 |
Drug Target Insights 2023; 17: 126-137 ISSN 1177-3928 | DOI: 10.33393/dti.2023.2660 REVIEW |
 |
Management of urinary tract infections in the era of antimicrobial resistance
ABSTRACT
Urinary tract infections (UTIs) are among the most common infections globally, imposing a substantial personal and economic burden on individuals and health resources. Despite international health concerns and sustained public awareness campaigns about the emergence of resistant microorganisms through the inappropriate therapeutic use of antimicrobial agents, the problem of antimicrobial resistance (AMR) is worsening, and AMR in UTIs represents a critical global healthcare issue. This narrative review summarizes evidence-based scientific material, recommendations from the current medical literature, and the latest clinical guidelines on antibiotic and antibiotic-sparing strategies for managing urological infections, including practical approaches to improve the management of patients with acute and recurrent UTIs (rUTIs) in routine clinical practice. Novel emerging therapies and prophylaxis options are described as potential alternatives to overcome the abuse and overuse of antibiotics and the practical application of the guideline recommendations and issues relating to best practice in managing UTIs.
Keywords: Antibiotic, Antibiotic sparing, Antimicrobial resistance, Prophylaxis, Recurrence, Urinary tract infections
Received: September 11, 2023
Accepted: November 21, 2023
Published online: December 20, 2023
Corresponding author:
Ria Pothoven
Florence Rijswijk
Laan van Vredenoord 1-7, 2289 DA Rijswijk
Postbus 1005, 2280 CA Rijswijk - The Netherlands
Andros Clinics Den Haag
Madame Curielaan 10
2289 ca Rijswijk - The Netherlands
Ria.Pothoven@Florence.nl
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Urinary tract infections (UTIs) are among the most common infections globally, imposing a substantial personal and economic burden on individuals and health resources (1,2). Data from the Global Burden of Disease Study 2019 (3,4) have been used to investigate the incidence, mortality, and disability-adjusted life years (DALYs) related to UTIs globally, by region, and by country from 1990 to 2019. Globally, there were an estimated 405 million cases, 237,000 deaths, and 5.2 million DALYs associated with UTIs in 2019, with a 2.4 times growth in deaths from 1990 to 2019, accompanied by an increasing age-standardized mortality rate over time (1,2). Specifically, the age-standardized incidence rate of UTIs increased from 4,715 individuals per 100,00 population in 1990 to 5,229 per 100,000 in 2019, and the number of deaths related to UTIs increased from approximately 99,000 in 1990 to 237,000 in 2019 (1,2).
The incidence of UTIs was more prominent in higher sociodemographic regions, together with an increasing mortality rate, whereas countries with lower sociodemographic development or a higher baseline disease burden showed notable rates of decline over the three decades. The burden of UTIs was higher in females and tended to increase with age, particularly in regions with a higher sociodemographic status (1).
UTIs present a critical global healthcare issue, particularly as, along with other infectious diseases, antimicrobial resistance (AMR) in UTIs is a continuing challenge. Despite global health concerns and sustained public awareness campaigns about the emergence of resistant microorganisms through the inappropriate therapeutic use of antimicrobial agents, the AMR problem is worsening (5-7).
In this narrative review we summarize the evidence from the scientific literature and recommendations from the latest clinical guidelines on the management of urological infections and suggest practical approaches to improve the management of patients with acute and recurrent UTIs (rUTIs). Novel emerging therapies and prophylaxis options are described as potential alternatives to overcome the abuse and overuse of antibiotics, including new clinical outcomes on the use of glycosaminoglycan (GAG) therapy in the management of rUTIs and the practical application of the guideline recommendations and issues relating to best practice in the management of UTIs.
Methods
The present review is based on relevant articles retrieved from PubMed/MEDLINE and Google Scholar guided by material presented at an interactive scientific workshop sponsored by IBSA Institut Biochimique SA (IBSA) during the 38th Annual European Association of Urology (EAU) Congress (EAU23) that was held from March 10 to 13, 2023, in Milan, Italy. The workshop addressed the management of UTIs against the background of increasing AMR, focusing on the widespread problem due to the over- or inappropriate use of antibiotics and the related threat of AMR in patients with UTIs.
Papers presented as part of the workshop were augmented by searching the databases for additional publications necessary to advance the discussion or provide additional evidence. Original research papers published in English in internationally recognized journals and online journals were selected preferentially, but review articles that added to the understanding of the management of UTIs in the era of AMR were also selected. Identified articles were reviewed for relevance, with preference given to recent papers.
The EAU presents programs of cutting-edge urological science at their annual Congress, including the presentation of the latest edition of the EAU Guidelines on Urological Infections. These widely respected clinical guidelines, which provide medical professionals with best evidence-based information and recommendations for preventing and treating UTIs and male accessory gland infections (8), reinforce the material presented in this review. The guidelines also address important public health aspects of infection control and antimicrobial stewardship, including the growing problem of resistance among uropathogenic bacteria, the overuse and misuse of antibiotics, and the lack of new antibiotics. The European Association of Urology Nurses (EAUN), which has been developing practice guidelines for European urology nurses since 2004, also hosted their annual meeting (EAUN23) in conjunction with EAU23, providing a forum for leading urology nursing and medical professionals to share evidence-based research of particular relevance to urological care from a nursing perspective.
A patient awareness campaign was also introduced at the Congress to inform and raise awareness of the correct recognition of symptoms and treatment of UTIs, involving patients, patient organizations, and physicians in managing bacterial infections according to the EAU guidelines. A video supporting the campaign, UTI and the use of antibiotics, clearly and directly explains several aspects of urological disorders and is available, along with other information, in the EAU Patient Information Portal at Online.
Management of UTIs in times of increasing AMR
There is increasing interest in infectious diseases, and the EAU Guidelines on Urological Infections have become the second most downloaded of all European clinical guidelines after Prostate Cancer guidelines. In parallel, the issue of AMR in urological infections, as in other infections, is increasingly recognized as an urgent public health priority (6,9).
An independent review on AMR commissioned in July 2014 by the UK Government to address the challenge of AMR estimated that failure to address the problem could lead to 10 million deaths globally per year by 2050, costing an estimated US$ 100 trillion if action is not taken (7). The latest predictive models of the World Health Organization (WHO) AMR Collaborators estimate that there were 4.95 million deaths associated with bacterial AMR globally in 2019 (6). This includes 1.27 million deaths directly attributable to bacterial AMR and 3.57 million associated with AMR. Just six pathogens were each responsible for over 250,000 deaths attributable to or associated with bacterial AMR: Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa (Fig. 1).
Together, they were responsible for 929,000 deaths (6). Of these, UTIs were the fourth most prevalent infectious syndrome associated with global deaths attributable to or associated with AMR, after lower respiratory infections and all related infections in the thorax, bloodstream infections, and peritoneal and intra-abdominal infections (6).
Classification and diagnosis
UTIs can be classified differently, but a system developed by the EAU Section of Infections in Urology and the European Society for Infections in Urology (ESIU) and adopted by the EAU provides a valuable classification tool. The EAU/ESIU system classifies UTIs according to the prevalence of risk factors (uncomplicated and complicated UTIs), localization (lower and upper UTIs), the frequency of occurrence (rare and recurrent), relapse or reinfection, and in women and men (8). That is, the system is based on the clinical presentation of the UTI, the anatomical level of the UTI, the grade of severity of the infection, the categorization of risk factors, the frequency of occurrence, and the availability of appropriate antimicrobial therapy (10).
For example, a young, premenopausal, nonpregnant woman with no known relevant anatomical and functional abnormalities within the urinary tract or comorbidities presenting with the typical symptoms of a UTI can be considered to have an uncomplicated UTI. By definition, all other UTIs are complicated UTIs, with a higher risk of developing a complicated course. This classification system for UTIs is summarized in Table I.
Even an uncomplicated UTI is not just a simple infection, as it involves symptoms and restricted activity and may necessitate sick leave or bed rest. A UTI occurring at least three times a year or twice in the previous 6 months is defined as an rUTI. However, contrary to historical practice, it is strongly recommended not to treat cases of asymptomatic bacteriuria, where the presence of bacteria is found in a urine culture taken as part of a routine clinical visit in a patient without symptoms (8). In fact, there is evidence that asymptomatic bacteriuria is a common commensal colonization that may have a protective function against superinfecting symptomatic UTI, and treating it may be harmful in some cases (8,11).
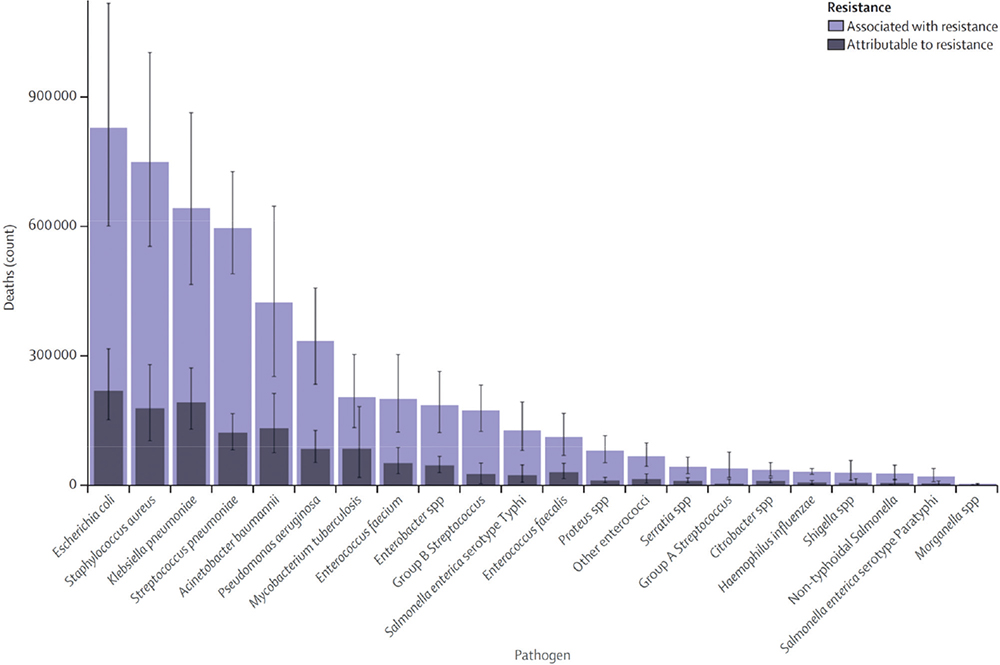
Fig. 1 - Global deaths attributable to or associated with antimicrobial resistance by pathogen in 2019 (6).
Reproduced from The Lancet, 2022;399(10325), Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Pages 629-655, published by Elsevier Ltd as an Open Access article under the CC BY 4.0 license.
| Classification | |
|---|---|
| Uncomplicated UTIs | Acute, sporadic, or recurrent lower (uncomplicated cystitis) and/or upper (uncomplicated pyelonephritis) UTI, limited to nonpregnant women with no known relevant anatomical and functional abnormalities within the urinary tract or comorbidities. |
| Complicated UTIs | All UTIs that are not defined as uncomplicated, meaning in a narrower sense UTIs in a patient with an increased chance of a complicated course, that is, all men, pregnant women, patients with relevant anatomical or functional abnormalities of the urinary tract, indwelling urinary catheters, renal diseases, and/or with other concomitant immunocompromising diseases, for example, diabetes. |
| Recurrent UTIs | Recurrences of uncomplicated and/or complicated UTIs, with a frequency of at least three UTIs/year or two UTIs in the last 6 months. |
| Catheter-associated UTIs | Catheter-associated UTI (CA-UTI) refers to UTIs occurring in a person whose urinary tract is currently catheterized or has had a catheter in place within the past 48 hours. |
| Urosepsis | Urosepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection originating from the urinary tract and/or male genital organs (54). |
UTI = urinary tract infection.
Reproduced from Bonkat G, Bartoletti R, Bruyere F, et al. EAU Guidelines on Urological Infections. Edn. presented at the EAU Annual Congress, Milan, Italy, 2023. ISBN 978-94-92671-19-6 and published by uroweb.org as an Open Access article under the CC BY 4.0 license.
UTI is strongly gender biased, exhibiting one of the most prominent sex disparities among infectious diseases. Premenopausal women are 20-40 times more likely to have a UTI than men of the same age (12-15). Anatomical differences may account for some of this disparity. For instance, men have a longer urethra, and the distance between the anus and urethral opening is shorter in females. However, anatomical differences do not explain why young males and females have similar rates of rUTIs, and the rates of UTIs in males over 65 years increase substantially until they are nearly equivalent to those in older women (12,16). Changes in the incidence of UTIs in older men may, instead, be due to urodynamic changes such as prostatic hypertrophy or other factors, for example, biological or immunological changes occurring over time (16-19). Indeed, the differences in UTI susceptibility by gender are most evident in postpubescent adults younger than 50 years, a time when estrogen and testosterone are at their highest levels in females and males, respectively (12,14).
Zychlinsky Scharff and colleagues hypothesized that sex-based differences in immunity influence response to UTI, which they explored in an animal model designed to bypass anatomical differences, enabling innate and adaptive immune responses in infected female and male mice to be directly compared (19). They identified a cytokine pathway essential for bacterial clearance after infection with uropathogenic E. coli (UPEC), demonstrating that interleukin 17 (IL-17) specifically influenced the innate immune response to bacterial infection in a sex-dependent manner (19). Whether this finding is directly relatable to human infection outcomes remains to be demonstrated. However, it provides a possible explanation for the similarly high frequency of infection in older men and women and the increased severity associated with UTI in men, suggesting hormone-mediated suppression of the innate immune response. This is supported by significant sex differences in the expression of immune-mediating genes induced during UTI, including in the IL-17/IL-33 axis (19). The authors concluded that a better understanding of the immune system’s role in UTIs might contribute to developing nonantibiotic-based stratified therapies targeting sex-specific immune pathways in managing women and men (19).
In conclusion, while multiple factors, including genetics, age, and access to care, contribute to the gender differences in the incidence of UTIs, sex hormones have a powerful influence on the onset, severity, and patient outcomes (12,14).
UTI is a syndrome. The symptoms of urgency, frequency, fever, suprapubic pain, or dysuria, combined with the presence of bacteria in the urine, constitute a UTI. When a patient has symptoms without bacteriuria, other syndromes should be considered, such as overactive bladder (OAB) syndrome or interstitial cystitis/bladder pain syndrome (IC/BPS), which should not routinely be treated with antibiotics (20,21). Point-of-care testing by dipstick may help to clarify a diagnosis, but such analysis has drawbacks, and the characteristic clinical presentation is reliable for the diagnosis. A complete medical history and a short physical examination should still be taken. However, the diagnosis of a first episode of uncomplicated UTI can be symptom based, and there is usually no requirement for further diagnostic workup in women under 40 without other risk factors. Taking a urine culture is not recommended for the first episode but should be considered if presenting symptoms are not characteristic or when there is a failure to respond to antibiotics or the infection recurs within 1 month of antimicrobial therapy (8). A voided urine specimen, collected using a method to minimize contamination, is usually appropriate. An in-and-out catheter specimen is recommended if a voided specimen cannot be obtained. Any gram-negative organism isolated in counts ≥102 colony-forming units (CFU)/mL is considered relevant for this presentation in these patients. Of note, true bacteriuria is considered to be a count >10⁵ CFU/mL, both for pyelonephritis and cystitis, and the cut-off of 105 bacteria/mL remains the benchmark in many laboratories.
Data from international surveillance studies suggest that the most common pathogens associated with uncomplicated UTI are UPEC, which accounted for 76.7% of uncomplicated cystitis, Enterococcus faecalis (4.0%), Staphylococcus saprophyticus (3.6%), Klebsiella pneumoniae (3.5%), Proteus mirabilis (3.5%), and other bacteria (8.7%) (22,23). UPEC is also the most prevalent pathogen identified in complicated cystitis (43%), but to a much smaller extent than in uncomplicated UTI, and many other pathogens, including Klebsiella spp. (13%), Enterococcus spp. (10%), Pseudomonas aeruginosa (9%), Enterobacter spp. (7%), Proteus spp. (6%), S. aureus (3%), fungi (1%), and other bacteria, become important, as does the issue of AMR (22,24).
Treatment strategies
There are a variety of antibiotic strategies and antibiotic-sparing approaches to treating an episode of uncomplicated UTI (Fig. 2A).
A recent systematic review of randomized controlled trials of analgesics (nonsteroidal anti-inflammatory drugs [NSAIDs]/nonsteroidal antirheumatic drugs [NSARs]), herbal formulations, delayed prescription of antibiotics, and placebo therapy to prevent the overuse of antibiotics in women with uncomplicated UTIs found that antibiotic-sparing strategies can reduce antibiotic use by 60-70% (25). However, they may result in higher rates of incomplete recovery or therapy failure compared with immediate antibiotic use, and a higher incidence of secondary outcomes, such as pyelonephritis or febrile UTI (25-27). The use of antibiotic-sparing and other strategies in the treatment and prevention of rUTIs will be developed in the following sections.
The EAU Guidelines for antimicrobial therapy in uncomplicated UTI recommend first-line therapy with fosfomycin trometamol, different formulations of nitrofurantoin, or pivmecillinam. Table II summarizes the regimens and details the doses and duration of therapy. Of note, fluoroquinolone and quinolone antibiotics are no longer included in the treatment of uncomplicated UTIs, having been banned from use by the European Commission from 2019 because of serious disabling and potentially permanent side effects (28).
To guide the choice of antibiotic for uncomplicated UTI, it is important to be aware of the local resistance data. For example, surveillance study conducted in Europe and Brazil in the Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC) study identified E. coli as by far the most frequent uropathogen identified, present in 74.6% of urine cultures, followed by E. faecalis, S. saprophyticus, K. pneumoniae, and P. mirabilis, all at less than 5% and found that, although susceptibility rates varied considerably from country to country, rates for fosfomycin, pivmecillinam, and nitrofurantoin approached 100% overall (98.1%, 95.8%, and 95.2%, respectively) (23). Susceptibility to cotrimoxazole was lower (70.5%), which is why cotrimoxazole is not recommended as a first-line treatment in uncomplicated UTI.
In contrast, a recent study of the prevalence and resistance patterns of uropathogens in different regions of India found that, while E. coli was still the most prevalent pathogen identified (68.3% overall), there was a much higher prevalence of K. pneumoniae (17.7%) than in Europe, and resistance to nitrofurantoin was higher for both pathogens (5.8% and 45.4%, respectively) (29). Of note, none of the UPEC were resistant to fosfomycin. These differences emphasize the importance of local prevalence and resistance patterns to guide the choice of antibiotic.
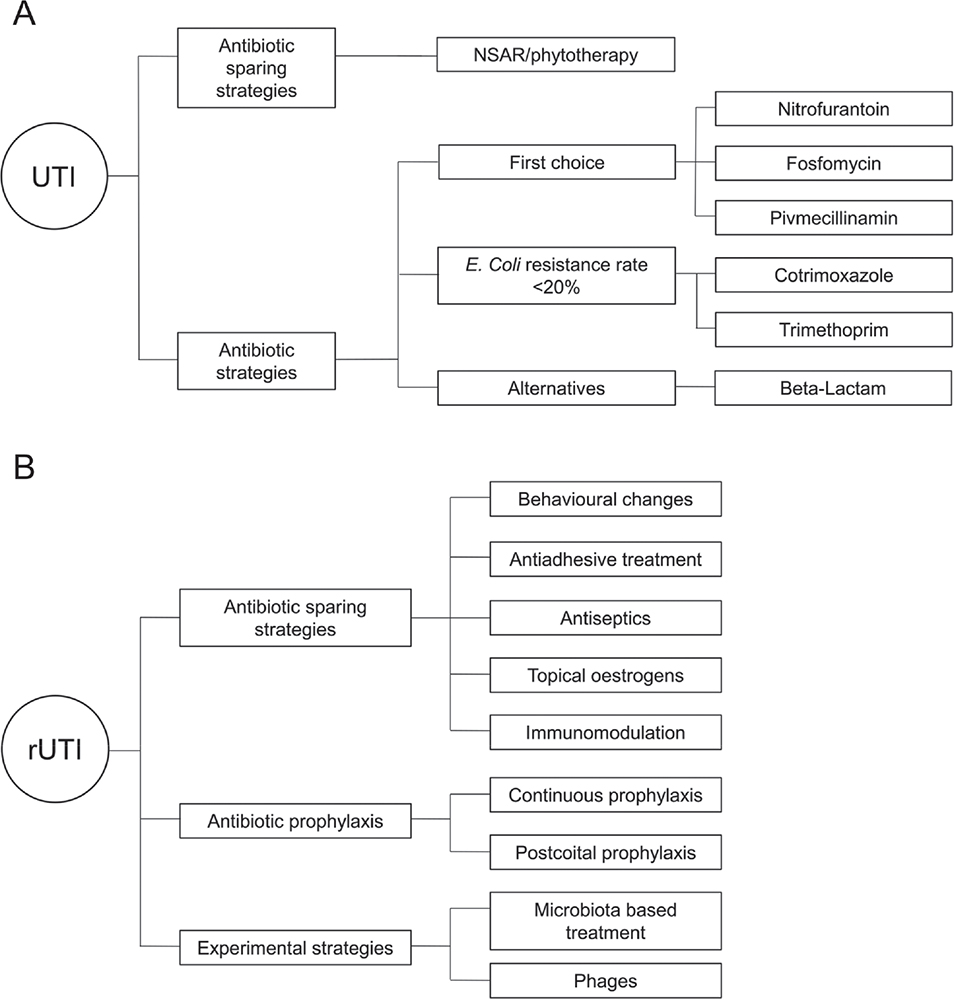
Fig. 2 - Treatment options for A) uncomplicated urinary tract infections (UTIs) and B) recurrent urinary tract infections (rUTIs).
| Antimicrobial | Daily dose | Duration of therapy | Comments |
|---|---|---|---|
| First-line women | |||
| Fosfomycin trometamol | 3 g SD | 1 day | Recommended only in women with uncomplicated cystitis. |
| Nitrofurantoin macrocrystal | 50-100 mg four times a day | 5 days | |
| Nitrofurantoin monohydrate/macrocrystals | 100 mg b.i.d. | 5 days | |
| Nitrofurantoin macrocrystal prolonged release | 100 mg b.i.d. | 5 days | |
| Pivmecillinam | 400 mg t.i.d. | 3-5 days | |
| Alternatives | |||
| Cephalosporins (e.g., cefadroxil) | 500 mg b.i.d. | 3 days | Or comparable |
| If the local resistance pattern for Escherichia coli is <20% | |||
| Trimethoprim | 200 mg b.i.d. | 5 days | Not in the first trimester of pregnancy |
| Trimethoprim-sulfamethoxazole | 160/800 mg b.i.d. | 3 days | Not in the last trimester of pregnancy |
| Treatment in men | |||
| Trimethoprim-sulfamethoxazole | 160/800 mg b.i.d. | 7 days | Restricted to men. Fluoroquinolones can also be prescribed in accordance with local susceptibility testing. |
b.i.d. = twice daily; SD = single dose; t.i.d. = three times daily.
Reproduced from Bonkat G, Bartoletti R, Bruyere F, et al. EAU Guidelines on Urological Infections. Edn. presented at the EAU Annual Congress, Milan, Italy, 2023. ISBN 978-94-92671-19-6 and published by uroweb.org as an Open Access article under the CC BY 4.0 license.
Prevention of recurrent cystitis: current state of the art
As noted, an rUTI is defined as an UTI occurring at least three times a year or twice in the previous 6 months. However, in real-world practice, only about 20% of episodes occur twice in 6 months or three times a year; in a recent European study (GESPRIT), 47.4% of women had at least six UTIs per year, and 14.4% had over 12 infections per year (30). In fact, before preventive measures were started, almost a third of women experienced at least ten episodes per year. The health and economic burden caused by rUTIs is significant; rUTIs impact patients’ daily activities and mental health, leading to high levels of frustration, anger, and dissatisfaction (30). Women may need bed rest, be absent from work, and require doctor visits and prescriptions for antibiotics.
There are many strategies for preventing the recurrence of UTIs, which can be summarized as antibiotic-sparing strategies, antibiotic prophylaxis, and experimental strategies (Fig. 2B).
Non-antimicrobial prophylaxis
In following a step-by-step approach to prophylaxis for rUTIs, avoidance of risk factors and behavioral modifications (increasing fluid intake, pre- or postcoital urination, and hygiene procedures) can be considered the first step. However, evidence for their benefits in reducing episodes of rUTI is limited. A recent study showed that increasing the daily water intake by about 1.5 L reduced cystitis episodes and antibiotic usage in premenopausal women with rUTIs (31). During 12 months of follow-up, the mean number of cystitis episodes in the 70 women randomized to the increased water intake group was 1.7 (95% confidence interval [CI], 1.5 to 1.8) vs 3.2 (95% CI, 3.0 to 3.4) in the 70 women in the control group who did not increase their usual water intake, a difference in means of 1.5 (95% CI, 1.2 to 1.8; p < 0.001). Over that time, the mean number of antibiotic regimens required to treat cystitis episodes was 1.9 vs 3.6, respectively (p < 0.001) (31).
Non-antimicrobial strategies avoid the risk of AMR and are the next step to consider before antimicrobial therapy is used. These strategies include hormonal replacement therapy, immunoactive prophylaxis, probiotics, cranberry, D-mannose, methenamine hippurate, and intravesical instillations of GAGs (8,32-34).
Hormonal replacement using topical (but not oral) estrogen therapy in postmenopausal women, intended to enhance vaginal and urethral flora, has been shown to have some beneficial effects in reducing rUTIs without systematic side effects, although local irritation and minor bleeding can occur (8,34-36). Topical estrogen administration by vaginal cream or pessary is more effective than placebo but less effective than antimicrobial prophylaxis.
A number of different immune cells are present in the bladder, including macrophages, dendritic cells, lymphocytes, monocytes, neutrophils, and eosinophils, suggesting a role for prophylaxis with immunoactive agents in UTIs (8,34). Immunoactive agents are understood to stimulate both the innate and adaptive immune systems to increase the production of bacteria-specific antibodies. OM-89 (Uro-Vaxom) is perhaps the most widely studied immunostimulant, consisting of an extract for oral administration of 18 strains of heat-killed UPEC. OM-89-S is a newer formulation prepared by a different lysis process. OM-89 (but not OM-89S) has shown significant efficacy in the prophylaxis of rUTIs and is included in the EAU guidelines as an option with a high level of evidence for preventing rUTI in women (8,33,34,37,38). A systematic review of the role of vaccines in the treatment of rUTIs concluded that OM-89 (usually at a dose of one oral tablet once daily for 3 months, and/or a booster of one tablet daily for the first 10 days of months 6-9) had a short-term role in preventing rUTIs, significantly reducing the risk of recurrence compared with placebo, with only mild side effects (33). However, vaccination therapies remain under-reviewed, and further research is needed.
When the balanced ecosystem of normal flora in the vagina is disturbed through repeated antibiotic use, spermicide use, or in postmenopausal women, the vagina may become a reservoir for uropathogenic bacteria (12,34,39). Probiotics may help to prevent rUTIs through many mechanisms, including restoration of the natural vaginal microbiota by increasing the level of protective Lactobacillus species, altering the pH of the vagina, inhibiting bacterial biofilm formation, downregulating inflammatory cytokines, and inhibiting the uptake of E. coli by vaginal epithelial receptors. While there are some contradictory findings, oral or vaginal administration of the lactobacillus strains L. rhamnosus GR-1, L. reuteri B-54 and RC-14, L. casei shirota, or L. crispatus CTV-05 have been shown to be effective for vaginal flora restoration and a beneficial effect in preventing rUTIs (8,12,34,36,39,40). However, the level of evidence is insufficient to allow definitive recommendations on the route of admission, optimal dosage, or treatment duration (8,35).
A number of meta-analyses and systematic reviews that have investigated the efficacy of cranberry-containing products have found a favorable benefit-to-harm ratio but limited evidence for their efficacy in protection against UTIs. However, a recent Cochrane Database systematic review of 50 randomized or quasi-randomized controlled trials (8,857 patients) of cranberry products vs placebo, no specific treatment, or other interventions for the prevention of UTIs supports their use to reduce the risk of symptomatic, culture-verified UTIs in women with rUTIs, children, and people susceptible to UTIs following interventions (41). Currently, the level of evidence for cranberry products compared to antibiotics or probiotics alone is very low, and the available evidence does not support their use in older patients, patients with bladder emptying problems, or pregnant women.
The monosaccharide isomer of glucose, D-mannose, is thought to act in the bladder by inhibiting the adhesion of UPEC to uroepithelial cells (34,36). Again, the overall level of evidence for efficacy in the prevention of rUTI is weak and contradictory, and, as with cranberry products, a good patient history to identify what has already been tried and to determine what triggers the UTI should first be taken. The patient should be informed that further studies are needed before D-mannose can be fully recommended (8). A recent Cochrane systematic review of randomized controlled trials also concluded that there is currently little to no evidence for or against D-mannose use in preventing rUTIs (42).
The urinary antiseptic methenamine hippurate (hexamine hippurate) has bacteriostatic activity via the production of formaldehyde from hexamine in an acid environment (34,36). The most recent Cochrane review of methenamine hippurate for preventing UTIs dates back to 2012 when it was concluded that methenamine hippurate might be effective short-term prophylaxis in patients without renal tract abnormalities (43). There was a 76% reduction in the incidence of UTIs (risk ratio 0.24; 95% CI, 0.07 to 0.89), comparable to antibiotic prophylaxis, but the quality of studies included in the analysis was mixed, and the data were heterogeneous. More recent studies have shown that long-term (6-12 months) methenamine hippurate is not inferior to daily low-dose antibiotics to prevent rUTI (26,44). In a study conducted at eight centers in the United Kingdom, 240 women with rUTIs were randomized to methenamine hippurate twice daily (n = 120) or daily low-dose antibiotics (nitrofurantoin, trimethoprim, or cefalexin; n = 120) for 12 months (26). During the 12-month treatment period, the incidence of UTIs requiring antibiotic treatment was 1.38 (95% CI, 1.05 to 1.72) episodes per person-year in the methenamine hippurate group and 0.89 (95% CI, 0.65 to 1.12) in the antibiotics group. The absolute difference of 0.49 (90% CI, 0.15 to 0.84) indicated noninferiority between the two strategies. Therefore, the current recommendations support using methenamine hippurate to prevent rUTIs in women without urinary tract abnormalities (8).
As disruption of the bladder lining (urothelium) and resultant loss of the protective GAG layer is considered to be a key factor promoting rUTIs, GAG therapy with intravesical instillations of hyaluronic acid (HA) or HA in combination with chondroitin sulfate (CS) has been suggested as an option to prevent rUTIs, particularly in patients where less invasive preventive approaches have been unsuccessful (8). The quality of evidence for the benefits of combination therapy is highest, as randomized controlled trials of the instillation of single-agent HA or CS are lacking. In a randomized trial in 57 women with rUTI, intravesical administration of 50 mL of HA 1.6% plus CS 2.0% (Ialuril®, IBSA) for 6 months, the decrease in mean UTI rate per patient-year at the end of the 12-month study was 86.6% ± 47.6 compared with 9.6% ± 24.6 for placebo (intravesical saline), a mean difference of 77% (95% CI, 72.3 to 80.8, p = 0.0002) (45). The mean time to UTI recurrence was also significantly shorter in the placebo group. Given the issue of antibiotic resistance, the instillation of GAGs may become a recommended prophylactic option to antibiotic prophylaxis when more data are available.
Another, retrospective, case-control study in 276 women treated for rUTIs at seven European centers compared intravesical administration of combination HA+CS with EAU-recommended standard of care (continuous or postcoital antimicrobial prophylaxis, immunoactive prophylaxis, prophylaxis with probiotics or cranberry, or a combination of these) (46). In this real-world setting, bladder instillation of combined HA and CS reduced the risk of UTI recurrences, compared with standard management (55.7% vs 62.1%, respectively), although the difference did not reach statistical significance (p = 0.313). However, when the adjusted odds ratio (OR) for developing a bacteriologically confirmed rUTI within 12 months was calculated, there was a 49% reduced risk of developing a recurrence in women treated with HA+CS compared with standard care (OR 0.51, 95% CI, 0.27 to 0.96) (46). Treatment adherence (≥5 instillations) was associated with improved benefits of HA+CS therapy.
Data from a systematic review and meta-analysis of randomized and nonrandomized trials found that HA plus CS decreased the rate of rUTI per patient-year by a pooled mean difference of 2.56 compared with controls (95% CI, 3.86, −1.26, p < 0.001) (47). The time to a first recurrence of a UTI was also increased by a mean of 130.05 days. However, patients should be informed that further studies are needed to confirm the results of existing trials (8). An oral formulation of HA, CS, quercetin, and curcumin (Ialuril® Soft Gels, IBSA) is available and may be appropriate in patients with pelvic pain, although hard data on usefulness in the prevention of rUTIs are limited.
Many other non-antimicrobial options have been investigated or are already in use as strategies to prevent rUTIs. A detailed review of these approaches is beyond the scope of this article. However, a comprehensive review by Paul Loubet and colleagues provides a useful overview of alternative therapeutic options to antibiotics (40). Among the approaches are small compounds, often molecules that are by-products of or mimic bacterial substrates, designed to occupy binding sites to prevent adherence of pathogens to the uroepithelium, directly target the protective capsule of bacteria, inhibit enzymes essential to UPEC, or reduce biofilm formation (40). The formation of biofilms, either directly on the urothelial surface or formed around indwelling catheters, is a common strategy adopted by UPEC, providing a favorable environment for bacterial colonization and a persistent source for bacterial access to the urinary tract, thus facilitating rUTIs (16,22). Vitamin C, Chinese medical herbs, and various vaccine approaches that target microbial adhesion, the bacterial capsule, toxins secreted by UPEC that act as virulence factors, or iron metabolism processes essential for bacterial growth and colonization are also alternative nonantibiotic therapeutic options for UTIs (34,40). Finally, although not commonly utilized in the West, bacteriophages, viruses that cause lysis of bacterial cells, including antibiotic-resistant UPEC, have been used in eastern European countries for decades (36).
Antibiotic prophylaxis
When non-antimicrobial interventions have failed, continuous low-dose antimicrobials and postcoital antimicrobial prophylaxis have been shown to reduce the rate of rUTIs (8,36) and are recommended for use (8). There is no significant difference between the two strategies. In both approaches, patients should be counseled on possible side effects. The optimal duration of continuous antimicrobial prophylaxis is uncertain, with studies reporting durations of from 3 to as long as 12 months; between 3 and 6 months is typical (8).
Intermittent self-start treatment (self-diagnosis and self-treatment) with a short course of antibiotic therapy is also effective and safe in women with rUTIs who have shown good compliance and motivation and can be an economical approach to preventing rUTIs (8,36).
The choice of antimicrobial agent is the same as that for acute uncomplicated UTI and should be based on local resistance patterns. Patients on antimicrobial prophylaxis may have been advised to increase the dose of the same antibiotic if they have a breakthrough infection. However, a more appropriate course of action would be for a culture to be taken to determine if switching to an antibiotic more helpful in the setting is indicated. If the patient has severe symptoms and wishes to start empirical treatment immediately, the recommended first-line antibiotics (i.e., fosfomycin trometamol, nitrofurantoin, or pivmecillinam) should be chosen (8).
Managing patients with UTIs in daily practice: the nurse’s perspective
Managing UTIs is a major problem for patients, caregivers, and the physicians and specialist nurses involved in daily clinical practice. As well as the economic cost, UTIs impose a considerable loss of quality of life for the patient (1,2,30,48).
Increasingly, public health policy is to keep older people in their homes for as long as possible, promoting self-care for everyone and providing home care services where necessary. For patients with UTIs, there is likely to be a preference for self-instilling bladder compounds at home, and visiting specialist nurses will teach self-catheterization and self-instillation procedures. A specialized urological team that can provide almost all urological care in the home, including managing UTIs, is effective in freeing up hospital care. In the absence of specialized in-home care, solutions must be found in the outpatient setting.
Ideally, seeing patients in their homes provides an insight into the most helpful approach to patient needs. UTIs often contribute to delirium, falls, and confusion in vulnerable older patients, even before a UTI is diagnosed (49,50). Therefore, prevention is the best solution; the challenge is achieving this. Close cooperation involving the nurse, physician, patient, caregivers, and even neighbors is fundamental. Knowledge and understanding of the relevant clinical guidelines for urological infections should be shared, and agreement on the relative roles and time points for interventions should be established. An open and proactive multidisciplinary approach should be adopted, sharing knowledge about patients and bringing the individual members’ experience and expertise to bear on the issue.
The patient and caregivers will benefit from being educated by the urologic nurse on prevention strategies. These include drinking enough water, following an adequate diet (e.g., limiting the amount of sugar, a substrate for bacteria, in the diet for diabetic patients), good hygiene procedures daily and after sex, solving any issues of incontinence as best as possible, encouraging patients to keep active and moving, and, where appropriate, good catheter management (8,30,51,52). This can be a big challenge with elderly and vulnerable people, particularly where dementia, depression, refusal of care, or living alone without support are issues. The problem can be different in every situation, and if a meaningful difference is to be achieved, it is essential to know what is acceptable to the patient and their level of compliance. Searching to find the best solution should always be the goal.
In daily practice, a cautious approach to antibiotics is appropriate, and their use is reserved only in case of infection after performing the necessary analysis. Antibiotics may initially be used if cystoscopy imaging shows the bladder to be very infected, with alternative treatments to antibiotic prophylaxis considered at a later time where appropriate, perhaps 3-4 months after initial antibiotic therapy. Despite reservations about its effectiveness, D-mannose may be appropriate to help reduce rUTI episodes in some patients, topical estrogens can be beneficial in postmenopausal women, and probiotics or GAG therapy may also be considered to help prevent rUTIs (8). If intravesical instillation of GAGs is applicable, high-dose HA and CS are preferred (8), and training on self-instillation should be given. Seven instillations are typically used, the first four at weekly intervals, then two at 2 weeks apart, and one a month later. However, if patients find pain relief from the treatment, it may be extended empirically in consultation with their needs.
A number of issues should be addressed to improve catheter management in rUTIs. It should be considered if catheterization is necessary, the risk of potential infections, how accepting the patient is of the procedure, issues of pain or blocking of catheter insertion, and the choice of catheterization methods. In all instances, the aim should be to find the best solution for the individual patient. In seeking alternative methods to standard catheterization, the Ialuadapter® device has been developed for instillation into the bladder (Fig. 3). It can be attached to Luer Lock or Luer Slip syringes and offers an efficient method of administering medicine into the bladder without needing a catheter. The adapter tip can be guided into the urethral orifice of female and male patients with minimal risk of pain or other complications (53).
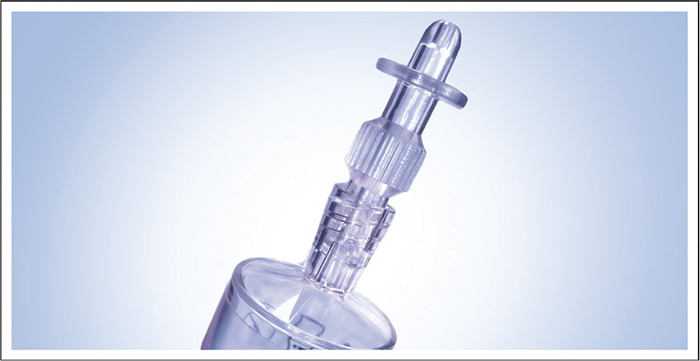
Fig. 3 - The Ialuadapter® device for use with a Luer Lock or Luer Slip syringe as an alternative to intravesical instillation by standard catheterization. Figure the property of Institut Biochimique SA (IBSA).
After an initial learning curve involving some practice with the Ialuadapter®, the use of the device for the instillation of HA and CS is a pain-free alternative to standard catheterization in both men and women, associated with fewer infections while providing simultaneous treatment of the bladder and the urethra. The method is also more effective and gives faster results in patients with bladder pain and urethritis. Although a residual urine volume of 50 mL is acceptable, the patient should ideally have an empty bladder before use.
| Parameter | N (%) |
|---|---|
| Gender | |
| Female | 78 (97.5) |
| Male | 2 (2.5) |
| Age of participants | |
| ≥50 years | 50 (62.5) |
| <50 years | 30 (37.5) |
| Urological indication | |
| BPS/IC | 64 (80.2) |
| rUTIs | 14 (17.4) |
| OAB syndrome | 2 (2.3) |
BPS/IC = bladder pain syndrome/interstitial cystitis; OAB = overactive bladder; rUTI = recurrent urinary tract infection.
In a 6-month study in the Andros Clinic in Rijswijk, around 170 instillations were performed with the device in 80 patients, predominantly in women with BPS/IC (80.2%), although 17.4% of patients had rUTIs and 2.3% OAB syndrome (Tab. III) (R Pothoven, data on file). The majority (62.5%) of patients were aged over 50, and only two men were included in the study.
The main reason patients tried the Ialuadapter® as an alternative to standard catheterization was an expectation of experiencing less pain during the procedure (40.8% of patients) (Fig. 4). Ease of use and anticipation of fewer infections were each given as reasons by around a quarter of patients, while 1.9% of patients had a fear of standard catheterization. Other reasons (fewer complications, less pain after instillation, more comfortable, lower volume of fluid to be given, and greater effect) were expressed by 5.8% of patients.
Eighty-two percent of patients over 50 and 63.3% of those under 50 wanted to continue using the Ialuadapter® (Fig. 5), mainly because of less pain (50.1% of patients overall). Other reasons were that the device was easier to use than a catheter (20.9%) and could be done at home (10.5%).
It was thought that a greater proportion of the younger patients were too tense and less able to relax than those over 50, leading to a higher rate of leakage after instillation, probably contributing to the lower percentage of patients under 50 wishing to continue to use the device. Of the 59 patients who continued using the Ialuadapter®, none experienced an rUTI. Research is ongoing to identify the patients best suited for instillation therapy.
Discussion and conclusions
UTIs impose a sizable personal and economic burden on individuals and health resources worldwide (1-4), and effectively managing UTIs is a major challenge faced not only by physicians and specialist nurses in their daily clinical practices but also by patients and their caregivers in the community.
While antibiotic treatment of symptoms suggestive of UTIs and rUTIs has clear benefits to patients, overuse and inappropriate use have led to a worldwide problem of AMR that has become an increasing threat to public health.
Programs of antimicrobial stewardship that aim to optimize clinical outcomes while reducing inappropriate antibiotic use have been developed to provide guidance encouraging prudent and appropriate antibiotic prescribing that minimizes the unintended consequences of antibiotic overuse, including antibiotic-related adverse events and the emergence of resistant bacterial strains (5,8). When antibiotic therapy is indicated for treating UTIs or rUTIs, choosing an appropriate antibiotic based on local resistance and susceptibility patterns is essential, guided by evidence-based clinical guidelines such as those compiled by the EAU, which recommends first-line therapy with fosfomycin trometamol, nitrofurantoin, or pivmecillinam as first-line antimicrobial therapy for uncomplicated UTIs (8).
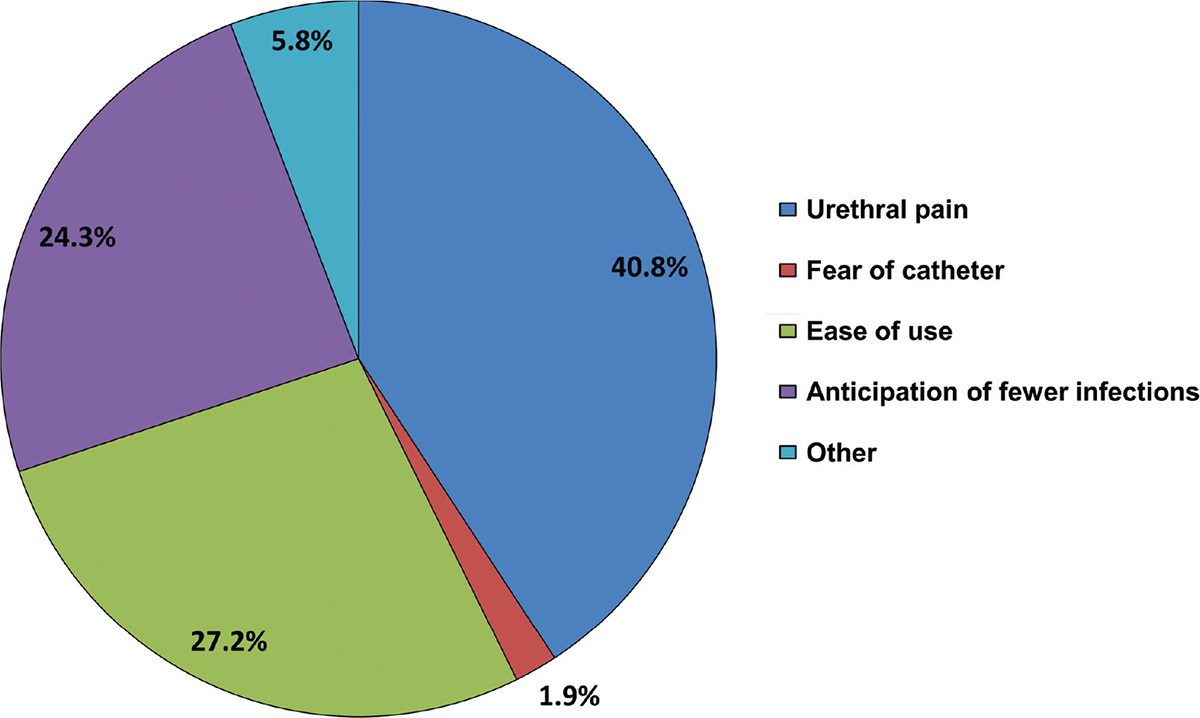
Fig. 4 - Reasons for trying the Ialuadapter® as an alternative to standard catheterization (n = 80). Data the property of the author.
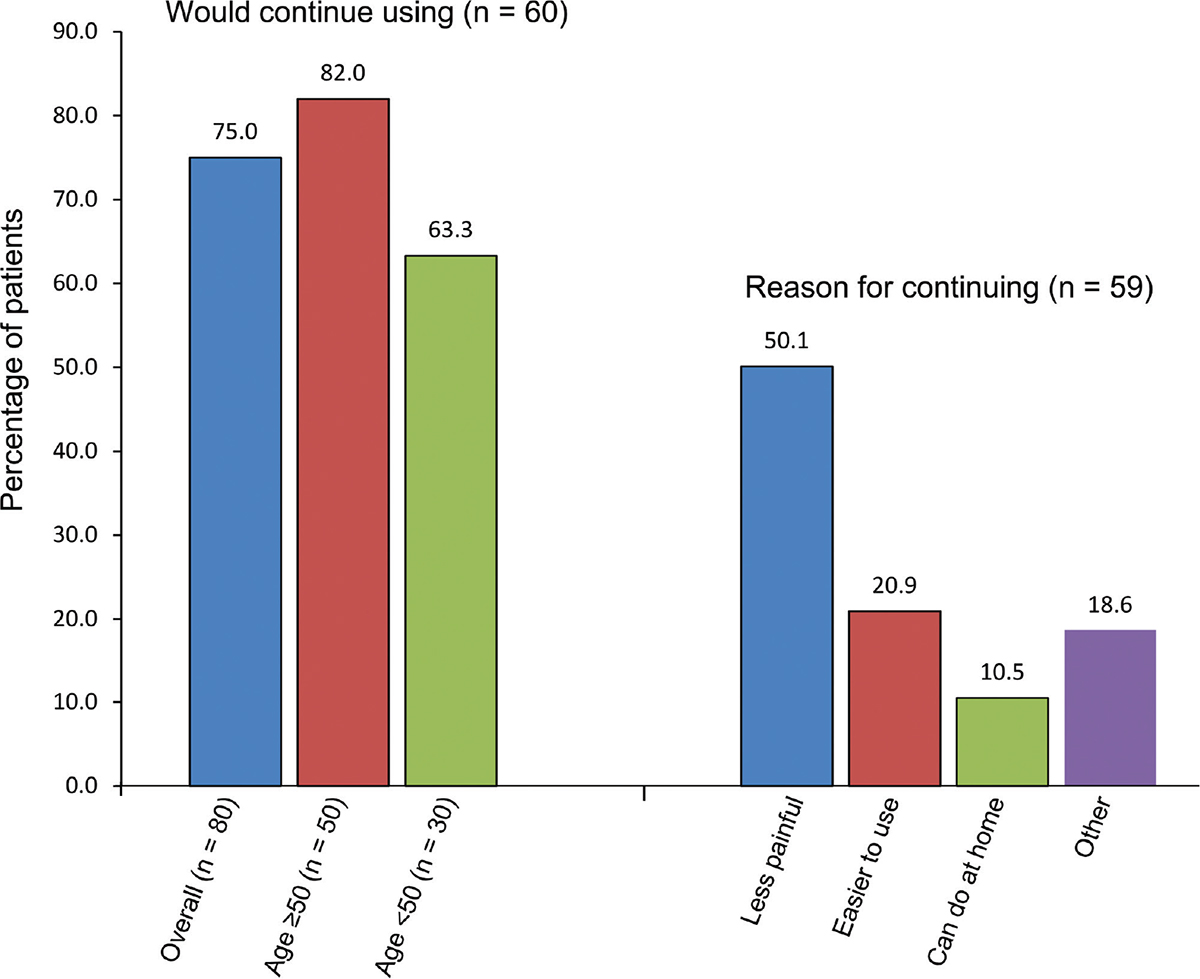
Fig. 5 - Patient feedback on using the Ialuadapter® device. Data the property of the author.
Appropriate non-antimicrobial strategies aimed at reducing the incidence of rUTIs should also be considered. Indeed, the increasing global burden of drug-resistant pathogens makes identifying effective non-antimicrobial strategies to reduce the burden of bacterial AMR an urgent priority (6). Strategies are available to reduce the dependence on antimicrobial use in uncomplicated UTIs, including methenamine hippurate, cranberry extract, D-mannose, probiotics, intravesical GAG therapy, and prophylactic vaccination.
Regardless of whether an antimicrobial or non-antimicrobial approach is taken, counseling patients about avoidance of risk factors and behavioral modifications can be a first step toward prophylaxis of rUTIs. Furthermore, the use of non-antimicrobial strategies should be based on physician expertise supported by clinical evidence, as reducing the overall use of antibiotics should not be at the expense of compromising clinical outcomes.
Further well-designed studies evaluating non-antimicrobial prophylaxis of rUTIs is needed to extend the level of evidence and fully define the place of non-antimicrobial strategies in routine clinical practice. Also important is the need to identify which patients might benefit most from non-antimicrobial treatment for acute UTIs or non-antimicrobial prophylaxis for rUTIs.
Acknowledgments
The authors thank Ray Hill, an independent medical writer who provided technical writing support funded by IBSA Institut Biochimique SA (Lugano, Switzerland).
Disclosures
Conflict of interest: The author has participated in workshops sponsored by IBSA and Goodlife Nederland.
Funding/support and role of the sponsor: The publication of this article was supported by an unconditional grant from IBSA.
References
- 1. Yang X, Chen H, Zheng Y, Qu S, Wang H, Yi F. Disease burden and long-term trends of urinary tract infections: a worldwide report. Front Public Health. 2022;10:888205. CrossRef PubMed
- 2. Zeng Z, Zhan J, Zhang K, Chen H, Cheng S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J Urol. 2022;40(3):755-763. CrossRef PubMed
- 3. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1223-1249. CrossRef PubMed
- 4. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1204-1222. CrossRef PubMed
- 5. Department of Health and Social Care (DHSC). UK antimicrobial resistance strategy and action plan. 2000. Online (Accessed March 2023).
- 6. Murray CJL, Ikuta KS, Sharara F, et al; Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. CrossRef PubMed
- 7. O’Neill J. Tackling drug-resistant infections globally: Final report and recommendations. Online (Accessed March 2023).
- 8. Bonkat G, Bartoletti R, Bruyere F, et al. EAU Guidelines on Urological Infections. Edn. presented at the EAU Annual Congress, Milan, Italy, 2023. ISBN 978-94-92671-19-6. Online (Accessed March 2023).
- 9. GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022 Dec 17;400(10369):2221-2248. CrossRef PubMed
- 10. Johansen TE, Botto H, Cek M, et al. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents. 2011;38(suppl):64-70. CrossRef PubMed
- 11. Köves B, Cai T, Veeratterapillay R, et al. Benefits and harms of treatment of asymptomatic bacteriuria: a systematic review and meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur Urol. 2017;72(6):865-868. CrossRef PubMed
- 12. Deltourbe L, Lacerda Mariano L, Hreha TN, Hunstad DA, Ingersoll MA. The impact of biological sex on diseases of the urinary tract. Mucosal Immunol. 2022;15(5):857-866. CrossRef PubMed
- 13. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653-660. CrossRef PubMed
- 14. Ingersoll MA. Sex differences shape the response to infectious diseases. PLoS Pathog. 2017;13(12):e1006688. CrossRef PubMed
- 15. Lipsky BA. Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med. 1989;110(2):138-150. CrossRef PubMed
- 16. Zeng G, Zhu W, Lam W, Bayramgil A. Treatment of urinary tract infections in the old and fragile. World J Urol. 2020;38(11):2709-2720. CrossRef PubMed
- 17. Dias SP, Brouwer MC, van de Beek D. Sex and gender differences in bacterial infections. Infect Immun. 2022;90(10):e0028322. CrossRef PubMed
- 18. Ligon MM, Joshi CS, Fashemi BE, Salazar AM, Mysorekar IU. Effects of aging on urinary tract epithelial homeostasis and immunity. Dev Biol. 2023;493:29-39. CrossRef PubMed
- 19. Zychlinsky Scharff A, Rousseau M, Lacerda Mariano L, et al. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight. 2019;5(13):e122998. PMID:31145099 CrossRef PubMed
- 20. Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2022;208(1):34-42. CrossRef PubMed
- 21. Nambiar AK, Arlandis S, Bø K, et al. European Association of Urology Guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur Urol. 2022;82(1):49-59. CrossRef PubMed
- 22. Wagenlehner FME, Bjerklund Johansen TE, Cai T, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17(10):586-600. CrossRef PubMed
- 23. Naber KG, Schito G, Botto H, Palou J, Mazzei T. Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008;54(5):1164-1175. CrossRef PubMed
- 24. Tandoğdu Z, Bartoletti R, Cai T, et al. Antimicrobial resistance in urosepsis: outcomes from the multinational, multicenter global prevalence of infections in urology (GPIU) study 2003-2013. World J Urol. 2016;34(8):1193-1200. CrossRef PubMed
- 25. Kaußner Y, Röver C, Heinz J, et al. Reducing antibiotic use in uncomplicated urinary tract infections in adult women: a systematic review and individual participant data meta-analysis. Clin Microbiol Infect. 2022;28(12):1558-1566. CrossRef PubMed
- 26. Harding C, Mossop H, Homer T, et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, non-inferiority trial. BMJ. 2022;376:e068229. CrossRef PubMed
- 27. Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015;351:h6544. CrossRef PubMed
- 28. European Medicines Agency (EMA). Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. EMA/795349/2018. 2018. Online. Accessed March 2023.
- 29. Mohapatra S, Panigrahy R, Tak V, et al. Prevalence and resistance pattern of uropathogens from community settings of different regions: an experience from India. Access Microbiol. 2022;4(2):000321. CrossRef PubMed
- 30. Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):107-117. CrossRef PubMed
- 31. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178(11):1509-1515. CrossRef PubMed
- 32. Kyriakides R, Jones P, Somani BK. Role of D-mannose in the prevention of recurrent urinary tract infections: evidence from a systematic review of the literature. Eur Urol Focus. 2021;7(5):1166-1169. CrossRef PubMed
- 33. Prattley S, Geraghty R, Moore M, Somani BK. Role of vaccines for recurrent urinary tract infections: A systematic review. Eur Urol Focus. 2020;6(3):593-604. CrossRef PubMed
- 34. Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15(12):750-776. CrossRef PubMed
- 35. Anger JT, Bixler BR, Holmes RS, Lee UJ, Santiago-Lastra Y, Selph SS. Updates to recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU Guideline. J Urol. 2022;208(3):536-541. CrossRef PubMed
- 36. Moussa M, Abou Chakra M, Dellis A, Moussa Y, Papatsoris A. Pharmacotherapeutic advances for recurrent urinary tract infections in women. Expert Opin Pharmacother. 2020;21(16):2011-2026. CrossRef PubMed
- 37. Wagenlehner FM, Ballarini S, Pilatz A, Weidner W, Lehr L, Naber KG. A randomized, double-blind, parallel-group, multicenter clinical study of Escherichia coli-lyophilized lysate for the prophylaxis of recurrent uncomplicated urinary tract infections. Urol Int. 2015;95(2):167-176. CrossRef PubMed
- 38. Vallée M, Bruyère F. Non-antimicrobial prophylactic measures in recurrent urinary tract infections. In: Bjerklund Johansen TEWF, Matsumoto T, Cho YH, Krieger JN, Shoskes D, Naber KG, eds. Urogenital infections and inflammations. German Medical Science GMS; 2022, Version 2022-02-03. Downloaded March 22, 2023, from Online.
- 39. Wawrysiuk S, Naber K, Rechberger T, Miotla P. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance-non-antibiotic approaches: a systemic review. Arch Gynecol Obstet. 2019;300(4):821-828. CrossRef PubMed
- 40. Loubet P, Ranfaing J, Dinh A, et al. Alternative therapeutic options to antibiotics for the treatment of urinary tract infections. Front Microbiol. 2020;11:1509. PMID:32719668 CrossRef PubMed
- 41. Williams G, Hahn D, Stephens JH, Craig JC, Hodson EM. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2023;4(4):CD001321. PubMed
- 42. Cooper TE, Teng C, Howell M, Teixeira-Pinto A, Jaure A, Wong G. D-mannose for preventing and treating urinary tract infections. Cochrane Database Syst Rev. 2022;8(8):CD013608. PubMed
- 43. Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10(10):CD003265. CrossRef PubMed
- 44. Botros C, Lozo S, Iyer S, et al. Methenamine hippurate compared with trimethoprim for the prevention of recurrent urinary tract infections: a randomized clinical trial. Int Urogynecol J. 2022;33(3):571-580. CrossRef PubMed
- 45. Damiano R, Quarto G, Bava I, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol. 2011;59(4):645-651. CrossRef PubMed
- 46. Ciani O, Arendsen E, Romancik M, et al. Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case-control study. BMJ Open. 2016;6(3):e009669. CrossRef PubMed
- 47. Goddard JC, Janssen DAW. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: systematic review and meta-analysis. Int Urogynecol J. 2018;29(7):933-942. CrossRef PubMed
- 48. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:1756287219832172. CrossRef PubMed
- 49. Krinitski D, Kasina R, Klöppel S, Lenouvel E. Associations of delirium with urinary tract infections and asymptomatic bacteriuria in adults aged 65 and older: A systematic review and meta-analysis. J Am Geriatr Soc. 2021;69(11):3312-3323. CrossRef PubMed
- 50. Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, Van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc. 2009;57(6):963-970. CrossRef PubMed
- 51. Kwok M, McGeorge S, Mayer-Coverdale J, et al. Guideline of guidelines: management of recurrent urinary tract infections in women. BJU Int. 2022;130(suppl 3):11-22. CrossRef PubMed
- 52. Pigrau C, Escolà-Vergé L. Recurrent urinary tract infections: from pathogenesis to prevention. Med Clin (Barc). 2020;155(4):171-177. CrossRef PubMed
- 53. Lovasz S. Minimally invasive device for intravesical instillation by urological syringe adapter (MID-ii U.S.A.) for catheter-free instillation therapy of the bladder in interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26(suppl 1):57-60. CrossRef PubMed
- 54. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. CrossRef PubMed