 |
Drug Target Insights 2023; 17: 110-113 ISSN 1177-3928 | DOI: 10.33393/dti.2023.2626 CASE REPORT |
 |
First-line tepotinib for a very elderly patient with metastatic NSCLC harboring MET exon 14 skipping mutation and high PD-L1 expression
ABSTRACT
Optimal treatment for metastatic non-small cell lung cancer (NSCLC) with mesenchymal epithelial transition gene (MET) exon 14 skipping mutation has not been established yet. MET inhibitors were demonstrated to be effective and tolerated in patients with this condition, while evidence on safety and efficacy of immunotherapy and/or chemotherapy in this population is limited. Here we report the case of an 86-year-old male with metastatic NSCLC harboring MET exon 14 skipping mutation and with high programmed cell death ligand 1 (PD-L1) expression (tumor proportion score ≥50%). The patient received the MET inhibitor tepotinib as first-line treatment, achieving a partial response, with G2 peripheral edema as adverse event that was successfully managed with temporary discontinuation, dose reduction, diuretics and physical therapy. After 31 months, the patient is still receiving tepotinib, with an ongoing response. Tepotinib is a valuable therapeutic option for first-line treatment of older patients with NSCLC harboring MET exon 14 skipping mutation, even in the presence of high PD-L1 expression.
Keywords: Elderly, First-line treatment, MET 14-exon skipping mutation, MET inhibitor, Non-small cell lung cancer, Tepotinib
Received: July 3, 2023
Accepted: September 19, 2023
Published online: October 6, 2023
Corresponding author:
Alessandro Inno
Medical Oncology
IRCCS Ospedale Sacro Cuore Don Calabria
Via don A. Sempreboni 5
37024 Negrar di Valpolicella (VR) - Italy
alessandro.inno@sacrocuore.it
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Mesenchymal epithelial transition gene (MET) exon 14 (METex14) skipping mutations occur in approximately 3-4% of non-small cell lung cancer (NSCLC) (1). In the VISION trial, a phase 2, non-randomized open-label study, the MET inhibitor tepotinib achieved a response rate of 51.4% and a median duration of response (DOR) of 18 months among 313 patients with METex14 mutant advanced/metastatic NSCLC, both treatmet-naïve (n = 164) and previously treated (n ;= ;149) patients (2,3). Based on these results, tepotinib was approved by the Food and Drug Administration for the treatment of patients with metastatic NSCLC harboring METex14 skipping mutation regardless of the line of therapy (4), whereas the European Medicines Agency (EMA) approved tepotinib only for patients previously treated with immunotherapy and/or platinum-based chemotherapy (5).
Here we report the case of an 86-year-old male with METex14-mutated metastatic NSCLC and concomitant high programmed cell death ligand 1 (PD-L1) expression treated with first-line tepotinib that achieved a deep and durable response with manageable toxicity.
Case description
Following an accidental fall, a former 86-year-old male smoker underwent a head and chest computed tomography (CT) scan, which showed a lung tumor in the right lower lobe. The 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT confirmed the presence of a lung tumor and showed bone, left adrenal and peritoneal metastases (Fig. ;1). Stage was cT1c N0 M1c, IVB according to the American Joint Committee on Cancer (AJCC) 8th edition. The pathology examination of a CT-guided biopsy of the lung mass diagnosed lung adenocarcinoma with sarcomatoid transformation. The immunohistochemical evaluation showed high PD-L1 expression with a tumor proportion score (TPS) ≥50%, and next-generation sequencing (NGS) found METex14 skipping mutation. Comorbidities included trigeminal neuralgia, prostatic hypertrophy, eye maculopathy, and bilateral knee and right hip replacement.
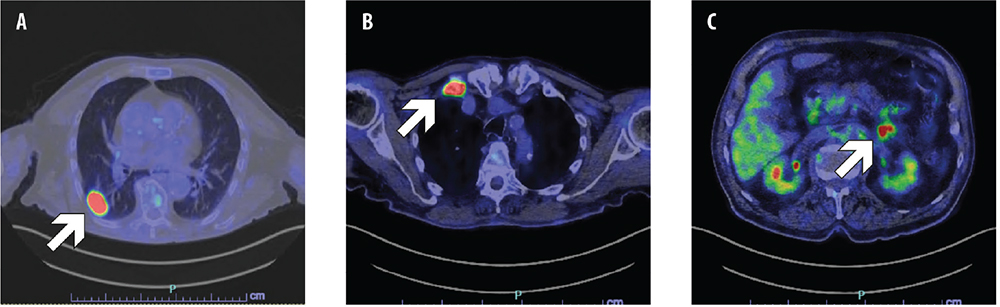
Fig. 1 - Positron emission tomography-computed tomography (PET-CT) scan showing primary lung tumor (A), bone metastasis in the first right rib (B), and peritoneal metastasis (C).
First-line tepotinib 500 mg/day was started through an Early Access Program. The patient achieved partial response at the first tumor assessment after 3 months of treatment. Further response was observed at months 6, 9 and 12, then stable disease was observed (Fig. 2). During the treatment, the patient was diagnosed also with a prostate carcinoma (stage cT2 cN0, Gleason Score 4+4), therefore androgen deprivation therapy was added, without safety concerns. After 6 months of treatment with tepotinib, the patient developed treatment-related G2 peripheral edema; therefore, tepotinib was temporarily discontinued, and diuretics were administered, with complete regression of edema. Tepotinib was then restarted at 250 mg/day. After 16 months of treatment, however, tepotinib was discontinued again due to recurrent G2 peripheral edema. The patient received a further course of diuretics combined with physical therapy (i.e., compression stockings, retrograde massage), achieving edema improvement at G1; then, tepotinib was restarted at 250 mg/day. After 23 months of treatment, tepotinib was reduced at 250 mg every other day because of worsening edema, and it is currently ongoing (after 31 months).
Discussion
For patients with metastatic NSCLC without driver molecular alterations, immunotherapy with or without chemotherapy represents the standard of care in the first-line setting (6). Particularly, for patients with high PD-L1 expression (TPS ≥50%), immunotherapy alone with anti-PD-(L)1 antibodies as single agent represents the treatment of choice. Indeed, in the phase III KEYNOTE-024 trial, the anti-PD-1 antibody pembrolizumab as single agent achieved a median overall survival (OS) of 26.3 months, and a 5-year OS rate of 31.9%, compared respectively with 13.4 months and 16.3% of platinum-based chemotherapy as first-line treatment of metastatic NSCLC with TPS ≥50% (7). Similar results are available also for other anti-PD-(L)1 agents (8,9). Moreover, the addition of immunotherapy to the first-line platinum-based chemotherapy improved survival over chemotherapy alone, regardless of PD-L1 expression levels ;(10).
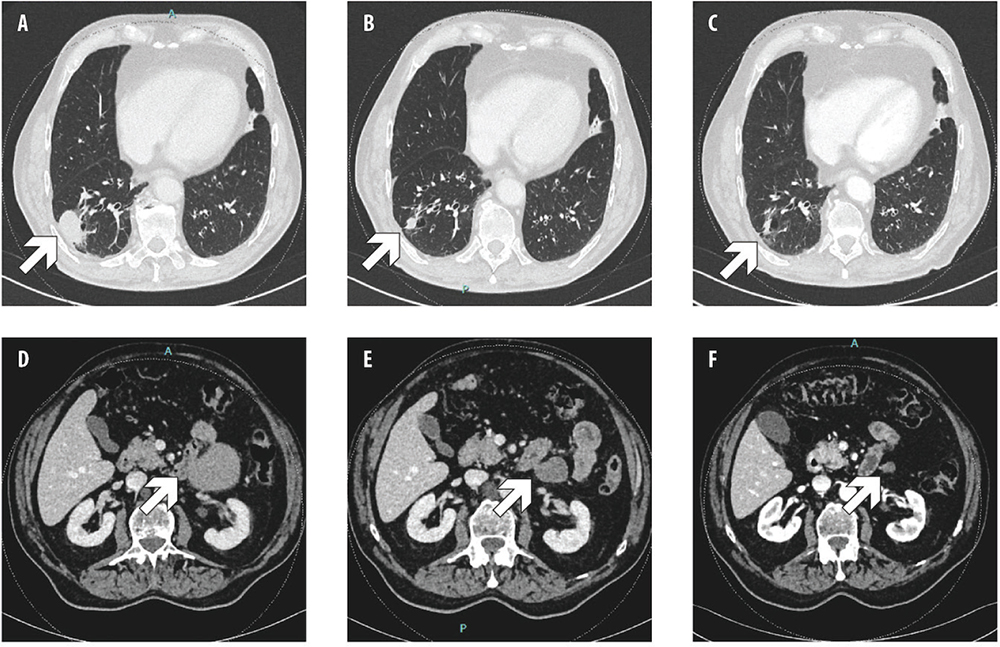
Fig. 2 - Computed tomography (CT) scan showing, respecively, primary lung tumor and peritoneal metastasis at baseline (A, D), after 12 weeks (B, E) and 1 year (C, F) of tepotinib.
For patients with NSCLC harboring METex14 skipping mutations, however, the optimal treatment strategy has not yet been established. In fact, there are no data from randomized studies on the efficacy of immunotherapy and/or chemotherapy in the specific population of patients with METex14 mutant NSCLC. Retrospective studies showed conflicting results, with some studies suggesting a limited activity of immunotherapy in this population regardless of PD-L1 expression level (11,12), whereas other studies reported a similar activity of immunotherapy among MET mutant and wild-type cancers (13,14). However, no randomized comparisons of immunotherapy, either with or without chemotherapy, vs. MET inhibitors are available for patients with METex14 mutant metastatic NSCLC as front-line treatment.
Of note, METex14-mutated NSCLC is mainly found in elderly subjects (15). Elderly patients were generally underrepresented in clinical trials investigating immunotherapy with or without chemotherapy in NSCLC; therefore, the benefit/risk balance of these regimens should be carefully assessed individually when treating older patients in daily clinical practice. In fact, a recent pooled analysis showed that addition of chemotherapy to immunotherapy in patients with PD-L1 ≥50% older than 75 years was not beneficial (16). Chemotherapy alone may represent another option for elderly patients with advanced NSCLC. However, a joint analysis of two randomized trials on NSCLC older than 70 years reported limited activity of chemotherapy in this population, with a median progression-free survival (PFS) of 3 ;months for single-agent chemotherapy (either gemcitabine or pemetrexed) and 4.6 months with the addition of cisplatin. Interestingly, in this joint analysis the addition of cisplatin to single-agent chemotherapy did not significantly prolong OS (17). Thus, the use of platinum-based chemotherapy, with or without immunotherapy, may be questionable in elderly patients.
In clinical trials, selective MET inhibitors including tepotinib, capmatinib and savolitinib have recently shown meaningful activity against METex14-mutated NSCLC. Particularly, in the phase II VISION study, among 152 METex14-mutated NSCLC patients treated with tepotinib, the response rate by inde-pendent review was 46%, and median PFS was 8.5 months. This trial enrolled both treatment-naive and preatreted patients. Interestingly, in treatment-naive patients (n = 164), objective response rate (ORR) was 57.3% and median DOR was 46.4 months, whereas among pretreated patients, ORR was 45.0% and median DOR was 12.6 months, suggesting that tepotinib might be more beneficial as front-line treatment. In this study, the toxicity profile of tepotinib was acceptable, with peripheral edema reported as main grade 3 toxicity. In this study, 43% of patients received tepotinib as the first-line treatment (2,3). In the phase II GEOMETRY mono-1 study, among 97 METex14-mutated NSCLC patients treated with capmatinib, the ORR was observed in 41% of 69 patients who had previously received one or two lines of therapy and in 68% of 28 previously untreated patients; the median DOR was 9.7 months and 12.6 months, respectively (18). A Chinese phase II study evaluated savolitinib in 70 patients with METex14-mutated NSCLC after ≥1 line of standard treatment or deemed unsuitable for standard treatment, reporting an ORR of 42.9% (19).
Our patient achieved a partial response to tepotinib, with a PFS of 31+ months and DOR of 28+ months, that is consistent with data from VISION trial and compares favorably with data of first-line immunotherapy or chemotherapy. Based on this observation, we believe that MET inhibitors represent an effective and well-tolerated therapy for metastatic, METex14 mutant NSCLC in the first-line setting. Unfortunately, the EMA approval of MET inhibitors only for patients with progressive disease after chemotherapy and/or immunotherapy is likely to limit across Europe the access to treatment for very elderly patients unfit to receive first-line chemotherapy and/or immunotherapy before MET inhibitors.
Acknowledgments
Medical writing services were provided by Laura Brogelli, Valentina Attanasio, and Aashni Shah (Polistudium srl, Milan, Italy).
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: The development of this publication was financially supported by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany through an independent medical writing grant. The views and opinions described in this publication do not necessarily reflect those of the grantor.
Author Contributions: Collection and interpretation of data: All authors; manuscript drafting: AI; approval to submit: all authors.
Data Availability Statement: Data described in this article are available from the corresponding author upon reasonable request and according to ethical restrictions.
Ethical approval: The patient provided informed written consent to the publication of the anonymous clinical description.
References
- 1. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543-550. CrossRef PubMed
- 2. Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET Exon 14 skipping mutations. N Engl J Med. 2020;383(10):931-943. CrossRef PubMed
- 3. Mazieres J, Paik PK, Garassino MC, et al. Tepotinib treatment in patients with MET exon 14-skipping non-small cell lung cancer: long-term follow-up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;e231962. CrossRef PubMed
- 4. European Medicine Agency. Tepmetko. Online. Accessed October 2022.
- 5. U.S. Food and Drug Administration. Tepmekto 2021. Online. Accessed October 2022.
- 6. Reck M, Remon J, Hellmann MD. First-Line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40(6):586-597. CrossRef PubMed
- 7. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39(21):2339-2349. CrossRef PubMed
- 8. Jassem J, de Marinis F, Giaccone G, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. 2021;16(11):1872-1882. CrossRef PubMed
- 9. Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592-604. CrossRef PubMed
- 10. Liu T, Wu S, Fang W, et al. Identifying optimal first-line immune checkpoint inhibitors based regiments for advanced non-small cell lung cancer without oncogenic driver mutations: a systematic review and network meta-analysis. PLoS One. 2023;18(4):e0283719. CrossRef PubMed
- 11. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321-1328. CrossRef PubMed
- 12. Sabari JK, Leonardi GC, Shu CA, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085-2091. CrossRef PubMed
- 13. Mayenga M, Assié JB, Monnet I, et al. Durable responses to immunotherapy of non-small cell lung cancers harboring MET exon-14-skipping mutation: a series of 6 cases. Lung Cancer. 2020;150:21-25. CrossRef PubMed
- 14. Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and safety of anti-PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol. 2020;15(4):628-636. CrossRef PubMed
- 15. Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493-1502. CrossRef PubMed
- 16. Akinboro O, Vallejo JJ, Nakajima EC, et al. Outcomes of anti-PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC) with PD-L1 score ≥ 50%: FDA pooled analysis. J Clin Oncol. 2022;40:16_suppl, 9000. CrossRef
- 17. Gridelli C, Morabito A, Cavanna L, et al. Cisplatin-based first-line treatment of elderly patients with advanced non-small-cell lung cancer: joint analysis of MILES-3 and MILES-4 phase III trials. J Clin Oncol. 2018;36(25):2585-2592. CrossRef PubMed
- 18. Wolf J, Seto T, Han JY, et al; GEOMETRY mono-1 Investigators. Capmatinib in MET Exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944-957. CrossRef PubMed
- 19. Lu S, Fang J, Li X, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med. 2021;9(10):1154-1164. CrossRef PubMed