 |
Drug Target Insights 2024; 18: 1-3 ISSN 1177-3928 | DOI: 10.33393/dti.2024.2670 ORIGINAL RESEARCH ARTICLE |
 |
Long-term response with the atypical reaction to nivolumab in microsatellite stability metastatic colorectal cancer: A case report
ABSTRACT
Immunotherapy has become an integral part of a comprehensive treatment approach to metastatic colorectal cancer (mCRC). Nivolumab (Opdivo) is a human immunoglobulin G4 monoclonal antibody that blocks the interaction between the programmed cell death 1 (PD-1) receptor and its ligands 1/2 (PD-L1/PD-L2), leading to inhibition of T-cell proliferation, cytokine secretion, and enhanced immune response. The US Food and Drug Administration (FDA) has approved this drug for use in high microsatellite instability (MSI-high)/deficiencies in mismatch repair (dMMR) advanced CRC patients. However, its efficacy is extremely limited in microsatellite stability (MSS)/mismatch repair proficient (pMMR) patients. We report a case of a 42-year-old man diagnosed with MSS/pMMR mCRC who has achieved a durable response to nivolumab after a progression under chemotherapy with antiangiogenic treatment. We observed for the first time an atypical response after 8 months of nivolumab treatment, with the regression of previous primary pulmonary lesions and the presence of new para-aortic lymph node lesions. This report demonstrates that a subset of pretreated mCRC patients with the MSS/pMMR phenotype may benefit from nivolumab and these patients need more attention.
Keywords: Dissociated response, iRECIST, Metastatic colorectal cancer, Microsatellite stability, Nivolumab
Received: July 19, 2023
Accepted: January 8, 2024
Published online: January 23, 2024
Corresponding author:
Nataliya Babyshkina
nbabyshkina@mail.ru
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Background
Immunotherapy has become an integral part of a comprehensive treatment approach to metastatic colorectal cancer (mCRC). Nivolumab, one of the first immune checkpoint inhibitors, was approved by the US Food and Drug Administration (FDA) in 2017 for use in patients whose tumors harbor deficient mismatch repair (dMMR), or high microsatellite instability (MSI-high) and have progressed after conventional chemotherapy. The nivolumab action is directed at the programmed cell death 1 (PD-1) receptor, a member of the CD28 superfamily, which is expressed on the surface of activated T and B lymphocytes. Activation of PD-1/programmed cell death ligands 1/2 (PD-L1/PD-L2) on the tumor and the tumor microenvironment leads to enhanced immunosuppressive effects (1). Nivolumab binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, inhibiting the PD-1-mediated immune response.
Nivolumab approval was based on data from CheckMate 142 multicenter phase 2 study investigating its efficacy and safety in the cohort of MSI-high/dMMR mCRC patients who had progressed during or after prior treatment with a fluoropyrimidine-, oxaliplatin-, or irinotecan-based chemotherapy. In this study, 68.9% of patients responded to treatment, with 31.1% achieving an objective response rate (2). Further studies have confirmed that nivolumab provides a long-term overall survival in MSI-high/dMMR mCRC (3). Recent clinical trials data suggest that the combination of nivolumab with multikinase or histone deacetylase inhibitors demonstrates promising synergistic activity in patients with microsatellite stability (MSS)/mismatch repair proficient (pMMR) mCRC, which is detected in approximately 95% of all mCRC cases (4,5). However, the molecular features of MSS/pMMR that lead to enhanced tumor immunogenicity and sensitivity to immune checkpoint inhibitors are to be discussed.
The use of immune checkpoint inhibitors, including nivolumab, has introduced new atypical response patterns, such as dissociated response. The most common definition of a dissociated response is the coexistence of both responding and non-responding lesions within the same patient (6). However, there is no established terminology as well as standard criteria of definition for dissociated response; different terms such as mixed or heterogeneous response are used. Although various types of dissociated response to nivolumab have been described in many solid tumors (7-9), there are no data regarding such response in mCRC patients with MSS/pMMR phenotype.
Here we report a case diagnosed with MSS/pMMR mCRC who has achieved a durable response to nivolumab with atypical reaction after progression following first- and second-line chemotherapy with antiangiogenic therapy.
Case report
Patient information
A 42-year-old man, never smoker, with abdominal pain accompanied by nausea was taken by ambulance to the local hospital in August 2017, where intestinal obstruction was verified. His medical history included сhronic cholecystitis, chronic pancreatitis, and mixed etiology of hepatitis. The patient underwent abdominal stoma surgery. Biopsy revealed a highly differentiated adenocarcinoma. The patient independently visited Tomsk Cancer Research Institute for further examination, where he underwent left hemicolectomy and colostomy suturing. Pathologic verification of resection specimens confirmed moderately differentiated adenocarcinoma with mucinous features and foci of severe fibrosis, as well as comedo necrosis and with an accompanying lymphocytic infiltrate and invasion into the serous membrane. The patient was diagnosed with splenic flexure colon cancer, T4N3M0, stage IIIC. However, a month later, during an additional examination in October 2017, supraclavicular lymph node metastases on both sides were detected. Due to definitive evidence of disseminated disease, the patient was upstaged to stage IV. Molecular testing of the tumor revealed the KRASp.G12D mutation.
MSS/pMMR phenotype
Immunohistochemistry analysis of MMR protein expression (MLH1, MSH2, MSH6, and PMS2) demonstrated a homogeneous pattern of their proficiency in colon tumor tissue (Fig. 1A). Polymerase chain reaction (PCR) assay confirmed the MSS phenotype for three different colon tumor specimens (Fig. 1B). With respect to PD-L1 status, a weak expression (TPS-1% and CPS-6.0) in several colon tumor specimens was independently detected by two researchers.
Chemotherapeutic intervention
Patient was started on a first-line FOLFOX plus bevacizumab regimen, which consisted of bevacizumab 5 mg/kg on day 1, oxaliplatin 85 mg/m2 on day 1, leucovorin 400 mg/m2 on day 1, and 5-fluorouracil 400 mg/m2 bolus injection followed by 46-hour continuous infusion of 5-fluorouracil 2,400 mg/m2 on day 1. After four cycles of treatment, the partial response per RECIST 1.1 was recorded. In June 2018, the patient completed 14 cycles of chemotherapy and following the chest computed tomography (CT) images showed the appearance of randomly located focal nodules of up to 7 mm in size consistent with lung metastases. The levels of tumor markers such as CEA, CA19-9, and CA242 did not exceed the normal values, being 0-3.40 ng/mL for CEA, 0-34 U/mL for CA19-9, and 0-20.0 U/mL for CA242.
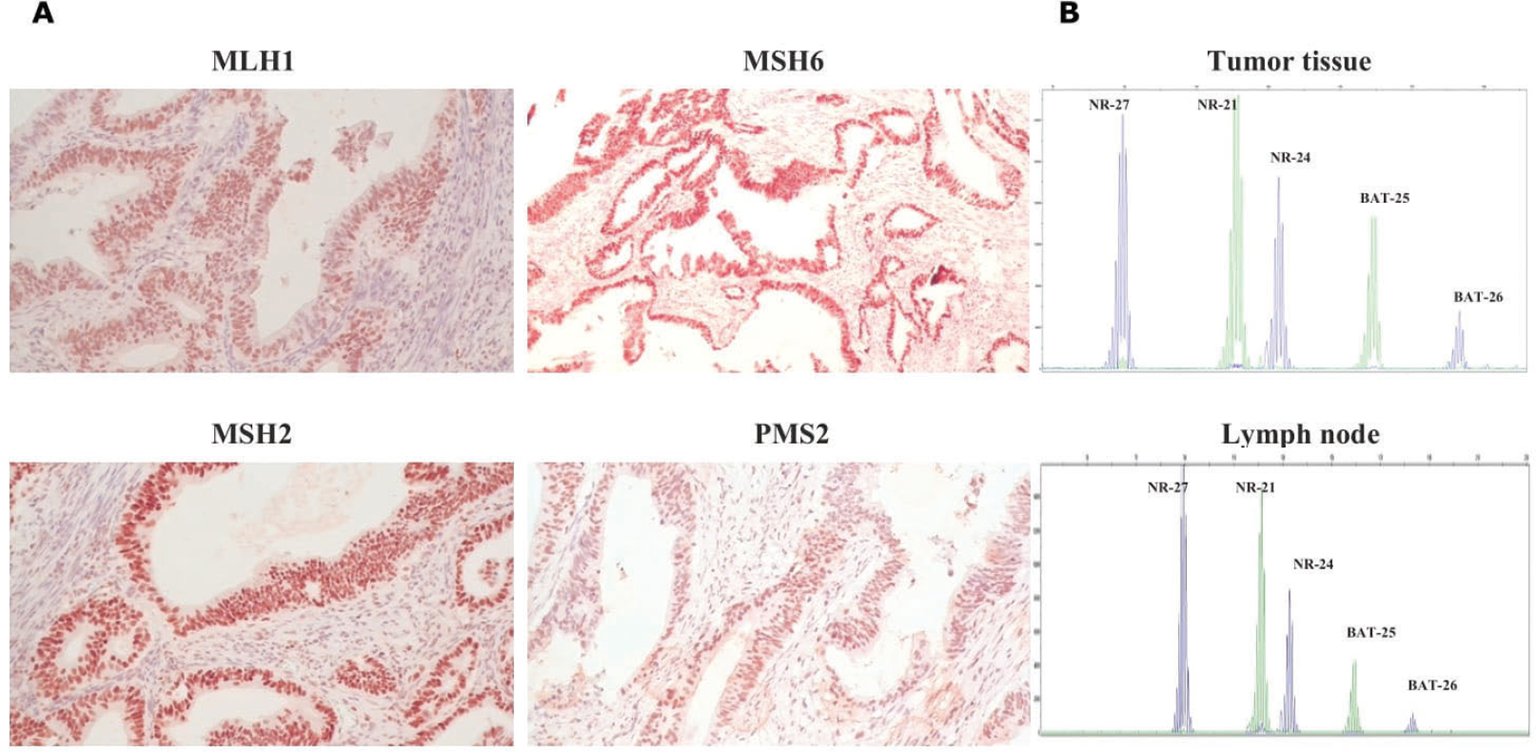
Fig. 1 - Immunohistochemical staining of MMR proteins. Positive expression of MLH1, MSH2, MSH6, and PMS2 in the colon tumor tissue (magnification ×200) (A); MSI test electropherograms with NR-27, NR-21, NR-24, BAT25, and BAT26 markers in the colon tumor and lymph node specimens (B). MMR = mismatch repair; MSI = microsatellite instability.
Second-line treatment with FOLFIRI and aflibercept (aflibercept 4 mg/kg on day 1, irinotecan 180 mg/m2 on day 1, leucovorin 400 mg/m2 on day 1, 5-fluorouracil 400 mg/m2 bolus injection followed by 46-hour continuous infusion of 5-fluorouracil 2,400 mg/m2) was initiated. Repeat CT images after three cycles showed no growth of focal nodules in the lung. This treatment was associated with adverse events such as of grade 2 hypertension, nausea, and thrombocytopenia. CT assessment after seven cycles of treatment confirmed a stable response. Due to an acute exacerbation of mixed etiology hepatitis (chronic hepatitis B with serum hepatitis B virus DNA titer >105 copies/mL and toxic hepatitis) and an abnormal alanine aminotransferase exceeding nine times the upper limit of normal at baseline, subsequent treatment was interrupted. The patient received hepatoprotective and detoxification treatment. Approximately 2 months later, CT scans showed an increase of nodule size in the lung (Fig. 2A).
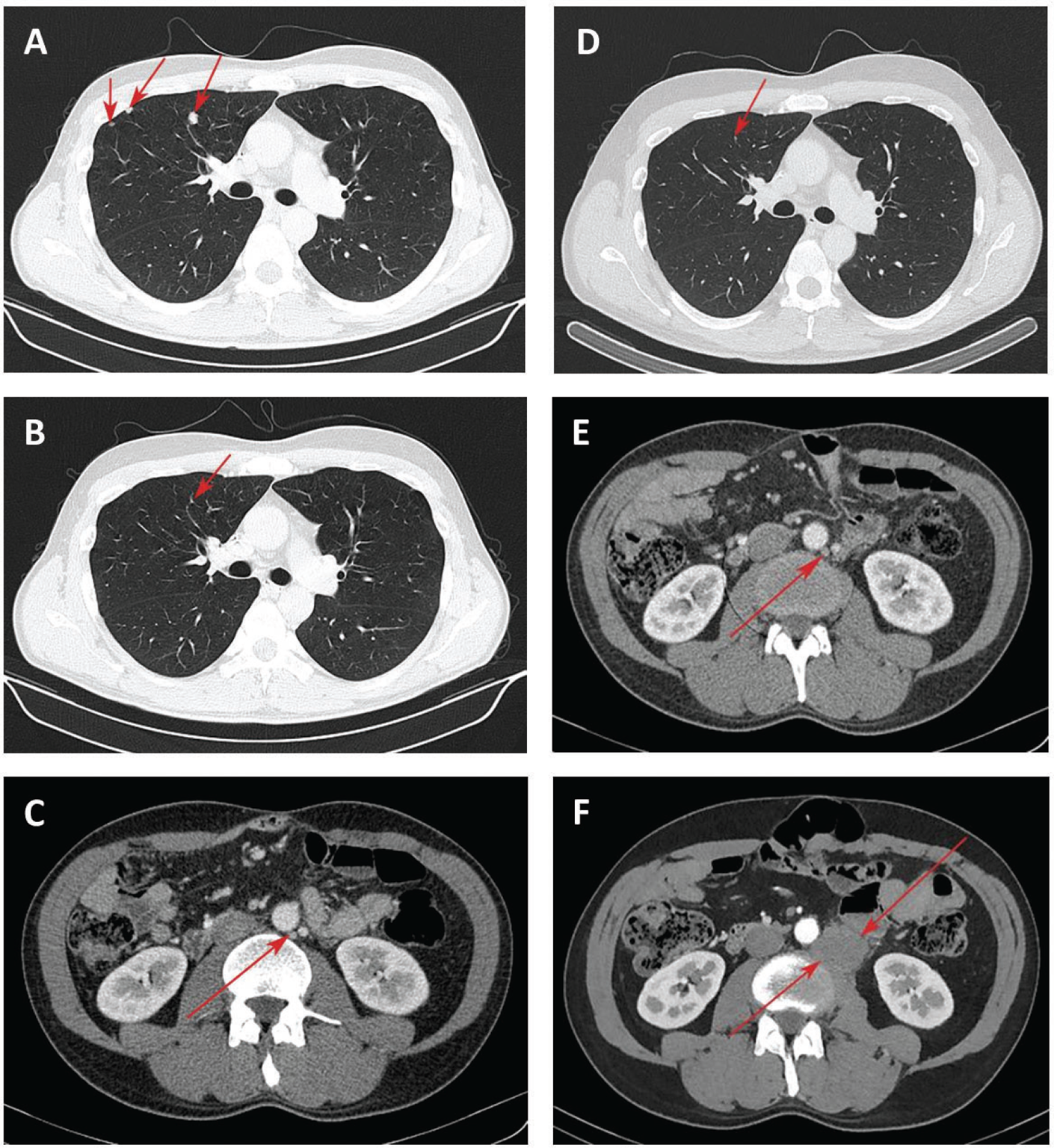
Fig. 2 - Chest computed tomography (CT) of the lung lesions and para-aortic lymph node lesion. Focal nodules in the lung at start of nivolumab treatment (A); 5 months after nivolumab treatment, reduction of lung nodules (B) and para-aortic lymph node lesion up to 12 mm (C); 8 months after nivolumab treatment, disappearance of lung lesions (D) and lack of growth of para-aortic lymph node lesion (E); increase of para-aortic lymph node lesion 40 months after nivolumab treatment (F).
Nivolumab administration
Given the fast progression and PD-L1 status, treatment with nivolumab at a dose 240 mg was started in December 2018. Three months later, most of the previously identified focal nodules in the lung were not clearly visualized by CT, the remaining lesions decreased in size up to 1-2 mm. In May 2019, chest CT scans confirmed the reduction of lung nodules (Fig. 2B). However, a para-aortic lymph node (PALN) on the left up to 15×12 mm was found (Fig. 2C). Using the iRECIST as a modified immune RECIST criterion (10), the response was classified as a dissociated response. MSS phenotype in lymph node was further verified by PCR (Fig. 1B).
Further administration of nivolumab allowed achieving positive dynamics in terms of the disappearance of lesions in the lung (Fig. 2D) and the absence of the growth of PALN metastases in August 2019 (Fig. 2E). An immune stable disease (iSD) per iRECIST was recorded until September 2021, when we had to discontinue treatment due to the patient being infected with COVID-19. The administration of nivolumab was resumed; however, in April 2022, CT scans revealed an increase in the size of PALN by 7 mm compared with the baseline (Fig. 2F). The decision to initiate FOLFOX plus bevacizumab therapy (bevacizumab 5 mg/kg on day 1, oxaliplatin 85 mg/m2 on day 1, leucovorin 400 mg/m2 on day 1, and 5-fluorouracil 400 mg/m2 bolus injection followed by 48 hours continuous infusion of 5-fluorouracil 2,400 mg/m2 on day 1) was taken by a multidisciplinary staff. Due to the experience of hypersensitivity reaction to oxaliplatin after three cycles, the patient was switched to the bevacizumab in combination with capecitabine regimen (bevacizumab 5 mg/kg and capecitabine 2,500 mg/day, days 1 and 14). Currently, the patient has completed 10 cycles of this therapy with good tolerance.
Conclusion
We have reported a patient with MSS/pMMR mCRC who has achieved a complete response to nivolumab after a progression on chemotherapy and antiangiogenic treatment, with a response duration of 32 months. Based on the presence of PD-L1 weak expression, the potential immunogenic features of the tumor leading to benefit from PD-1/PD-L1 targeting therapy could be suggested. Present case report demonstrates that a subset of pretreated mCRC patients with MSS/pMMR phenotype may benefit from nivolumab. We will undertake a high-throughput transcriptome sequencing of both colon tumor and lymph node specimens to establish possible molecular background for a dissociated response.
Disclosures
Conflict of interest: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval: This study was performed in line with the principles of the Helsinki Declaration. Approval was granted by the Ethics Committee of the Cancer Research Institute, Tomsk National Research Medical Center (Date: 01/19/22).
Informed consent: Written informed consent was obtained from the patient.
Financial support: The study was supported by the Russian Science Foundation, grant #22-15-00212; Online.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Evgeny Grigoryev, Tatyana Dronova, Polina Gervas, Alexey Dobrodeev, Dmitry Kostromitskiy, Victor Goldberg, and Sergey Afanasiev. The first draft of the manuscript was written by Nataliya Babyshkina and Nataliya Popova, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1. Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5(1):16-18. CrossRef PubMed
- 2. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. CrossRef PubMed
- 3. Overman MJ, Bergamo F, McDermott RS, et al. Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): Long-term survival according to prior line of treatment from CheckMate-142. J Clin Oncol. 2018;36(4_suppl):554–554. CrossRef
- 4. Fakih M, Raghav KPS, Chang DZ, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023;58:101917. CrossRef PubMed
- 5. Saunders MP, Graham J, Cunningham D, et al. CXD101 and nivolumab in patients with metastatic microsatellite-stable colorectal cancer (CAROSELL): a multicentre, open-label, single-arm, phase II trial. ESMO Open. 2022;7(6):100594. CrossRef PubMed
- 6. Humbert O, Chardin D. Dissociated response in metastatic cancer: an atypical pattern brought into the spotlight with immunotherapy. Front Oncol. 2020;10:566297. CrossRef PubMed
- 7. Wong A, Vellayappan B, Cheng L, et al. Atypical response patterns in renal cell carcinoma treated with immune checkpoint inhibitors-navigating the radiologic potpourri. Cancers (Basel). 2021;13(7):1689. CrossRef PubMed
- 8. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38-47. CrossRef PubMed
- 9. Sato Y, Morimoto T, Hara S, et al. Dissociated response and clinical benefit in patients treated with nivolumab monotherapy. Invest New Drugs. 2021;39(4):1170-1178. CrossRef PubMed
- 10. Seymour L, Bogaerts J, Perrone A, et al; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. CrossRef PubMed