary tract infections. Reporting them on ECBU reports will be of epidemiological interest.
Conclusion
This study has provided information on the completeness of ECBU reports. The high frequency of non-compliance is a cause for concern and is of concern to all prescribers in the hospital. Close collaboration between prescribers and biologists would help to control non-compliance in the drafting of examination reports.
Conflicts of interest
The authors declare that they have no competing interests.
Authors’ contributions
Luka Diarra collected the data and wrote the article. Moussa Mariko contributed to the data analysis. All other authors reviewed and approved the final manuscript.
Funding
The authors received no funding for this work.
Acknowledgements
The authors sincerely thank all the staff of Sikasso Hospital for their contributions. Special thanks to the general management of the hospital for their support throughout the process.
References
- 1. Adeoti MF, Silue A, Sawadogo D, Dosso M, Sess ED. Reflection on good practice in writing the medical analysis bulletin. Immunol Anal Biol Spec. 2004;19(6):370-373. Online CrossRef
- 2. Yacouba A, Kiello K, Kiba-Koumare TCRA, Kabre E, Sakande J. Writing quality of the laboratory request forms at Yalgado Ouédraogo teaching hospital, Burkina Faso. Int J Biol Chem Sci. 2019;13(6):2683-2690. Online CrossRef
- 3. Jnah A, Yagoubi M, Seffar M, El Hamzaoui S, Hamamouchi J, Zouhdi M. Control of non-conformities in the pre-analytical phase at the Bacteriology Laboratory of the Ibn Sina University Hospital in Rabat (Morocco). Tunis Med. 2022;100(3):247-254. Online PubMed
- 4. Djobo K, Yacouba A, Alhousseini D, et al. Multicentre analysis of the editorial quality of biological analysis bulletins in Niger. Pan Afr Med J. 2022;43(59). Online
- 5. Bahachimi A. Pre-analytical non-conformities in the biomedical laboratory of the Mali hospital 2020. Online
- 6. Gérôme P, Dusseau JY, Masseron T, Bercion R. The pre-analytical phase in bacteriology. Revue Française des Laboratoires. 2001;2001(335):23-30. Online CrossRef