 |
Drug Target Insights 2022; 16: 81-87 ISSN 1177-3928 | DOI: 10.33393/dti.2022.2520 REVIEW |
Focus on Antimicrobial Resistance (AMR)
|
Antibiotic-resistant bacteria originating from the gut may modulate the mucosal immune response during sepsis and septic shock
ABSTRACT
The enrichment and diversity of gut microbiota play an important role in sepsis, but the role of gut microbiota composition and early-life colonization in sepsis and septic shock has not yet been characterized. The impact of gut microbiota diversity on host immunological disorders and future treatments of inflammatory diseases are not yet fully elucidated. Further, the association between the microbiota and immune development in sepsis remains unknown, and the underlying mechanisms are not well understood. The altered composition of gut microbiota during sepsis is profoundly associated with a loss of commensal bacteria and an overgrowth of potentially pathogenic bacteria, especially AMR bacteria. Disruptions of gut microbiota diversity are directly associated with susceptibility to sepsis and a higher risk of adverse outcomes. Several studies have confirmed that a mutual association between gut microbiota and the host is important for the metabolism of essential nutrients for the organism, for gut development, and for the maturation and development of a fully functional immune system. Therefore, understanding the gut microbiota diversity, composition, and function during various inflammatory conditions and sepsis may provide a comprehensive knowledge of the mechanisms behind the pathogenesis of gut-derived infection in diseases and the design of new treatment options (e.g., probiotics or fecal microbiota transplantation).
Emerging evidence displays an important role of gut microbiota and their derived metabolites in modulating the host mucosal immune response and determining the susceptibility to, as well as outcomes of sepsis.
Keywords: Immune response, Inflammation, Metabolites, Microbiota, Sepsis
Received: November 16, 2022
Accepted: December 30, 2022
Published online: December 31, 2022
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Antimicrobial resistance (AMR) also known as drug resistance, is a naturally occurring process that happens when germs like bacteria, fungi, viruses, and parasites develop the ability to defeat the drugs designed to kill them (1). Microbes change over time and no longer respond to antibiotics and other antimicrobial drugs, making infections harder to treat and increasing the risk of disease spread, severe illness, and death (2). Resistant infections can be difficult, and sometimes impossible, to treat. When the microorganisms become resistant to most of the antibiotics and other medications commonly used to treat the infections they cause, they are often referred to as “superbugs.” AMR is considered one of the leading public health threats of the 21st century (3). About 700,000 deaths have been reported due to drug-resistant infections (4). About 2.8 million people suffer from acquired drug-resistant infections across the globe and 35,000 patients die annually in the United States due to infections alone (5). It is responsible for an estimated 33,000 deaths per year in the European Union (EU) (6). In 2019, the World Health Organization (WHO) reported that, if left unchecked, the anticipated deaths due to AMR would rise to 10 million by 2050 (5). These infections have significant economic and human costs. Economic projections suggest that by 2050, the economic costs of healthcare-associated infections (HAIs) to the US healthcare system will range from 28 to 45 billion dollars per year (7). Further, recent data released by the UK government argued that AMR could kill 10 million people per year by 2050 (8). The WHO and numerous other groups anticipated that AMR is a global issue that requires a coordinated action plan to address. The way AMR infections are spreading could make many bacterial pathogens resistant to emerging antibiotics and more lethal in the future than they are today (9).
India is considered the AMR capital of the world. India has one of the largest numbers of antibiotic-resistant pathogens worldwide (10). The highest burden of multidrug-resistant tuberculosis cases has been reported in different parts of India, as alarmingly high-resistance bacterial cases (11). India is one of the largest consumers of antibiotics worldwide and consumption and sales of antibiotics continue to rise rapidly. Despite the decline in the number of cases of communicable diseases, the consumption of antibiotics continues to increase (12). On the one hand, emerging new multidrug-resistant (MDR) organisms pose newer diagnostic and therapeutic challenges in front of policymakers and health care workers, while on the other hand India is still striving to battle old enemies such as malaria, cholera, and tuberculosis (13). Infectious disease remains a leading cause of mortality in India. About 50,000 newborns lose the battle to sepsis annually due to pathogens resistant to first-line antibiotics (14). Two million deaths are anticipated in India due to AMR by the year 2050 (13). There are several factors such as illiteracy, congestion, poverty, malnutrition, and excessive antibiotic use that contribute AMR situation being worse in India. The lack of awareness about the pathogenesis of infectious diseases and their spread among the public and inaccessibility to healthcare further compound the situation. Easy availability of over-the-counter (OTC) antimicrobial drugs and self-prescription of these drugs without any professional knowledge regarding the dose and duration of treatment significantly contribute to AMR. The lack of tertiary care hospital facilities to diagnose patients with MDR, a significant load of resistant infections, and unregulated sales of antibiotics have contributed to a speedy rise in resistant infections in India. This has an enormous socioeconomic impact due to a large number of deaths and increased costs due to protracted stay in the hospital. Despite the high burden of AMR cases and the continuous rise in resistance cases, India spends only 4.7% of its total Gross Domestic Product (GDP) on health. However, the government shared only one-fourth (1.15%) of its GDP, making the task massive (15). The contribution of the Government of India to health is very poor (16). In 2017 the Government of India adopted a National Action Plan (NAP) on AMR.
The gut microbiota is essentially required to maintain gut homeostasis by mutually interacting with intestinal epithelial cells and mucosal immune cells (17). During prolonged inflammation, this interaction could become pathological due to changes in the composition and diversity of gut microbiota (18). The loss of diversity and compositions of gut microbiota may lead to disruption of intestinal homeostasis and deleterious clinical manifestations (19). Recent studies reported the key role of microbiota and their metabolites in the development of gut-derived infection, sepsis, and multiple organ dysfunctions in sepsis (20). Therefore, it is important to understand the gut microbiota composition, diversity, and functions of their metabolites during sepsis and other inflammatory conditions. The present review article may provide a more inclusive understanding of the mechanisms of gut-derived infection in the pathogenesis of sepsis and the design of new treatment options. Here, we present current knowledge and key concepts linking gut microbiota to the development and function of the immune system. Through this article, we discuss how AMR causes the defective host immune system activation by modulating the gut microbiota, the current progress in the field, and identify the need for experimental studies investigating the use of these treatments in sepsis management. Finally, we highlighted the challenges and perspectives of microbiome-targeted approaches in studying disease pathogenesis and developing new microbiome-related treatments.
Gut microbiota in health and disease
The mammalian gut contains highly diverse and wide varieties of the microbial community called the microbiome, which includes mostly bacteria, viruses, fungi, etc. Gut microbiota includes about 1,000 to 1,500 bacterial species (21). Gut microbiota is highly dynamic and varies from one individual to another individual. The diversity of gut microbiota can be imagined from the data obtained from an individual that contains only about 160 bacterial species (22). It indicates that the composition of gut microbiota is highly diverse among individuals and depends on nutrition, environmental changes, and genetic inheritance (21,22). Nutrition and environmental factors are very important in determining bacterial richness and diversity among individuals (23). A direct mutual association between gut microbiota and the host is reported in several studies. The gut provides a favorable condition for the growth and development of microbiota, and the gut microbiota supports the maturation of the mucosal immune system and metabolic system by providing beneficial nutrients such as vitamins and short-chain fatty acids (SCFAs) (24). Therefore, understanding the association between the gut microbiota and its metabolites with the intestinal immune system is vital for the development and maturation of the mucosal immune system.
A change in the richness and diversity of the microbiome profile in the gut is known as dysbiosis. The dysbiosis of gut microbiota is closely linked to several diseases, such as type 2 diabetes, obesity, hypertension, necrotizing enterocolitis (NEC), inflammatory bowel disease (IBD), etc. (18,25). Gut dysbiosis leads to the development of gut barrier dysfunction and bacterial translocation. It impairs the ability to maintain mucosal membrane function and contributes to systemic inflammation (26). When dysbiosis occurs, bacteria and bacterial endotoxins or toxins can leak from the gut, along with food particles. This systemic translocation of bacteria and bacterial products is responsible for other clinical manifestations in critically ill patients (27). The different bacterial genera present in the gut are likely to affect the intestinal environment of the hosts and alter the metabolic patterns and influence the occurrence of diseases. The altered gut microbiota is also associated with metabolic parameters, sex hormones, and the mediators of the gut-brain axis (22,28). Several studies have confirmed the role of gut microbiota in sepsis, but the direct association of gut microbiota diversity with the pathogenesis of disease and outcomes has not yet been fully understood (20). Figure 1 shows the association of gut microbiota dysbiosis with changed metabolites and immune system dysregulation in critical illness and disease.
Role of gut microbiota in sepsis and septic shock
Sepsis, defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, affects 1.7 million people annually in the United States (14). About 20%-30% of patients die annually across the globe due to sepsis (29). It is recognized as a global health emergency by WHO (30). The overwhelming inflammatory response is a hallmark property of sepsis. The exaggerated inflammatory response that occurs during sepsis may lead to immune suppression and dysregulated immune response (31). These dysregulated host immune responses during sepsis may lead to the dysfunction of multiple organ systems, which includes the cardiovascular, renal, pulmonary, hepatic, and gastrointestinal systems (31). Sepsis-induced hyperactivation of immune cells and immune suppression may be considered the main contributors to the pathophysiology of sepsis (32). Immune suppression often increases the individual’s susceptibility to secondary infections, further increasing the risk of death (33). The recent development of molecular-based sequencing tools has exposed the importance of gut microbiota diversity in human health and disease (34). Several studies have reported gut dysbiosis with a sharp decrease in diversity, overgrowth of pathogenic bacteria, and loss of commensal bacteria (21). Recent studies have elucidated key immune pathways that are modulated by gut microbiota and their metabolites (24). The altered gut microbiota during sepsis may influence inflammatory responses and increase gut barrier permeability, which could enable the translocation of pathogenic bacteria to the systemic circulation and distant organs. The increased gut permeability during inflammatory conditions and sepsis may lead to the translocation of enteric bacteria from the gut to the systemic circulation served as the motor of multiple organ dysfunction syndromes (MODS) and, subsequently, cause acute septic responses (35). The altered gut microbiota composition and diversity during inflammatory conditions and subsequently enhanced translocation of gut microbiota may cause mucosal immune dysfunction (35). The loss of membrane integrity and translocation of enteric bacteria and its metabolites from the gut to the systemic circulation is a hallmark property of sepsis and other inflammatory diseases (17). Further, the antibiotics treatment may change the composition and diversity of gut microbiota that may lead to the translocation of enteric bacteria and their metabolites across the epithelium, which may provide vital information about the importance of the microbiota in host resistance against pathogens (36).
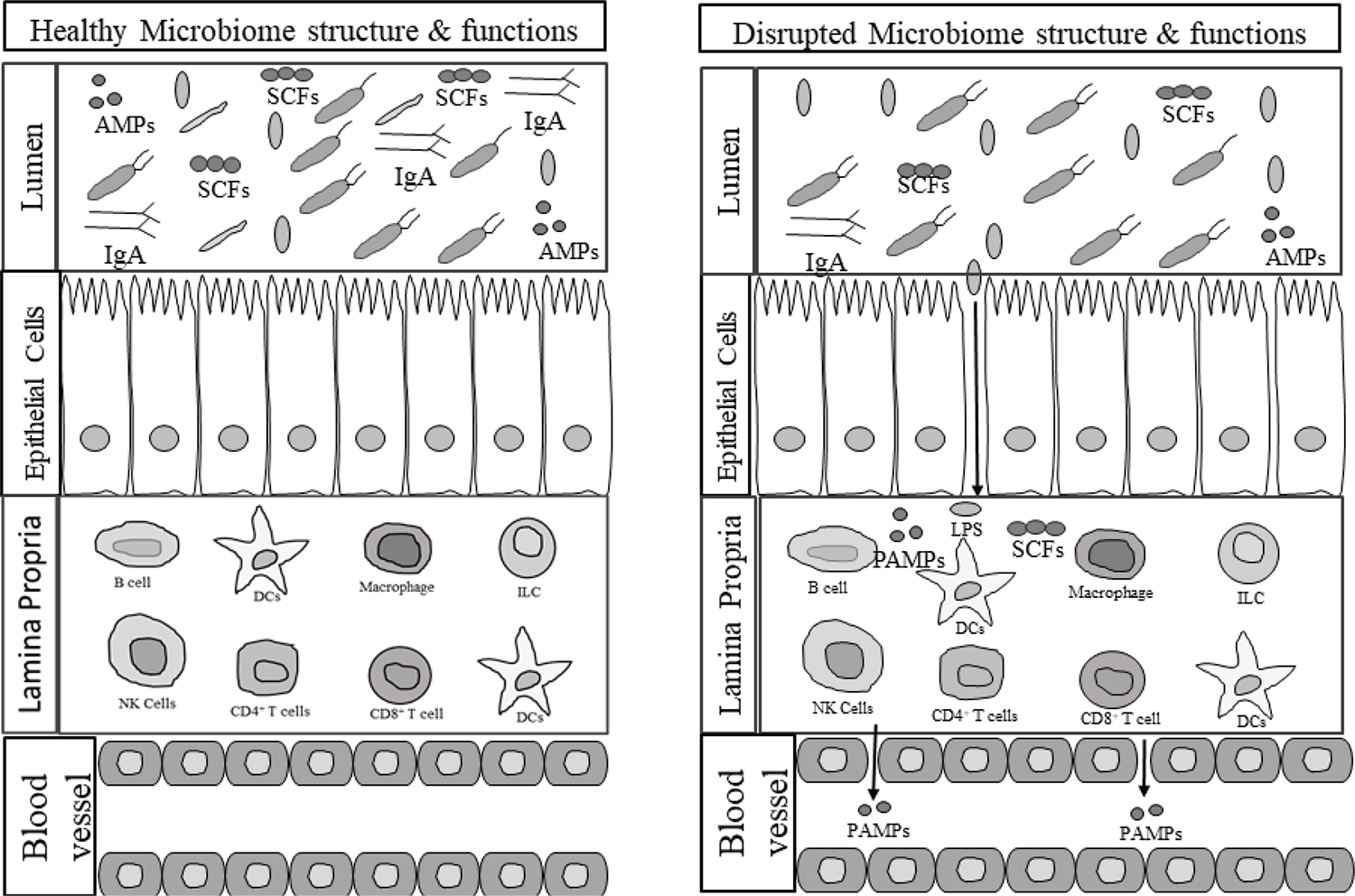
Fig. 1 - Gut microbiome dysbiosis predisposes to selection for pathogenic bacteria that leads to immune dysregulation, and decreased production of beneficial metabolites by the gut microbiome. AMPs = antimicrobial peptides; DCs = dendritic cells; ILC = innate lymphoid cell; LPS = lipopolysaccharides; PAMPs = pathogen associated molecular patterns; SCFAs = short-chain fatty acids
Despite the recent findings of gut dysbiosis to sepsis outcomes, the precise mechanisms underlying the protective effects of gut microbes in sepsis have not been well defined. Further, the role of gut microbiota and their metabolites in modulating the host mucosal immune response is not well known. More importantly, at present, we do not have diagnostic kits or therapies directed at the gut microbiome that could be implemented in the clinical management of sepsis. Patients in the early stages of sepsis manifest differences in their microbiome composition and diversity as compared to critically ill stages of sepsis (20). As compared to survivors, non-survivors with sepsis have pathogenic and antibiotic-resistant bacterial species such as Clostridia species and Enterococcus species (37). Use of excessive antibiotics for the treatment of sepsis patients inclines to gut dysbiosis and a state of immune suppression, with subsequent poor outcomes in the later course of hospitalization during sepsis (38). A study conducted in the murine model of sepsis confirmed the translocation of bacteria Klebsiella pneumoniae from the gut to the systemic circulation (35). A prospective cohort study conducted on 71 preterm infants with sepsis showed domination of the gut microbiota with bacilli and decreased abundance of anaerobic bacteria (39). A recent study highlighted that gut dysbiosis with an accumulation of bacilli (largely coagulase-negative staphylococci) and their fermentation metabolites could precede late-onset sepsis (40). A significant reduction of commensal bacteria and overgrowth of enteric bacteria may lead to overwhelming inflammation and inflammatory diseases. Several studies have highlighted the importance of diverse and balanced intestinal microbiota in enhancing the host’s immunity to intestinal and systemic pathogens, and disturbing this balance is likely to increase the susceptibility to sepsis (14,17). However, the role of gut microbiota and its association with the pathogenesis of sepsis is not yet fully understood. Moreover, association studies of the gut microbiota with clinical parameters and the outcome of patients with sepsis are urgently needed.
Effects of gut microbiota in modulating the mucosal immune response
The major components of the immune system that are involved in protecting the host against diverse pathogens are immune cells, tissues, organs, soluble mediators such as cytokines, and cell receptors. The gastrointestinal tract is considered the most important immunological organ in the body because it harbors up to 70% of the body’s lymphocyte population. The intestine mucosal immune system is an integral component of innate and adaptive immunity that includes three different mucosal lymphoid structures: Peyer’s patches (PP), the lamina propria (LP), and the epithelia. Beneath the epithelium, the LP harbors dendritic cells (DCs), which are potent antigen-presenting cells (APCs), and the gut-associated lymphoid tissue (GALT), which includes PP, lymphocytes, and intraepithelial lymphocytes (IELs). IELs are the first immune cells that encounter invading pathogens through an epithelial surface of the intestinal tract, urinogenital tract, and respiratory tract. The mucus layer present on the surface of epithelial cells along with secreting antimicrobial peptides (AMPs) in response to bacteria or pathogens primarily contributes to the intestinal innate host defense system (1). The intestinal epithelial cells are directly involved in defense against invading pathogens and also send signals to the mucosal immune system by producing soluble mediators such as cytokines and chemokines (41). The innate lymphoid cells (ILCs) located in the epithelial cells also work as the first line of defense and get activated in response to stimuli. Once ILCs get activated it produces various soluble mediators such as cytokines and chemokines that are essentially required for the development and maturation of the mucosal immune system (42). The composition and diversity of gut microbiota are vital in maintaining intestinal homeostasis in mammals. Dysbiosis of gut microbiota occurs due to excessive use of antibiotics during inflammatory conditions and sepsis may lead to the uncontrolled production of inflammatory mediators and overwhelming activation of innate immune cells. In addition, the adaptive immune system of the gut contributes to intestinal barrier defense by secreting immunoglobulins (Ig). The secreted Ig by the activated B cells in response to invading pathogens into the intestinal lumen neutralize the pathogenic microorganism and protect the mucosal tissue (43). The role of gut microbiota in shaping the host mucosal immune response is confirmed by several studies (24). The various metabolites such as SCFAs and tryptophan decomposition metabolites produced by gut microbiota are required to stimulate ILCs and enhance gut integrity (44). IELs are considered an important player in the adaptive immune response against invading pathogens (45). They are rich in αβ+ and γδ+ T-cell populations that are required to protect against germs and pathogens during inflammation (46). IELs showed a diverse immune response when they get activated with stimuli. Once IELs are activated, they express cytokines such as interferon-γ and growth factor, to protect epithelial cells from injury.
Recently, the association of gut microbiota with the development of host immunity has been confirmed by several studies. Colonization of commensal bacteria in early life is important for the metabolism of essential nutrients required for the host, for gut development, and for the maturation of the innate and adaptive immune system (47). A study conducted in germ-free (GF) animals showed that colonization of gut microbiota in the early stages of life is crucial for the optimum development and maturation of the immune system (48). Early studies on GF animals showed that the lack of colonization of commensal microbes is associated with significant intestinal defects in immune cell development and functions (49). Intestinal microbial colonization during the early-life stage is critical for the development of αβ and γδ IELs, induction of mucosal IgA antibodies, and Th17 cells (47). These immune dysfunctions are restored by microbial colonization, most notably with segmented filamentous bacteria and other commensal bacteria. Any abnormalities that lead to gut dysbiosis severely affected the development of intestinal mucosal immunity and make individuals more susceptible to secondary infections. A small animal study conducted in GF mice showed comparatively smaller mesenteric lymph nodes, PP, and reduced numbers of immune cells such as CD4+ T cells, CD8+ T cells, IgA-producing plasma cells, and intraepithelial T-cell receptors in mice having sepsis as compared to the control (14).
Challenges, pitfalls and future aspects in immune-microbiome research
Despite the impressive achievement that has greatly enhanced our understanding of gut microbiota diversity and its association with immune system development, many challenges remain in disentangling microbiome-immune system interactions in homeostasis and disease (50). Several mechanistic studies are required to explore the role of the commensal microbiome in modulating the host’s innate and adaptive immunity in health and disease. Recent studies conducted in animals show a bidirectional relationship exists between microbiome perturbation and immune dysregulation (48). Early-life colonization of gut microbiota and metabolites causing immune development, activation, and chronic inflammation conversely may shape the dysbiotic configuration and functions of microbial communities. However, a direct causal association between the richness and diversity of gut microbiota with immune development before the onset or during the early stages of the disease has not been established in most medical conditions. In the context of septic patients, a large human study cohort is required to find microbiota composition, diversity, and dysbiotic changes before, during, and after the occurrence of sepsis to identify the protective commensals and microbiota potentially associated with susceptibility to sepsis and worse outcomes. In addition, a multidimensional approach, including metabolomics, proteomics, single-cell transcriptomics, epigenomics, and meta-genomics, is required to elucidate how the gut microbiome and immune system are cross-regulated during sepsis. Finally, the microbiome composition and immune responses are highly variables among human individuals and disease states. This inherent inter-individual variability of the gut microbiome and associated complexity constitutes a major experimental challenge. This increases the likelihood of precision medicine concerning microbiota. It intrigued us to predict the personalized, host immune responses based on gut microbiome profiles in terms of treatment and prognosis. Therefore, the microbiota is a next-generation medicine and may facilitate the development of personalized microbiome-targeted treatments for immunological disease.
Conclusions
In summary, the intestinal microbiota is essentially required for the development and maturation of host immunity and contributes to maintaining intestinal homeostasis. Recent studies have shown the pivotal role of intestinal microbiota in modulating the host cellular immune response to stimuli and enhancing mucosal immunity. The mutual association between the gut microbiota and the host is required for the maturation and development of host gut immunity. The host provides a suitable environment for the growth of the microbiome, and subsequently, the gut microbiota facilitates the development and maturation of the mucosal immune system. The altered composition and diversity of gut microbiota especially AMR bacteria due to antibiotics treatment can lead to the translocation of enteric bacteria from the gut to the systemic circulation and cause the pathogenesis of sepsis. The interaction between the gut microbiota and mucosal immune system is key for controlling normal homeostasis and inflammatory response. Impaired communication between these two is associated with the pathogenesis of several inflammatory diseases and sepsis, and it highlights the importance of exploring the function of microbiota in such diseases.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contribution: All authors contributed equally to this manuscript.
References
- 1. Bhopale GM. Antimicrobial peptides: a promising avenue for human healthcare. Curr Pharm Biotechnol. 2020;21(2):90-96. CrossRef PubMed
- 2. Droz N, Hsia Y, Ellis S, Dramowski A, Sharland M, Basmaci R. Bacterial pathogens and resistance causing community acquired paediatric bloodstream infections in low- and middle-income countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2019;8(1):207. CrossRef PubMed
- 3. Samreen, Ahmad I, Malak HA, Abulreesh HH. Environmental antimicrobial resistance and its drivers: a potential threat to public health. J Glob Antimicrob Resist. 2021;27:101-111. CrossRef PubMed
- 4. Brinkac L, Voorhies A, Gomez A, Nelson KE. The threat of antimicrobial resistance on the human microbiome. Microb Ecol. 2017;74(4):1001-1008. CrossRef PubMed
- 5. Unemo M, Lahra MM, Escher M, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe. 2021;2(11):e627-e636. CrossRef PubMed
- 6. Diallo OO, Baron SA, Abat C, Colson P, Chaudet H, Rolain JM. Antibiotic resistance surveillance systems: a review. J Glob Antimicrob Resist. 2020;23:430-438. CrossRef PubMed
- 7. Naylor NR, Atun R, Zhu N, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7(1):58. CrossRef PubMed
- 8. Paladino JA, Sunderlin JL, Price CS, Schentag JJ. Economic consequences of antimicrobial resistance. Surg Infect (Larchmt). 2002;3(3):259-267. CrossRef PubMed
- 9. Murray CJL, Ikuta KS, Sharara F, et al; Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. CrossRef PubMed
- 10. Bhardwaj N, Mathur P, Behera B, Mathur K, Kapil A, Misra MC. Antimicrobial resistance in beta-haemolytic streptococci in India: a four-year study. Indian J Med Res. 2018;147(1):81-87. CrossRef PubMed
- 11. Veeraraghavan B, Walia K. Antimicrobial susceptibility profile & resistance mechanisms of Global Antimicrobial Resistance Surveillance System (GLASS) priority pathogens from India. Indian J Med Res. 2019;149(2):87-96. CrossRef PubMed
- 12. Broom A, Doron A. Antimicrobial resistance, politics, and practice in India. Qual Health Res. 2020;30(11):1684-1696. CrossRef PubMed
- 13. Ramachandran R, Muniyandi M. Rapid molecular diagnostics for multi-drug resistant tuberculosis in India. Expert Rev Anti Infect Ther. 2018;16(3):197-204. CrossRef PubMed
- 14. Stoll BJ, Puopolo KM, Hansen NI, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174(7):e200593. CrossRef PubMed
- 15. Mutua F, Sharma G, Grace D, Bandyopadhyay S, Shome B, Lindahl J. A review of animal health and drug use practices in India, and their possible link to antimicrobial resistance. Antimicrob Resist Infect Control. 2020;9(1):103. CrossRef PubMed
- 16. Verma A, Sahay S. Healthcare needs and programmatic gaps in transition from pediatric to adult care of vertically transmitted HIV infected adolescents in India. PLoS One. 2019;14(10):e0224490. CrossRef PubMed
- 17. Adelman MW, Woodworth MH, Langelier C, et al. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit Care. 2020;24(1):278. CrossRef PubMed
- 18. Scheithauer TPM, Rampanelli E, Nieuwdorp M, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11:571731. CrossRef PubMed
- 19. Chen Y, Zhou J, Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. 2021;11:625913. CrossRef PubMed
- 20. Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2(2):135-143. CrossRef PubMed
- 21. Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473-493. CrossRef PubMed
- 22. Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11(7):E1613. CrossRef PubMed
- 23. Sakkas H, Bozidis P, Touzios C, et al. Nutritional status and the influence of the vegan diet on the gut microbiota and human health. Medicina (Kaunas). 2020;56(2):88. CrossRef PubMed
- 24. Amoroso C, Perillo F, Strati F, Fantini MC, Caprioli F, Facciotti F. The role of gut microbiota biomodulators on mucosal immunity and intestinal inflammation. Cells. 2020;9(5):E1234. CrossRef PubMed
- 25. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek. 2020;113(12):2019-2040. CrossRef PubMed
- 26. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. 2014;111(42):E4485-E4493. CrossRef PubMed
- 27. Marizzoni M, Cattaneo A, Mirabelli P, et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis. 2020;78(2):683-697. CrossRef PubMed
- 28. Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46(1):77-89. CrossRef PubMed
- 29. Fleischmann C, Scherag A, Adhikari NKJ, et al; International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259-272. CrossRef PubMed
- 30. Rhee C, Jones TM, Hamad Y, et al; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2):e187571. CrossRef PubMed
- 31. Rossaint J, Zarbock A. Pathogenesis of multiple organ failure in sepsis. Crit Rev Immunol. 2015;35(4):277-291. CrossRef PubMed
- 32. Gupta DL, Bhoi S, Mohan T, Galwnkar S, Rao DN. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine. 2016;88:214-221. CrossRef PubMed
- 33. Gupta DL, Sharma A, Soni KD, Kazim SN, Bhoi S, Rao DN. Changes in the behaviour of monocyte subsets in acute post-traumatic sepsis patients. Mol Immunol. 2021;136:65-72. CrossRef PubMed
- 34. Chen R, Wang J, Zhan R, Zhang L, Wang X. Fecal metabonomics combined with 16S rRNA gene sequencing to analyze the changes of gut microbiota in rats with kidney-yang deficiency syndrome and the intervention effect of You-gui pill. J Ethnopharmacol. 2019;244:112139. CrossRef PubMed
- 35. Zhou X, Li J, Guo J, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6(1):66. CrossRef PubMed
- 36. Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22(6):458-478. CrossRef PubMed
- 37. Shimizu K, Yamada T, Ogura H, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22(1):239. CrossRef PubMed
- 38. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260-268. CrossRef PubMed
- 39. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496-503. CrossRef PubMed
- 40. Graspeuntner S, Waschina S, Künzel S, et al. Gut dysbiosis with bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin Infect Dis. 2019;69(2):268-277. CrossRef PubMed
- 41. Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell. 2017;171(4):783-794.e13. CrossRef PubMed
- 42. Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int J Mol Sci. 2021;22(14):7618. CrossRef PubMed
- 43. Li Y, Jin L, Chen T. The effects of secretory IgA in the mucosal immune system. BioMed Res Int. 2020;2020:2032057. CrossRef PubMed
- 44. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716-724. CrossRef PubMed
- 45. Ma H, Qiu Y, Yang H. Intestinal intraepithelial lymphocytes: maintainers of intestinal immune tolerance and regulators of intestinal immunity. J Leukoc Biol. 2021;109(2):339-347. CrossRef PubMed
- 46. Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol. 2019;10:861. CrossRef PubMed
- 47. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539-544. CrossRef PubMed
- 48. Hapfelmeier S, Lawson MAE, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705-1709. CrossRef PubMed
- 49. Hernández-Chirlaque C, Aranda CJ, Ocón B, et al. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohn’s Colitis. 2016;10(11):1324-1335. CrossRef PubMed
- 50. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341-352. CrossRef PubMed
