 |
Drug Target Insights 2023; 17: 31-38 ISSN 1177-3928 | DOI: 10.33393/dti.2023.2510 REVIEW |
 |
Association between cardiovascular diseases and periodontal disease: more than what meets the eye
ABSTRACT
Cardiovascular diseases (CVDs) are inflammatory diseases of coronary arteries accompanying atheroma formation that can spawn impairment and, in severe cases, death. CVDs are the leading cause of death in the world. In recent decades, investigators have focused their impact on CVD by periodontal disease (PD). PD is a risk factor that can trigger the formation, maturation, and instability of atheroma in the arteries. Two mechanisms have been proposed to explain this relationship: periodontopathic pathogens explicitly invade the circulation or indirectly increase systemic levels of inflammatory mediators. It has been suggested that improvement in disease state has a positive effect on others. This review summarizes evidence from epidemiological studies as well as researches focusing on potential causation channels to deliver a comprehensive representation of the relationship between PD and CVD.
Keywords: Cardiovascular diseases, Periodontal disease, Periodontal therapy, Risk factor, Systematic review, Systemic diseases
Received: October 25, 2022
Accepted: November 10, 2022
Published online: February 2, 2023
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, claiming an estimated 17.9 million lives each year. CVD is an encyclopedic term for heart and blood vessel disorders. Atherosclerosis is an underlying cause of CVD. Atherosclerosis is a chronic vascular inflammatory condition characterized by lipid deposition (plaque) in the arterial wall (1). Atherosclerotic formation and its advancement could diminish arterial blood flow and cause ischemia in tissues or organs, as well as endorse clotting.
On the bright side, cardiovascular mortality has decreased. If combating infectious diseases was the public health success story of the first half of the 20th century, then the decline in mortality rates from CVD is the success story of the last four decades: a sharp decline in mortality rates, aided by rapid advances in both areas of prevention and treatment, including drastic reductions in smoking, improvements in the treatment and control of hypertension, and the widespread use of statins.
Periodontitis is the sixth most common disease in humans, affecting 740 million people worldwide. Periodontitis is a bacterially induced chronic tissue destructive inflammation of the teeth. This periodontal microbiota causes the release of proinflammatory mediators both locally and systemically. As the paradigm of chronic infection in dental pathology, periodontal disease (PD) shares several pathogenic pathways with CVDs. As a result of the low‐grade state of systemic inflammation posed by periodontitis, it is considered to be strongly associated with CVDs (2).
There is robust association between CVD and PD. The delineating focus of the relationship has been the periodontal pathogens from the oral cavity, which directly exacerbate CVD in which chronic periodontal inflammation at the site of infection increases circulating levels of inflammatory mediators, and bacteria dispersed into the circulation provokes host inflammatory arbiter, which unswervingly alters other systemic diseases.
Several studies have been conducted to determine whether PD is associated with risk factors for CVD (3). C-reactive protein (CRP), homocysteine, fibrinogen, high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) have all been studied as CVD markers (4,5).
From a public health standpoint, CVD is the most significant of all the systemic conditions associated with PD, accounting for high mortality rates in most countries. Because multiple intervention studies, meta-analyses, and systematic reviews have been published in this area, this review aims to answer the following questions: Is there a link between CVD and periodontitis? What does the literature tell us after more than two decades of research, and why is this such a difficult question to answer? What does the Bradford-Hill criteria suggest about this unique association? What are our current circumstances, and what are our prospects for the future?
Mechanism and etiopathogenesis
To explain how PD influences CVD, two mechanisms have been proposed. First, periodontopathic flora directly annex endothelial cells via a direct mechanism (6). Polymerase chain reaction assays for atherosclerotic plaques support this theory. Streptococcus mutans was found to be the most common bacteria in cardiovascular specimens containing thrombus tissues (78%), followed by Aggregatibacter actinomycetemcomitans (7). Other bacteria found in atherosclerotic lesions in coronary arteries include Tannerella forsythia, Prevotella intermedia, and predominantly Porphyromonas gingivalis. Still, it remains unclear how the existence of periodontopathogenic organisms impacts atherosclerosis intracellularly, but few pathogens, such as P. gingivalis, may induce formation of foam cells or tenacity in cells, resulting in tributary inflammation and endothelial dysfunction (8,9).
The second proposed mechanism is the indirect pathway where PD causes increases in the levels of inflammatory cytokines. PD induces an inflammatory response, which results in elevation in levels of various inflammatory mediators, including interleukin 8, interleukin 6, interleukin 1 and tumor necrosis factor, which are also linked to atherosclerotic vascular disease. Some can speed up the production and emission of fibrinogen and CRP. Moreover, bacterial lipopolysaccharides plunge the flow and elicit strong immune response (Fig. 1). These elements influence atherosclerosis by acting on endothelial cells, increase the oxidative stress, and harmonize the lipid metabolism. This is confirmed by a previous study in which endothelial dysfunction was found in patients with periodontitis.
To jot down, it is intelligible that due to PD, inflammation persuades which can immigrate the periodontopathic organisms or leak out the inflammatory cytokines into the circulatory system which might either lead to systemic inflammation or periodontal pathogens may end up in vascular tissues (Fig. 2) which has a final ultimatum – formation, maturation, and exacerbation of atheromatous plaque.
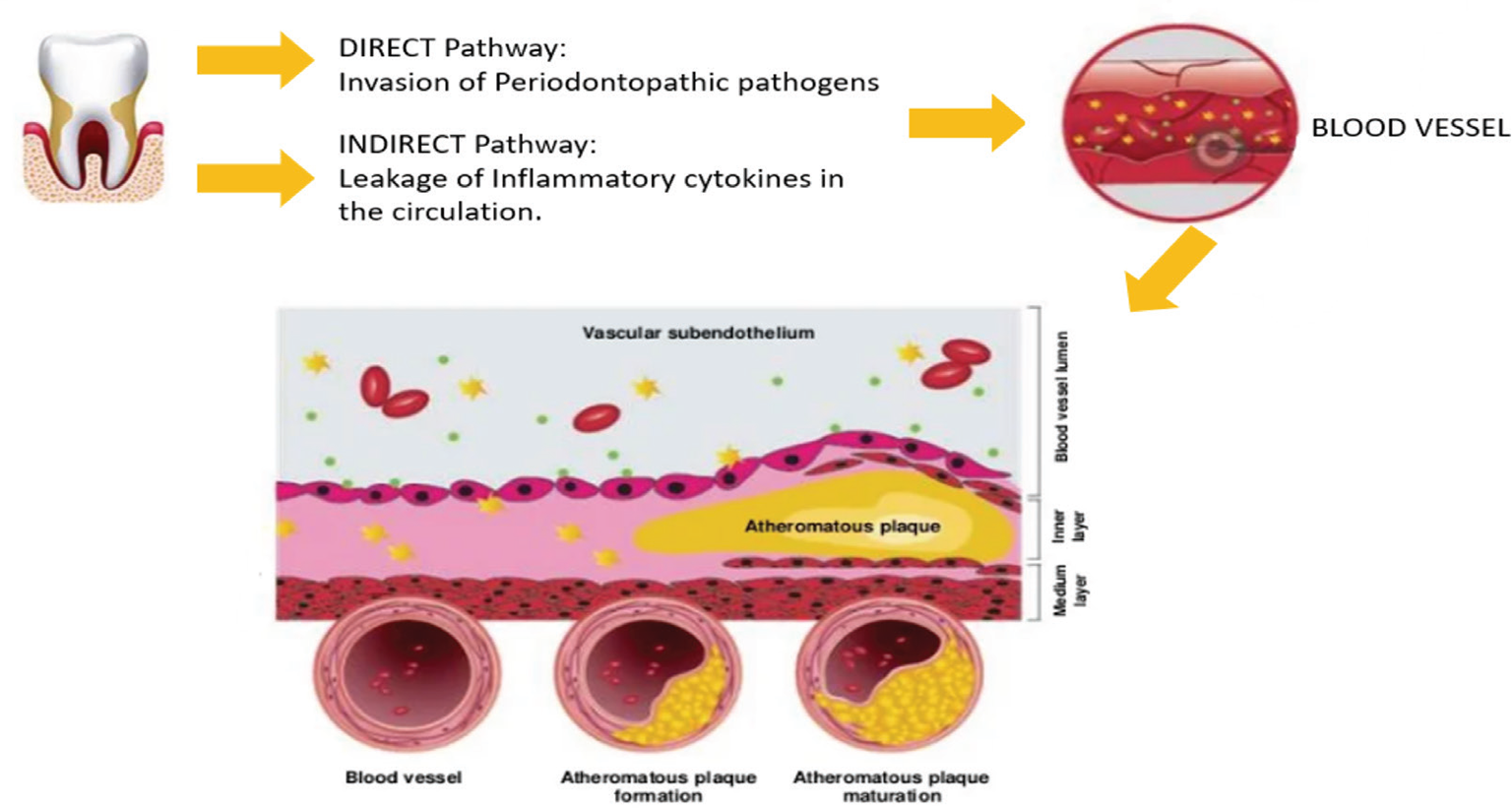
Fig. 1 - Direct and indirect relationship between periodontal disease and cardiovascular disease.
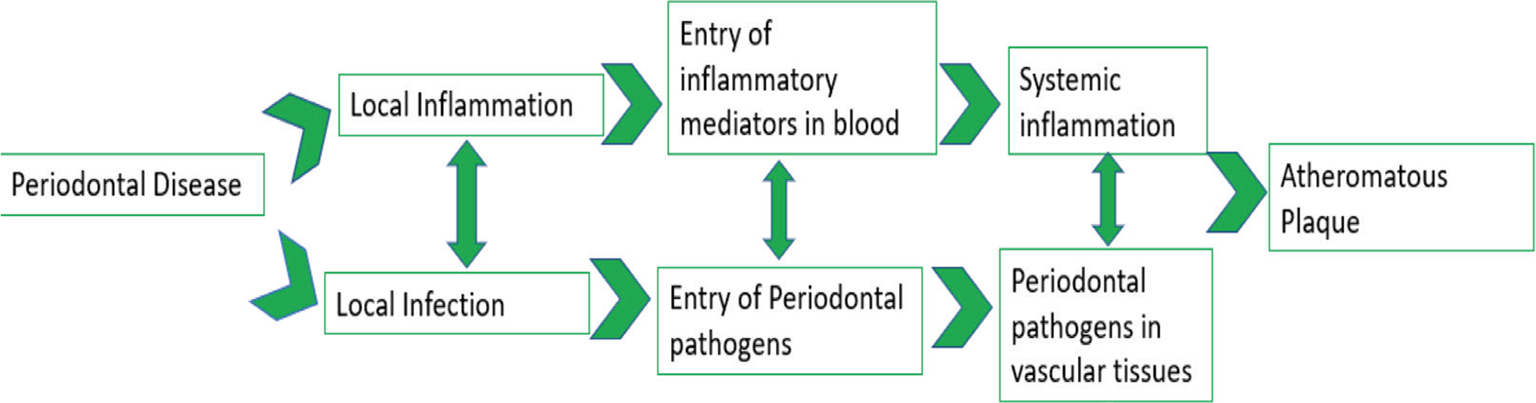
Fig. 2 - Etiopathogenesis flowchart.
Epidemiological studies and literature review of the PD and systemic disease connection
Certainly, contemporary evidences have imparted useful knowledge on common biomarkers of CVD and PD, which has prognostic as well as diagnosing aptitude to crucially decrease the menace of abominable cardiac episode in an untimely manner (Tab. I).
Cross-sectional and case-control studies
Genco and colleagues investigated the link between certain subgingival periodontopathogens and myocardial infarction (MI) (20), comparing 233 controls with 97 nonfatal MI patients. Noninfected individuals were compared with infected individuals. For MI, odds ratio for the presence of T. forsythia was 2.99 and for P. gingivalis was 2.52. These results support the idea that specific pathogenic bacteria found in PD may also be associated with MI.
Arbes et al (NHANES III) studied the relationship between PD and coronary heart disease (CHD) and found that with increase in the severity of periodontitis the likelihood of suffering an MI increased. The study thus confirmed with other studies the link and displayed an undeviating robust association amidst increased severity of periodontitis and CVD (21).
Longitudinal studies
DeStefano and colleagues reviewed NHANES-I data as well as a 15-year epidemiologic follow-up. They discovered periodontitis was one significant predictor of CVD in 9,760 men and women (22). These relationships were unaffected by age, gender, body mass index, education, marital status, poverty index, race, blood pressure, alcohol consumption, diabetes status, and serum cholesterol levels.
Beck and coworkers conducted a study of 921 men who did not have coronary artery disease at baseline. A total of 40 had stroke, 59 died of coronary artery disease, and 207 men developed coronary artery disease during an 18-year follow-up period. Odds ratios for total periodontal bone loss and CHD, fatal CHD and stroke, CVD risk factors and age were 1.5, 2.8, and 1.9, respectively. Accordingly, the odds of suffering a vascular event or CHD were 0.5-2.8 times higher in individuals with radiographically proven periodontitis (23).
Hujoel et al conducted a longitudinal study that found no link between chronic heart disease and periodontitis (24). These authors evaluated the NHANES-I study and the results of their 21-year follow-up. It is worth noting that DeStefano et al (22) used the same database and discovered a link between CVD and PD in NHANES-I study 15 years later.
Hujoel et al adjusted extensively for potential confounders, which may have explained the lack of a relationship after adjustment (24). Hujoel et al may have adjusted too heavily for factors strongly associated with infection, such as PD. It is also possible that periodontal status of subjects was significantly misclassified over time. In addition, because of treatments and extractions over time, the authors may have misclassified subjects who had PD at baseline. The misclassification being non-differential, which would have been worsened through 21-year follow-up period, could brace the null hypothesis of the research that there is no link between CVD and PD.
Joshipura and colleagues discovered that after controlling for other risk factors, the association between self-reported history of PD and incidence of heart disease was no longer significant (25). However, these researches merit further discussion as they were derived from a well-characterized, large longitudinal study. The majority of studies that found a correlation discovered that the amount of PD was significant. It would be impossible to quantify the extent of PD present in Joshipura’s research as they answered a “yes or no” query about PDs. Furthermore, discrepancy based on self-reported PD is possible.
The most recent longitudinal study, published by Howell and colleagues (26), was a double-blind, randomized, placebo-controlled trial of beta-carotene and aspirin for the prevention of CVD and cancer in the United States in 22,071 male physicians. The study outcomes were nonfatal MI, stroke, and death from CVD. After controlling for the treatment and age (beta-carotene and aspirin), the researchers discovered a positive non-significant trend (95% confidence interval [CI], relative risk [RR] = 1.13).
Although most evidence from case-control and longitudinal researches advocates a link between CVD and periodontitis, the link seems to be dwindling. There is insufficient evidence to conclude that the associations are contributory.
Observational studies: from systematic review to meta-analysis
Scannapieco et al (27), in a comprehensive systematic review, concluded that there is moderate evidence of a relation between MI, CVD, atherosclerosis, and PD, but the causation was uncertain.
Khader et al (5), in a meta-analysis, combined two cross-sectional studies and six cohort and found a lower RR of 1.15 (95% CI [1.06-1.25]).
Nine cohort researches compiled by Janket et al (28) in a meta-analysis suggests that, for future CVD and cyclic vomiting syndrome episodes, PD is a determined risk factor and discovered that chronic periodontitis patients had a 19% surged peril for advancing such events. People under the age of 65 were at a higher risk (44%).
Another meta-analysis of observational studies by Alessandra Blaizot et al investigated the relationship between CVD and periodontitis exposure (29). Researches done between 1989 and 2007 were recovered from seven databases via electronic and manual searches. The MOOSE meta-analysis guidelines for observational studies were followed (30). There were 47 observational studies among the 215 epidemiological studies, 29 of which could be combined using meta-analysis methodology. There was increased risk (about 34%) of developing CVD in individuals having periodontitis than in individuals who are not exposed with periodontitis, where 1.34 was the RR of these seven cohort researches (p = 0.0001). This finding suggests that people with Parkinson’s disease are at higher risk for developing CVD.
Interventional studies
Owing to many factors, for example, financial, ethical, or methodological, the periodontal intervention and its efficacy as primary prevention for CVDs such as Ischemic Heart Disease (IHD) and death haven’t yet been studied (50).
Consequently, CVD proxy markers have been thoroughly investigated, and periodontal intervention shows substantial effect on these markers, as shown in Table II. There is limited affirmation on durable effect of periodontal intervention on these proxy markers. In addition, the impact of periodontal intervention on the scientific conclusions of these markers of CVD remains to be still investigated.
Previous research has found that rigorous periodontal intervention transiently impairs the endothelial function role, which raises levels of serum inflammatory markers, probably due to liberation of inflammatory mediators or bacterial organisms in bloodstream. Nevertheless, after a few weeks of periodontal intervention the levels of inflammatory makers and periodontal parameters also seem to lower or decrease (51,52,53,54). Furthermore, 6 months after periodontal treatment, carotid intimal-medial thickness is reduced. Several interventions and researches have been done as shown in Table II, which has been published in the past few years, and they all brace up for the hypothesis that periodontal intervention reduces cardiovascular risk factor and thus has an impact on CVD events (55).
Imminent interventional studies are required toward better understanding of the association between PD and CVD, mainly the biological effects of PD on the atherogenic cascade by influencing the vascular endothelium. Further enduring intervention researches are needed, ideally utilizing similar methods to assess CVD events, to determine whether the reported benefits of periodontal intervention can actually decipher the reduction in incidence of CVD.
Microbiological studies
Clinically, it is extremely strenuous to determine the causal agent of atherosclerosis. First, endothelial damage advances by masking the causative agent and progresses without symptoms. Second, multiple contributing factors cause atherosclerosis, and these influencers may coexist, making it difficult to determine the causative factor (56,57). In addition, studies of interventions have shown mixed results. Sometimes after periodontal intervention there is enhancement, whereas sometimes there is brief deterioration of the symptoms and sometimes there is no change.
However, seven rules must be met for promoting atherosclerosis by the periodontal pathogens, which are enlisted below (50).
Evidence 1: Systemic vascular tissues can provide a pathway for periodontal pathogens. Numerous researches have demonstrated that periodontopathic bacteria could cause bacteremia by entering the systemic circulation (50,58,59,60,62). A previous systematic review found that bacteremia after periodontal procedures could be atop 50% (63,64). Table III summarizes the prevalence of periodontopathic pathogens in systemic circulation after periodontal intervention in atheromatous lesions, with and without periodontal procedures in periodontitis patients. Following periodontal procedures, periodontopathic organisms may infiltrate the circulation, being a determinant of atherosclerosis.
Evidence 2: Affected tissues accommodate periodontal pathogens. Several studies have provided sufficient evidence that DNA, RNA, and antigen sequencing can be used to identify different periodontal species in atheromatous lesions (65,66,67). Analysis shows that periodontitis patients are at increased risk for emergent atherosclerosis.
Evidence 3: In the affected site, live periodontal pathogens will be present. This proof requires the detection of live periodontopathic bacteria. Live A. actinomycetemcomitans and P. gingivalis were isolated from atherosclerotic samples in multiple studies (68,69).
Evidence 4: Invasion of the affected cell with in vitro evidence. Several in vitro researches show periodontopathic organisms can invade various types of host cells. According to many studies P. gingivalis is responsible for infiltrating the endothelial cells in studies, the significance as well as the mechanism of the specific strain type are being investigated further (70,71,72).
Evidence 5: Provides indication that periodontal pathogen can encourage atherosclerosis in diseased animal model. In 2012, the European Federation of Periodontology and the American Academy of Periodontology published a review that found proof that periodontal pathogens can endorse atherosclerosis (56). P. gingivalis is shown to promote atherosclerosis in murine (73), rabbit (74), and pig models (75). Furthermore, when hyperlipidemic mice were orally infected with Fusobacterium nucleatum, T. forsythia, P. gingivalis, Treponema denticola, and other expedient organisms from this specific class were found in aorta, atherosclerotic plaque, and oral epithelium (76,77).
Evidence 6: Pathology is significantly less when caused by noninvasive mutants, according to in vitro and in vivo evidence where the incursion of vascular cells and tissues by bacterial strains is been investigated. Noninvasive fimA-deficient mutant of P. gingivalis exhibits fewer proinflammatory mediators than the invasive wild-type strain of P. gingivalis (73).
Evidence 7: Fulfill a modified version of Koch’s postulate to show that a human atheroma isolate causes disease in animal models. To do this, isolate the periodontopathogen from a human atheroma and induce atheroma formation in an animal model after inoculation. P. gingivalis were isolated from atherosclerotic samples. There is also evidence that suffused pathogenic bacteria can cause atherosclerosis. The evidence is still, however, considered incomplete as the bacterial strain utilized were not isolated from human atherosclerotic samples (68,76,77).
Apart from Evidence 7, there are abundant researches available to support Evidences 1 to 6. Nonetheless, the first six proofs embrace the notion that periodontal pathogens are linked to CVD.
Neoteric substantiation of CVD and PD
Febbraio et al (88) concluded that there is an association between oral health and CVD, but causality has yet to be established. However, studies show improvements in cardiovascular risk factors following periodontal treatments, although with relatively short follow-up periods. Rational evidence suggests that good oral health contributes to overall and heart health. The best bet is to continue to remind patients that a healthy mouth contributes to a healthy heart.
Fazal et al (19) concluded that Non Surgical Periodontal Therapy (NSPT) lowers cardiac biomarker concentrations in patients with chronic periodontitis and may reduce the risk of CVD in the future. However, Paul et al (89) suggest a contradictory result in a review that therapeutic periodontal interventions cannot be used to prevent heart disease or stroke.
Larvin et al (90) found an increased risk of CVD in people with PD in a systematic review and meta-analysis. Males and people with severe PD are at the highest risk of developing CVD, indicating potential target populations for future public health interventions and inspection.
In a cross-sectional observational single-center study, Lazureanu et al (91) concluded that increasing patients’ awareness of oral healthcare measures resulted in better outcomes and improved oral-health-related quality of life.
In a 13-year follow-up study, Tiensripojamarn et al (92) show that severe periodontitis is linked to a higher incidence of CHD, independent of existing cardiovascular risk factors.
Periodontitis Grade B/C was linked to a higher overall cardiovascular risk, and this association was not explained by smoking confounding in participants aged 65-74 years, according to Petrenya et al (93). They also recommended that patients with periodontitis, particularly those with extensive alveolar bone loss, use NORRISK 2 score for cardiovascular risk assessment. Individuals with a high cardiovascular risk profile should have routine periodontal examinations. In addition to adequate, evidence-based periodontal intervention, cessation of smoking and blood pressure normalization are essential in lowering cardiovascular risk in individuals with PD.
What do the Bradford-Hill criteria imply for the relationship between CVD and PD?
The Canadian Dental Hygienists Association (CDHA) published a position paper that used the Bradford-Hill criteria to determine whether there is sufficient evidence for a causal relationship between PD and CVD (87). The Bradford-Hill criteria analysis found no evidence of a link between PD and CVD. Although the link between PD and CVD is well established, the findings of the CDHA’s recent position paper show that, while an association exists, the nature of that link is unknown, and there is insufficient evidence for that association to be causal at this time.
Conclusion
Epidemiologic researches have now substantiated that there may be a link between PD and CVD. Although research continues to point to a connection between CVD and oral health, causality has not yet been established. Despite the fact that the follow-up times in most studies are brief, numerous studies show an improvement of CVD risk factors after periodontal interventions. The association of good oral health and general and heart health is proved by reasonable evidence.
In the coming decades, medical and dental professionals must be capable of better planning of preventive interventions as constant researches approve and account the forte of the consortium between CVD and PD. Scientific data assembled thus far would seem to support the continued value of interventional periodontal therapy not just for oral health but for overall health as well.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contribution: All authors contributed equally to this manuscript.
References
- 1. Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56.
- 2. Nesse W, Dijkstra PU, Abbas F, et al. Increased prevalence of cardiovascular and autoimmune diseases in periodontitis patients: a cross‐sectional study. J Periodontol. 2010 Nov;81(11):1622-1628.
- 3. Sanz M, Del Castillo AM, Jepsen S, et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol. 2020 Mar 1;47(3):268-288.
- 4. Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160:2749-2755.
- 5. Khader YS, Albashaireh ZS, Alonari MA. Periodontal diseases and the risk of coronary heart and cerebrovascular diseases: a meta-analysis. J Periodontol. 2004;75:1046-1053.
- 6. Ford PJ, Gemmell E, Chan A, et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. 2006;21:206-211. CrossRef
- 7. Nakano K, Nemoto H, Nomura R, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. 2009;24:64-68. CrossRef
- 8. Pucar A, Milasin J, Lekovic V, et al. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J Periodontol. 2007;78:677-682. CrossRef
- 9. Roth GA, Moser B, Huang SJ, et al. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J Thromb Haemostasis. 2006;4:2256-2261. CrossRef
- 10. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C‐reactive protein level. J Periodontol. 2001 Sep;72(9):1221-1227.
- 11. Singh N, Chandel S, Singh H, Agrawal A, Savitha AN. Effect of scaling & root planing on the activity of ALP in GCF & serum of patients with gingivitis, chronic and aggressive periodontitis: a comparative study. J Oral Biol Craniofac Res. 2017 May 1;7(2):123-126.
- 12. Delange N, Lindsay S, Lemus H, Finlayson TL, Kelley ST, Gottlieb RA. Periodontal disease and its connection to systemic biomarkers of cardiovascular disease in young American Indian/Alaskan natives. J Periodontol. 2018 Feb;89(2):219-227.
- 13. Mohammed AA, Youssef JM, Metwally SS, Anees MM. Evaluation of the serum ceruloplasmin level before and after non-surgical periodontal therapy in patients with chronic periodontitis. Stomatological Dis Sci. 2018 Mar 14;2:3.
- 14. Ilea A, Lazăr AC, Roșca D, et al. Periodontitis in a group of patients with cardiovascular disease. Anatomy Physiol Biochem Int J. 2018;5(4):1-6. CrossRef
- 15. Leira Y, Blanco J. Brain natriuretic peptide serum levels in periodontitis. J Periodontal Res. 2018 Aug;53(4):575-581.
- 16. Ameen M, Attia AM, Felimban A, et al. Evaluation of cardiac biomarkers in smokers and non-smokers with chronic periodontitis. Int J Health Sci. 2020 May;14(3):26.
- 17. Gupta M, Chaturvedi R, Jain A. Role of cardiovascular disease markers in periodontal infection: understanding the risk. Indian J Dent Res. 2015 May 1;26(3):231.
- 18. Boyapati R, Vudathaneni V, Nadella SB, Ramachandran R, Dhulipalla R, Adurty C. Mapping the link between cardiac biomarkers and chronic periodontitis: a clinico-biochemical study. J Indian Soc Periodontol. 2020 Jul;24(4):309.
- 19. Fazal I, Shetty B, Yadalam U, Khan SF, Nambiar M. Effectiveness of periodontal intervention on the levels of N-terminal pro-brain natriuretic peptide in chronic periodontitis patients. J Circ Biomark. 2022 Oct 3;11:48-56.
- 20. Genco RJ, Wu T, Grossi S, Faulkner K, Zambon JJ, Trevisan M. Periodontal microflora related to the risk of myocardial infarction: a case-control study (abstract 2811). J Dent Res 1999;78(special issue):457.
- 21. Arbes SJ, Slade GD, Beck J. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res 1999;78:1777-1782.
- 22. DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688-691.
- 23. Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123-1137.
- 24. Hujoel P, Drangsholt M, Spiekerman C, DeRouen T. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406-1410.
- 25. Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res 1996;75:1631-1636.
- 26. Howell TH, Ridker PM, Ajani UA, Hennekens CH, Christen WG. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardiol. 2001;37:445-450.
- 27. Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease and stroke: a systematic review. Ann Periodontol. 2003;8:38-53.
- 28. Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:559-569.
- 29. Blaizot A, Vergnes JN, Nuwwareh S, Amar J, Sixou M. Periodontal diseases and cardiovascular events: meta-analysis of observational studies. Int Dent J. 2009;59:197-209.
- 30. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012.
- 31. Caúla AL, Lira-Junior R, Tinoco EM, Fischer RG. The effect of periodontal therapy on cardiovascular risk markers: a 6-month randomized clinical trial. J Clin Periodontol. 2014;41:875-882. CrossRef
- 32. Vidal F, Cordovil I, Figueredo CM, Fischer RG. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol. 2013;40:681-687. CrossRef
- 33. Bresolin AC, Pronsatti MM, Pasqualotto LN, et al. Lipid profiles and inflammatory markers after periodontal treatment in children with congenital heart disease and at risk for atherosclerosis. Vasc Health Risk Manage. 2013;9:703-709. CrossRef
- 34. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551-2567. CrossRef
- 35. Bokhari SA, Khan AA, Butt AK, et al. Nonsurgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. 2012;39:1065-1074. CrossRef
- 36. Banthia R, Jain P, Banthia P, Belludi S, Parwani S, Jain A. Effect of phase I periodontal therapy on pro-coagulant state in chronic periodontitis patients – a clinical and haematological study. J Irish Dental Assoc. 2013;59:183-188.
- 37. Kiany F, Hedayati A. Evaluation of serum anti-cardiolipin antibodies after non-surgical periodontal treatment in chronic periodontitis patients. Odontology. 2015;103:203-209. CrossRef
- 38. Gupta B, Sawhney A, Patil N, et al. Effect of surgical periodontal therapy on serum C-reactive protein levels using ELISA in both chronic and aggressive periodontitis patient. J Clin Diagnostic Res. 2015;9:Zc01-Zc05. CrossRef
- 39. Graziani F, Cei S, Orlandi M, et al. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: a randomized clinical trial. J Clin Periodontol. 2015;42:843-852. CrossRef
- 40. Houcken W, Teeuw WJ, Bizzarro S, et al. Arterial stiffness in periodontitis patients and controls. A case-control and pilot intervention study. J Hum Hypertens. 2016;30:24-29. CrossRef
- 41. Torumtay G, Kirzioglu FY, Öztürk Tonguç M, Kale B, Calapoglu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res. 2016;51:489-498. CrossRef
- 42. Siddeshappa ST, Nagdeve S, Yeltiwar RK, Parvez H, Deonani S, Diwan V. Evaluation of various hematological parameters in patients with periodontitis after nonsurgical therapy at different intervals. J Indian Soc Periodontol. 2016;20:180-183. CrossRef
- 43. Arvanitidis E, Bizzarro S, Alvarez Rodriguez E, Loos BG, Nicu EA. Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy. Thromb J. 2017;15:5. CrossRef
- 44. Zhou QB, Xia WH, Ren J, et al. Effect of intensive periodontal therapy on blood pressure and endothelial microparticles in patients with prehypertension and periodontitis: a randomized controlled trial. J Periodontol. 2017;88:711-722. CrossRef
- 45. de Souza AB, Okawa RT, Silva CO, Araújo MG. Short-term changes on C-reactive protein (CRP) levels after non-surgical periodontal treatment in systemically healthy individuals. Clin Oral Investig. 2017;21:477-484. CrossRef
- 46. Jockel-Schneider Y, Bechtold M, Haubitz I, et al. Impact of anti-infective periodontal therapy on parameters of vascular health. J Clin Periodontol. 2018;45:354-363. CrossRef
- 47. Saffi MAL, Rabelo-Silva ER, Polanczyk CA, et al. Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis. (2018) 24:1349-1357. CrossRef
- 48. Morozumi T, Yashima A, Gomi K, et al. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J Periodontal Res. 2018;53:536-544. CrossRef
- 49. Moeintaghavi A, Arab HR, Moghaddam MA, Shahmohammadi R, Bardan BY, Soroush Z. Evaluation of effect of surgical and nonsurgical periodontal therapy on serum C-reactive protein, triglyceride, cholesterol, serum lipoproteins and fasting blood sugar in patients with severe chronic periodontitis. Open Dent J. 2019;13:15-21. CrossRef
- 50. Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontology 2000. 2020;83:66-89. CrossRef
- 51. Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911-920. CrossRef
- 52. Teeuw WJ, Slot DE, Susanto H, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014;41:70-79. CrossRef
- 53. D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269-273. CrossRef
- 54. D’Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. 2006;151:977-984. CrossRef
- 55. Piconi S, Trabattoni D, Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2009;23:1196-1204. CrossRef
- 56. Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol. 2013;40(Suppl.)14:S30-S50. CrossRef
- 57. Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!” – epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dental Res. 2010;89:879-902. CrossRef
- 58. Joshipura K, Zevallos JC, Ritchie CS. Strength of evidence relating periodontal disease and atherosclerotic disease. Compend Contin Educ Dent. 2009;30:430-439.
- 59. Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419-S420. CrossRef
- 60. Ratto-Tespestini A, Chaparro PJ, Romito G, et al. Comparison of independent and dependent culture methods for the detection of transient bacteremia in diabetic subjects with chronic periodontitis. Biomédica Revista del Instituto Nacional de Salud. 2016;36:156-161. CrossRef
- 61. Balejo RDP, Cortelli JR, Costa FO, et al. Effects of chlorhexidine preprocedural rinse on bacteremia in periodontal patients: a randomized clinical trial. J Appl Oral Sci. 2017;25:586-595. CrossRef
- 62. Dhotre S, Jahagirdar V, Suryawanshi N, Davane M, Patil R, Nagoba B. Assessment of periodontitis and its role in viridans streptococcal bacteremia and infective endocarditis. Indian Heart J. 2018;70:225-232. CrossRef
- 63. Horliana ACRT, Chambrone L, Foz AM, et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 2014;9:e98271. CrossRef
- 64. Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82:1469-1477. CrossRef
- 65. Figuero E, Lindahl C, Marín MJ, et al. Quantification of periodontal pathogens in vascular, blood, and subgingival samples from patients with peripheral arterial disease or abdominal aortic aneurysms. J Periodontol. 2014;85:1182-1193. CrossRef
- 66. Serra e Silva Filho W, Casarin RC, Nicolela EL Jr, et al. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS One. 2014;9:e109761. CrossRef
- 67. Armingohar Z, Jørgensen JJ, Kristoffersen AK, Abesha-Belay E, Olsen I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol. 2014;6:1-13. CrossRef
- 68. Rafferty B, Jönsson D, Kalachikov S, et al. Impact of monocytic cells on recovery of uncultivable bacteria from atherosclerotic lesions. J Internal Med. 2011;270:273-280. CrossRef
- 69. Kozarov EV, Dorn BR, Shelburne CE, Dunn WA Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscleros Thromb Vasc Biol. 2005;25:e17-e18. CrossRef
- 70. Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337-5343. CrossRef
- 71. Dorn BR, Dunn WA Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792-5798. CrossRef
- 72. Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. 2015;7:28788. CrossRef
- 73. Gibson FC III, Hong C, Chou HH, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801-2806. CrossRef
- 74. Jain A, Batista EL Jr, Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infect Immun. 2003;71:6012-6018. CrossRef
- 75. Brodala N, Merricks EP, Bellinger DA, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscleros Thromb Vasc Biol. 2005;25:1446-1451. CrossRef
- 76. Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Zheng D, Lucas AR, Kesavalu L. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoE null Mice. PloS One. 2015;10:e0143291. CrossRef
- 77. Velsko IM, Chukkapalli SS, Rivera MF, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9:e97811. CrossRef
- 78. Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401-407. CrossRef
- 79. Lafaurie GI, Mayorga-Fayad I, Torres MF, et al. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J Clin Periodontol. 2007;34:873-879. CrossRef
- 80. Pérez-Chaparro PJ, Gracieux P, Lafaurie GI, Donnio P-Y, Bonnaure-Mallet M. Genotypic characterization of Porphyromonas gingivalis isolated from subgingival plaque and blood sample in positive bacteremia subjects with periodontitis. J Clin Periodontol. 2008;35:748-753. CrossRef
- 81. Castillo DM, Sánchez-Beltrán MC, Castellanos JE, et al. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. J Clin Periodontol. 2011;38:418-427. CrossRef
- 82. Waghmare AS, Vhanmane PB, Savitha B, Chawla RL, Bagde HS. Bacteremia following scaling and root planing: a clinico-microbiological study. J Indian Soc Periodontol. 2013;17:725-730. CrossRef
- 83. Sahrmann P, Manz A, Attin T, Zbinden R, Schmidlin PR. Effect of application of a PVP-iodine solution before and during subgingival ultrasonic instrumentation on post-treatment bacteraemia: a randomized single-centre placebo-controlled clinical trial. J Clin Periodontol. 2015;42:632-639. CrossRef
- 84. Marín MJ, Figuero E, González I, et al. Comparison of the detection of periodontal pathogens in bacteraemia after tooth brushing by culture and molecular techniques. Med Oral Patol Oral Cir Bucal. 2016;21:e276-e284. CrossRef
- 85. Elkaïm R, Dahan M, Kocgozlu L, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodontal Res. 2008;43:224-231. CrossRef
- 86. Gaetti-Jardim E, Marcelino SL, Feitosa ACR, Romito GA, Avila-Campos MJ. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. 2009;58:1568-1575. CrossRef
- 87. Lavigne SE, Forrest JL. An umbrella review of systematic reviews of the evidence of a causal relationship between periodontal disease and cardiovascular diseases: Position paper from the Canadian Dental Hygienists Association. Can J Dent Hyg. 2020 Feb;54(1):32.
- 88. Febbraio M, Roy CB, Levin L. Is there a causal link between periodontitis and cardiovascular disease? A concise review of recent findings. Int Dent J. 2022 Feb;72(1):37-51. CrossRef
- 89. Paul O, Arora P, Mayer M, Chatterjee S. Inflammation in periodontal disease: possible link to vascular disease. Frontiers Physiol. 2021 Jan 14;11:609614.
- 90. Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta‐analysis. Clin Exp Dent Res. 2021 Feb;7(1):109-122.
- 91. Lazureanu PC, Popescu FG, Stef L, Focsa M, Vaida MA, Mihaila R. The influence of periodontal disease on oral health quality of life in patients with cardiovascular disease: a cross-sectional observational single-center study. Medicina. 2022 Apr 24;58(5):584.
- 92. Tiensripojamarn N, Lertpimonchai A, Tavedhikul K, et al. Periodontitis is associated with cardiovascular diseases: a 13‐year study. J Clin Periodontol. 2021 Mar;48(3):348-356.
- 93. Petrenya N, Hopstock LA, Holde GE, Oscarson N, Jönsson B. Relationship between periodontitis and risk of cardiovascular disease: insights from the Tromsø Study. J Periodontol. 2022 Sep;93(9):1353-1365.