 |
Drug Target Insights 2023; 17: 90-91 ISSN 1177-3928 | DOI: 10.33393/dti.2023.2593 CASE REPORT |
 |
Activity of sotorasib against brain metastases from NSCLC harboring KRAS p.G12C mutation: a case report
ABSTRACT
In the CodeBreaK 100 phase 2 study, sotorasib was active for patients with metastatic non-small cell lung cancer (NSCLC) harboring Kirsten rat sarcoma viral oncogene homologue (KRAS) p.G12C mutation. However, patients with untreated and/or active brain metastases were excluded from the trial, and the activity of sotorasib in the setting of brain metastases should be further investigated. Here we report the case of a KRAS p.G12C mutant NSCLC patient with three brain metastases, of whom one was untreated and the other two had progressed after radiotherapy with symptoms requiring steroids, that responded to sotorasib. Our report suggests that sotorasib may be active against untreated or progressive brain metastases, supporting further evaluation of sotorasib in this setting.
Keywords: Brain metastases, Central nervous system, KRAS, NSCLC, Sotorasib
Received: April 27, 2023
Accepted: May 22, 2023
Published online: June 20, 2023
Corresponding author:
Alessandro Inno
Medical Oncology
IRCCS Ospedale Sacro Cuore Don Calabria
Via don A. Sempreboni 5
37024 Negrar di Valpolicella (VR) - Italy
alessandro.inno@sacrocuore.it
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Background
About 25-30% of non-small cell lung cancers (NSCLCs) harbor a mutation in the Kirsten rat sarcoma viral oncogene homologue (KRAS) gene. Particularly, KRAS p.G12C mutation is the most frequent KRAS mutation and it is found in approximatively 13% of NSCLC (1). Sotorasib, a specific inhibitor of KRAS p.G12C mutation, has demonstrated activity in pretreated metastatic NSCLC patients. In the CodeBreaK 100 trial, a single-group phase 2 study on 126 pretreated patients with metastatic, KRAS p.G12C mutant NSCLC, sotorasib led to a response rate of 37.1% (95% confidence interval (CI), 28.6-46.2), a median progression-free survival of 6.8 months (95% CI, 5.1-8.2), and a median overall survival of 12.5 months (95% CI, 10.0-not reached), with an acceptable safety profile (2).
Brain metastases represent a frequent complication of NSCLC. They are associated with deterioration of quality of life, poor prognosis, and low response rates to chemotherapy (3). For patients with brain metastases from oncogene addicted NSCLC, such as tumors with EGFR mutations or ALK rearrangements, target therapy achieves high intracranial response rate (4,5). However, there is still paucity of data regarding the activity of sotorasib against brain metastases from KRAS p.G12C mutant NSCLC. In fact, although in the CodeBreak 100 trial approximately 20% of patients had brain metastases at baseline, patients with active untreated brain metastases were excluded from the trial. More recently, a retrospective study reported six patients with active untreated brain metastases receiving sotorasib. Among four patients evaluable for response, confirmed intracranial response to sotorasib was observed in three patients, with a median duration of response of 4.1 months, and a median intracranial progression-free survival of 4.7 months (6).
Here we report a case of a patient with metastatic, KRAS p.G12C mutant NSCLC with both treated and untreated active brain metastases receiving sotorasib as second-line therapy.
Case report
In June 2017, a 72-year-old former Caucasian female smoker underwent upper right lung lobectomy with regional nodal dissection for lung adenocarcinoma, stage pT2a pN1. Comorbidities were: previous left nephrectomy for clear cell renal carcinoma, hypertension, type 2 diabetes, and meningioma. Molecular profile of NSCLC was: EGFR wild type, ALK negative, ROS1 negative, programmed death ligand (PDL) tumor proportion score (TPS) <1%, KRAS mutant p.G12C. At baseline staging, patient also had two synchronous intracranial metastases, in right parietal lobe and in right cerebellar hemisphere, both treated with stereotactic radiosurgery (21 Gy as single fraction). The patient also received first-line chemotherapy with carboplatin plus paclitaxel for four courses, from August to October 2017.
During the following surveillance program, the patient developed two lung metastases in the right middle lobe (March 2018) both treated with stereotactic radiotherapy (70 Gy in 10 fractions), a further histology-proven, adenocarcinoma lung metastasis KRAS mutant p.G12C in upper left lobe (September 2019) also treated with stereotactic radiotherapy (60 Gy in 10 fractions), and progressive right parietal and right cerebellar metastases treated with further radiation therapy, respectively 21 Gy in three fractions and 27 Gy in three fractions.
In March 2021 the patient experienced symptomatic intracranial disease progression, with a new brain metastasis in the right frontal lobe and increase in size of the other two known metastases and appearance of surrounding edema in the right parietal lobe, requiring steroid therapy. Positron emission tomography (PET)/computed tomography (CT) scan did not show extracranial disease. The patient was started on sotorasib, and the brain magnetic resonance imaging (MRI) after 2 months of treatment showed stability of the cerebellar metastasis, reduction in size of the previously treated parietal right metastasis with improvement of the surrounding edema, reduction in size of the previously untreated right frontal lobe metastasis, and no appearance of new brain metastases (Fig. 1). In March 2022 posterior fossa hemorrhage occurred due to bleeding of the cerebellar metastasis, which was treated with surgical evacuation of the hemorrhagic focus and metastasectomy. Histology examination of the cerebellar metastasis revealed radionecrosis with no residual viable cancer tissue. Treatment with sotorasib was continued and the disease remained stable until July 2022 when brain MRI showed oligoprogression due to increase in size of the right frontal metastasis, which was treated with stereotactic radiotherapy (24 Gy in three fractions). Sotorasib was continued and, after 27 months (May 2023), treatment is still ongoing, without safety concerns, and with stable intracranial disease at brain MRI and still no evidence of extracranial metastases at the PET/CT scan.
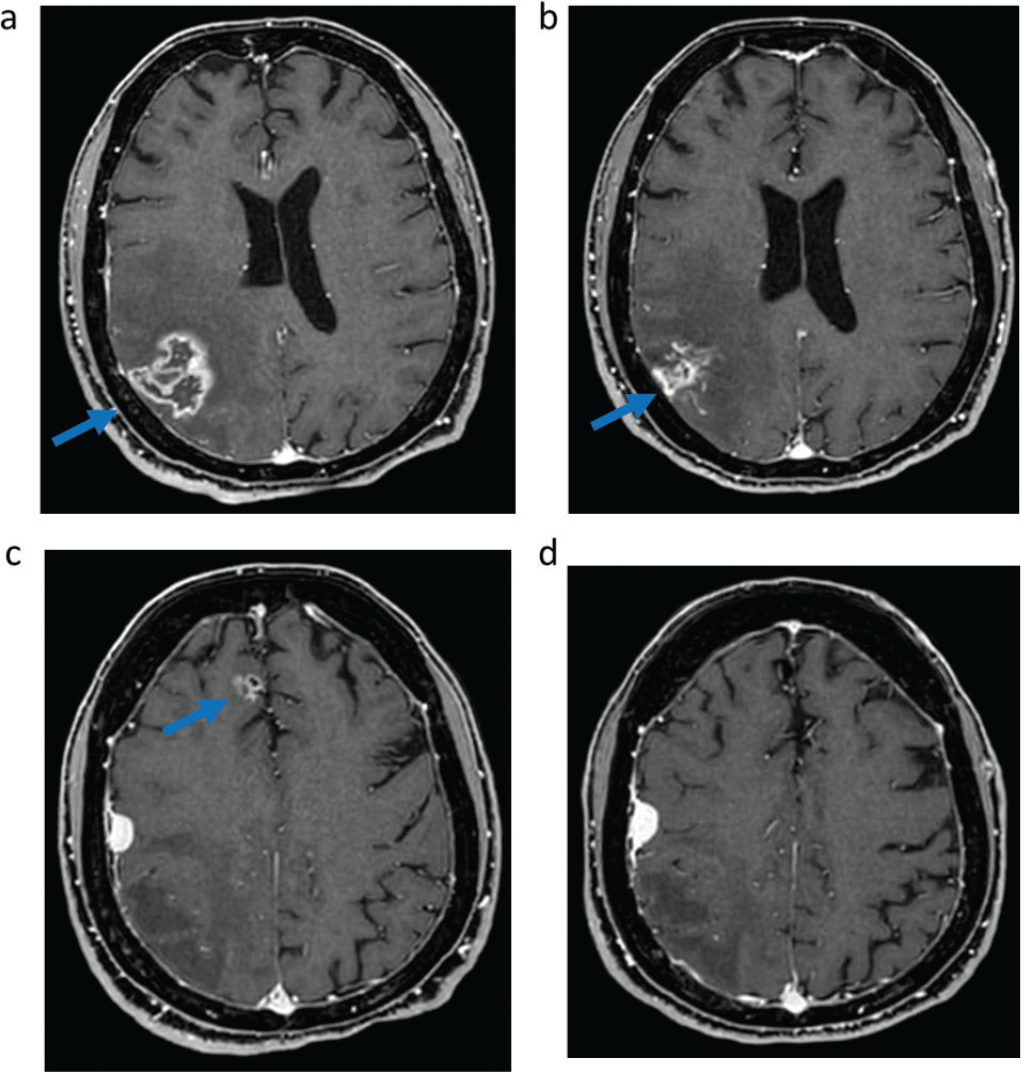
Fig. 1 - Intracranial response to sotorasib assessed with magnetic resonance imaging. T1-weighted imaging of right parietal metastasis before (A) and after (B) 6 months of sotorasib; Fluid-attenuated inversion recovery of right parietal metastasis surrounding edema before (C) and after (D) 6 months of sotorasib; T1-weighted imaging of right frontal metastasis before (E) and after (F) 6 months of sotorasib; T1-weighted imaging of right cerebellar metastasis before (G) and after (H) 6 months of sotorasib.
Conclusion
We have reported a case of intracranial response to sotorasib in a patient with both pretreated and untreated symptomatic brain metastases from KRAS p.G12C mutant NSCLC, with a duration of intracranial response of 16 months. An oligoprogressive brain metastasis was successfully managed with stereotactic radiotherapy while continuing sorafenib, with a time to treatment failure exceeding 27 months.
This report supports further investigation of sotorasib in the setting of KRAS p.G12C mutant NSCLC with untreated brain metastases.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contribution: All authors contributed equally to this manuscript.
References
- 1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535-554. CrossRef PubMed
- 2. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371-2381. CrossRef PubMed
- 3. Inno A, Di Noia V, D’Argento E, Modena A, Gori S. State of the art of chemotherapy for the treatment of central nervous system metastases from non-small cell lung cancer. Transl Lung Cancer Res. 2016;5(6):599-609. CrossRef PubMed
- 4. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;JCO2018783118(33):JCO2018783118. CrossRef PubMed
- 5. Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29(11):2214-2222. CrossRef PubMed
- 6. Lamberti G, Aizer A, Ricciuti B, et al. Incidence of brain metastases and preliminary evidence of intracranial activity with sotorasib in patients with KRASG12C-mutant non-small-cell lung cancer. JCO Precis Oncol. 2023;7(7):e2200621. CrossRef PubMed