 |
Drug Target Insights 2022; 16: 71-77 ISSN 1177-3928 | DOI: 10.33393/dti.2022.2504 ORIGINAL RESEARCH ARTICLE |
 |
MRSA carriage among healthcare workers in a Vietnamese intensive care unit: a prospective cohort study
ABSTRACT
Background: Little is known about the magnitude and patterns of methicillin-resistant Staphylococcus aureus (MRSA) carriage among intensive care unit (ICU) healthcare workers (HCWs), especially in lower-middle-income countries like Vietnam.
Materials and methods: A prospective cohort study was conducted on HCWs working in the adult ICU of the Hospital for Tropical Diseases in Vietnam between October 28 and December 20, 2019. These HCWs included physicians, nurses, and nursing assistants who were responsible for all essential medical activities and basic patient care. A questionnaire was used to collect participants’ information, including age, sex, profession, ICU working time, and underlying diseases. Hand and nasal swabs were collected weekly for 8 consecutive weeks for MRSA screening. Staphylococcal isolates were checked for catalase and coagulase and, for methicillin resistance using cefoxitin disk diffusion, then rechecked on the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Results: Among 55 HCWs, 16 (29.1%) carried MRSA in their noses or hands. MRSA intermittent hand carriage was documented in 2 (3.6%) HCWs. Among 53 HCWs undertaking nasal swabs, 13 (24.5%) were MRSA persistent and 3 (5.6%) were intermittent carriers. The MRSA carriage rate was highest among nursing assistants (50%, 4/8). More HCWs with underlying diseases were found to be MRSA carriers (31.8%, 7/22) compared with those without comorbidities (27.3%, 9/33).
Conclusion: MRSA carriage among HCWs is not rare. The findings highlight an urgent need to review and update the local infection prevention and control measures to prevent MRSA transmission from HCWs to patients.
Keywords: Healthcare workers, Intensive care unit, Methicillin-resistant Staphylococcus aureus carriage, Vietnam
Received: October 10, 2022
Accepted: December 19, 2022
Published online: December 31, 2022
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Colonization is the presence of an organism on or in a host with growth and multiplication to a sufficiently high concentration but does not invade the host’s tissues or cause disease (1). The causal relationship between prior colonization and subsequent infections has been well-established in high-resource clinical settings (1). Staphylococcus aureus is a well-described organism of the normal human flora, frequently colonizing the nose, pharynx, and skin (1). Most S. aureus isolates are sensitive to currently used antibiotics; thus, infections caused by this agent can be effectively treated. However, the emerging methicillin-resistant S. aureus (MRSA) has resulted in significant morbidity and mortality in susceptible patients (2). Notably, patients colonized with MRSA are more likely to develop S. aureus infection compared to methicillin-sensitive S. aureus (MSSA)-colonized or MSSA-non-colonized patients (2). In hospital settings, MRSA colonization among healthcare workers (HCWs) is a huge challenge because they may spread MRSA to their patients as a result of poor infection control practices (3-5). MRSA outbreaks in hospitals are epidemiologically associated with MRSA-colonized or MRSA-infected HCWs, especially those who had exfoliative skin conditions, skin infections, or respiratory tract infections (3,5). According to the current clinical practice guidelines, routine screening of HCWs for MRSA is not recommended. However, it is suggested that screening for MRSA can be beneficial in circumstances including (i) if transmission continues in a ward, despite active control measures; or if epidemiological aspects of an outbreak are unusual; or if there is evidence suggesting persistent MRSA carriage among HCWs; and (ii) if new MRSA carriers have been found among patients in a ward, and thus, HCWs with skin lesions should be identified and screened (5).
Currently, little is known about the magnitude of MRSA among HCWs, especially in low-resource settings like Vietnam, likely due to the lack of a routine screening program that is costly. To the best of our knowledge, available data focus on the magnitude of MRSA in those who are not intensive care unit (ICU) staff such as healthy adults, medical conference attendees, and ICU patients (6-8). Despite this, in 2006, an outbreak of severe community-acquired MRSA infections following routine immunization was reported in Ho Chi Minh City (HCMC), Vietnam (9). The outbreak investigation found that HCWs’ insufficient hand hygiene during routine injection led to the transmission of MRSA between children. A recent study conducted in the adult ICU at the Hospital for Tropical Diseases (HTD), HCMC, Vietnam, reported that 16.2% of patients acquired MRSA colonization during their ICU stay (10). This study also found that MRSA accounted for more than half (66.7%) of all S. aureus infections and suggested the role of HCWs in transmitting MRSA, leading to hospital-acquired infections (10). To strengthen MRSA prevention and control practices in Vietnam and other comparable settings, this study was conducted in the adult ICU of the HTD, which is among the largest local hospitals for infectious diseases in Vietnam, to examine the antimicrobial susceptibility profile of S. aureus isolates and the patterns of MRSA carriage among HCWs.
Materials and methods
Study design
A prospective cohort study was conducted in the 20-bed adult ICU of the HTD in Vietnam between October 28 and December 20, 2019. All ICU HCWs were invited to participate in the study. These HCWs included physicians, nurses, and nursing assistants who were responsible for all essential medical activities and basic patient care. A written informed consent was obtained, and the study was approved by HTD’s ethics committee (approval number 24/HDDD) and the University of New South Wales (approval number HC190730).
The adult ICU includes four pods in which there are five to seven patients in each pod. The HCW roster is divided into four different staff shifts (i.e., eight nurses and nursing assistants and three doctors per shift). In a normal working day, three shifts are on duty by turns (i.e., 8 hours per shift), and one shift is off. Within a shift, all medical staff are further split into four small groups to care for patients in the four corresponding ICU pods. According to the local policy, staff within each shift are rotated every 8 weeks, so that all staff have an equal chance to work across the ICU and share the same responsibilities. According to previous studies conducted at the same ICU, weekly swabs are sufficient to detect potential bacterial colonization among study participants (6,10). Therefore, to ensure that all staff’s potential bacterial colonizations were captured when working in different ICU pods, swabs were taken weekly during the 8-week study period. A questionnaire was used to collect participants’ characteristics, including age, sex, profession (medical doctor, nurse, and nursing assistant), ICU working time, and underlying diseases (sinusitis, skin diseases, diabetes, and others). The questionnaire was developed based on the available literature regarding the sources and vectors of MRSA as well as risk factors for MRSA carriage in healthcare settings (3,11).
Swabbing procedure
Hand and nasal swab samples were taken weekly using the Sterile Transport Swab (Jiangsu Kangjian Medical Apparatus Co., Ltd., China) for S. aureus screening. The swabbing procedure was based on the HTD’s infection control guidelines. A qualified study nurse performed hand and nasal swabs of participants at the start of each work shift. The study nurse put on gloves and a surgical mask to prevent contamination of the samples. For hand swabbing, HCWs washed their hands and let them dry according to the World Health Organization guidelines on hand hygiene in healthcare (12). Then, a moist and sterile swab was rotated across the palm and back of both hands as well as fingertips, fingernails, and between fingers. For nasal swabbing, another swab was inserted about 2 cm into the anterior nares of both nostrils of HCWs and rotated a few times against the nasal mucosa until it was covered in secretions.
Microbiological methods
Blood agar (bioMérieux) was used to isolate S. aureus from swabbing samples. S. aureus was confirmed based on its morphology and hemolytic activity. When grown in culture, several staphylococcal colonies could develop. However, due to resource constraints, a maximum of two staphylococcal colonies were included in this study in case several staphylococcal colonies were isolated. The selection of these two colonies was based on their levels of predominance (13). Staphylococcal colonies were checked for catalase and coagulase, and for methicillin resistance using cefoxitin disk diffusion (14), then rechecked on the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDITOF, Bruker Daltonics, United States). Catalase assay was used to detect the catalase enzyme that releases oxygen from hydrogen peroxide (H2O2), which helps differentiate between staphylococci (catalase positive) and streptococci (catalase negative). To detect the presence of catalase in bacteria in the culture, several drops of 3% H2O2 were added to the culture. The rapid formation of bubbles indicates catalase-positive culture. Coagulase assay was used to detect the coagulase enzyme that converts fibrinogen (soluble) to fibrin (insoluble), which helps differentiate between S. aureus (coagulase positive) and other staphylococci (coagulase negative). The coagulase assay used in this study was the coagulase slide test to detect the bound coagulase of S. aureus. A suspension of the isolated colony is emulsified on a slide with a drop of rabbit plasma. Clumping of the organisms indicates the presence of bound coagulase. The principle of MALDITOF is that bacterial cells are ionized into charged molecules, then their mass-to-charge ratio is measured and analyzed by a mass spectrometer. Every bacterial genus/species has a distinctive protein spectrum that can be compared with a database software so that the nearest organism can be identified (15,16). The process is rapid, sensitive, and economical in terms of both labor and costs involved. No control MRSA strain was used in the study. Testing for susceptibility to eight most commonly used antibiotics including penicillin, oxacillin, vancomycin, erythromycin, ciprofloxacin, sulfamethoxazole-trimethoprim, rifampin, and clindamycin was performed using the Kirby/Bauer disk diffusion method and the 2015 Clinical and Laboratory Standards Institute (CLSI) guidelines. These microbiological methods have been validated elsewhere (6,10).
S. aureus carriage patterns
S. aureus carriage reported in our study included MSSA and MRSA carriage. S. aureus carriage was classified into three different categories: persistent carriage, intermittent carriage, and noncarriage. Given each study participant was swabbed weekly for 8 consecutive weeks, persistent carriage was defined as ≥2 positive consecutive cultures of either hand or nasal swabs with S. aureus. Intermittent carriage referred to the isolation of S. aureus in less than two positive consecutive cultures of either hand or nasal swabs. All negative cultures of hand and nasal swabs were categorized as noncarriage.
Statistical analysis
Descriptive analyses were performed and consisted of frequency and percentage (95% confidence interval [CI]) for categorical data, and median (interquartile range [IQR]) for continuous data using R statistical software. Chi-squared test was used to examine the significant relationship between categorical variables. The comparison of continuous variables was performed using Mann-Whitney U-test. Alpha was set at 5% level.
Results
Study participants’ characteristics
Most HCWs working in the adult ICU (92%, 55/60) participated in the study, including all 11 doctors (100%), 36 nurses (87.8%, 36/41), and all 8 nursing assistants (100%) (Tab. I). Two-thirds of participants were female (67.3%, 37/55). Most of the participants (87.3%, 48/55) were younger than 41 years, and 45.5% (25/55) of them have been working for more than 5 years in the adult ICU. Gastritis and sinusitis (29.2%, 16/55) were the most common underlying diseases, but none of those having these diseases experienced any acute symptoms during the study period.
| Characteristics | Summary statistics* |
|---|---|
| Age (years) | 32 (27-36) |
| Age groups | |
| ≤30 | 23 (41.8) |
| 31-40 | 25 (45.5) |
| ≥41 | 7 (12.7) |
| Male | 18 (32.7) |
| Profession | |
| Medical doctors | 11 (20) |
| Nurses | 36 (65.5) |
| Nursing assistants | 8 (14.5) |
| Working time in ICU (years) | 5 (1.2-11) |
| Period of time working in ICU | |
| <1 | 11 (20) |
| 1-5 | 19 (34.6) |
| 6-10 | 9 (16.4) |
| 11-15 | 12 (21.8) |
| >15 | 4 (7.2) |
| Healthcare workers’ underlying diseases | |
| Gastritis | 11 (20) |
| Sinusitis | 5 (9.2) |
| Diabetes mellitus | 2 (3.6) |
| Gastroesophageal reflux disease | 1 (1.8) |
| Chronic colitis | 1 (1.8) |
| Rheumatoid arthritis | 1 (1.8) |
| Thyroid cancer | 1 (1.8) |
| No underlying diseases | 33 (60) |
*Median (interquartile range) for continuous variables and n (%) for categorical variables.
Antimicrobial susceptibility of S. aureus isolates
A total of 128 S. aureus isolates were cultured and included 123 (96.1%) from nasal swabs and 5 (3.9%) from hand swabs. MRSA accounted for 71.1% (91/128) of all S. aureus isolates (Fig. 1), with almost all (97.8%, 89/91) cultured from nasal samples. Almost three-quarters of 128 isolates were resistant to erythromycin (71.9%, 92/128) and clindamycin (70.3%, 90/128), while one-third (34.4%, 44/128) were resistant to ciprofloxacin. Vancomycin-resistant S. aureus was not detected. All S. aureus strains were fully sensitive to sulfamethoxazole-trimethoprim and rifampicin.
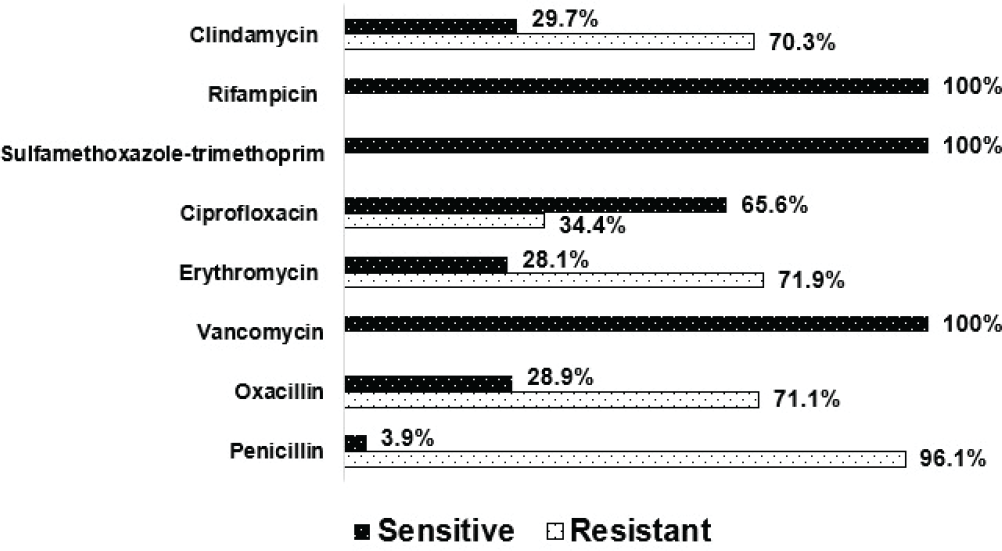
Fig. 1 - Antimicrobial susceptibility of Staphylococcus aureus isolates from 55 healthcare workers working in the adult intensive care unit.
MRSA carriage patterns
All 55 participants agreed to have hand swabs taken, while 2 refused to undertake nasal swabs. The proportion of MRSA hand carriers increased with hand swabbing frequency from 0% in the first 4 weeks to 3.6% (2/55) in the last 4 weeks of the study period. Similarly, MRSA nasal carriers were detected in 18.9% (10/53) of study participants in the first week and increased to 22.6% (12/53) at the end of the study period.
A total of 20 (36.4% of 55) HCWs carried S. aureus (MSSA and MRSA) in their noses or hands. MRSA carriage accounted for 29.1% (16/55). All participants who tested positive for S. aureus were asymptomatic. Persistent carriage was not detected from hand swabs. S. aureus intermittent carriers were documented in 7.2% (4/55) of participants, of whom 2 were MRSA intermittent carriers (Tab. II). For nasal swabbing, 34% (18/53) of participants were found to be S. aureus persistent carriers, of whom 13 (24.5% of 53) were MRSA persistent carriers. Five participants were S. aureus intermittent carriers, and 3 of them were MRSA intermittent carriers, all from nasal swabs. Additionally, of the 18 HCWs with S. aureus persistent nasal carriage, 3 were found to be S. aureus intermittent hand carriers, and S. aureus isolates recovered from their nasal and hand swab cultures shared the same antimicrobial susceptibility profile.
| Swab taken | S. aureus carriage categories | n (%) | 95% CI | |
|---|---|---|---|---|
| Hand swab
(n = 55) |
Persistent carriage | MRSA or MSSA | 0 | |
| Intermittent carriage | MRSA | 2 (3.6) | 1-12.3 | |
| MSSA | 2 (3.6) | 1-12.3 | ||
| Noncarriage | 51 (92.8) | 82.7-97.1 | ||
| Nasal swab
(n = 53) |
Persistent carriage | MRSA | 13 (24.5) | 14.9-37.6 |
| MSSA | 5 (9.5) | 4.1-20.3 | ||
| Intermittent carriage | MRSA | 3 (5.6) | 1.9-15.4 | |
| MSSA | 2 (3.8) | 1-12.8 | ||
| Noncarriage | 30 (56.6) | 43.3-69.1 | ||
| Total
(n = 55) |
MRSA carriage | 16 (29.1) | 18.8-42.1 | |
| MSSA carriage | 4 (7.3) | 2.9-17.3 | ||
| Noncarriage | 35 (63.6) | 50.4-75.1 | ||
MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus.
Risk factors of MRSA carriage
The proportion of nursing assistants (50%, 4/8) who were MRSA carriers was higher than that of doctors (36.4%, 4/11) and nurses (22.2%, 8/36), but these differences were not statistically significant (p > 0.05). Similarly, more HCWs with underlying diseases were found to be MRSA carriers compared with those without comorbidities (31.8%, 7/22 vs. 27.3%, 9/33), although this difference was not statistically significant (p > 0.05). There was no statistically significant association between MRSA hand and nasal carriage and age, sex, profession, and underlying diseases (p > 0.05) (Tab. III).
| Characteristics† | MRSA (+)
(n = 16) |
MRSA (–)
(n = 39) |
p-Value* |
|---|---|---|---|
| Age (years) | 30.5
(26-36) |
32
(29.5-35.5) |
0.41 |
| Working time in ICU (years) | 2.25
(0.67-7.13) |
6
(2-11) |
0.16 |
| Male | 3 (18.8) | 15 (38.5) | 0.16 |
| Underlying diseases‡ | 7 (43.8) | 15 (38.5) | 0.72 |
| Profession | |||
| Medical doctors | 4 (36.4) | 7 (63.6) | 0.25 |
| Nurses | 8 (22.2) | 28 (77.8) | |
| Nursing assistants | 4 (50) | 4 (50) |
ICU = intensive care unit; IQR = interquartile range; MRSA = methicillin-resistant Staphylococcus aureus.
*Mann-Whitney U-test for continuous variables and chi-squared test for categorical variables.
†Median (IQR) for continuous variables and n (%) for categorical variables.
‡Gastritis, sinusitis, diabetes mellitus, gastroesophageal reflux disease, chronic colitis, rheumatoid arthritis, and thyroid cancer.
Discussion
The prevalence of MRSA carriage among our ICU HCWs was 29.1% (16/55, 95% CI: 18.8-42.1%). Specifically, 24.5% of HCWs were MRSA persistent nasal carriers. In Vietnam, previous studies found that the prevalence of MRSA colonization among healthy adults and ICU patients was 4.2% (28/662, 95% CI: 2.9-6.1%) (8) and 8.6% (72/838, 95% CI: 6.9-10.7%) (6), respectively, which is lower than the rate found in HCWs. Our findings are also in line with previous studies in Europe and the United States, which documented a higher rate of MRSA nasal colonization in HCWs compared with non-healthcare professionals (17,18). This can be explained by the increased exposure of HCWs to patients, hospital environment, and potential MRSA-contaminated objects including medical devices compared to non-healthcare individuals. Indeed, a multicenter study conducted in Nepal, which is a comparable country, found that MRSA can be isolated from the commonly used medical devices in ICU settings such as stethoscopes, pulse oximeters, ventilators, and defibrillators (19). The biofilm-forming property enables S. aureus to survive longer on the surfaces of these instruments, which are potential sources of nosocomial infections (19). A global review found that the estimated average MRSA carriage rate in HCWs was 5% (3). In detail, the rate of MRSA carriage among HCWs in Europe was 3.4% (95% CI: 3.1%-3.7%), the United States 4.2% (95% CI: 3.8%-4.7%), Africa 15.5% (95% CI: 13%-18.4%), the Middle East 6.1% (95% CI: 5.2%-7.2%), Australia and New Zealand 9.7% (95% CI: 8.5%-11.1%), and Asia excluding Vietnam 9.8% (95% CI: 8.4%-11.4%) (3). The prevalence of MRSA carriage in our HCWs was substantially higher than these reports and data from MRSA-endemic settings, where the prevalence in HCWs was 8.1% (95% CI: 7.4%-8.9%) (3). Globally, information on the burden of MRSA among ICU HCWs is scarce. Only 4.7% (95% CI: 4%-5.4%) of ICU staff from other regions were found to be MRSA carriers (3), which was lower compared with our data. Our findings further confirm the burden of MRSA in ICU settings in Vietnam, supported by high rates of MRSA colonization and infection in Vietnamese ICU patients (6,10). In light of this, Vietnam should be listed as a country with hyperendemic MRSA. Our study also indicates that MRSA carriage in HCWs in Vietnam is an urgent health problem that needs to be addressed, though this warrants further large-scale studies.
Nurses are associated with a higher risk of MRSA colonization. A meta-analysis showed that the risk of MRSA colonization among nurses was 2.6 (95% CI: 1.8-3.7) times higher than other healthcare staff including doctors and nursing assistants (17). This is probably due to the more frequent and close contact of nurses with patients compared with other healthcare staff. However, we noticed a higher proportion of nursing assistants who were colonized with MRSA compared with doctors and nurses, although this difference was not statistically significant. Suboptimal infection control practices have been indicated as a risk factor for MRSA carriage in HCWs (20). Despite the availability of local infection control guidelines, recent studies found that infection control compliance among HCWs is suboptimal in Vietnam (20,21). Especially, nursing assistants are found to have a lower infection control knowledge compared with other healthcare staff (22). This may explain the high proportion of MRSA carriage among our nursing assistants. To address this, infection control education programs should be tailored to meet the nursing assistants’ level of knowledge, and an audit program to measure infection control practice, especially hand hygiene, should be reinforced. Indeed, tailored infection control programs have been proven to be effective in comparable developing countries (23).
We also found that HCWs with gastritis or sinusitis had a higher prevalence of MRSA carriage compared with those without these comorbidities, although this association was not statistically significant. It is documented that HCWs with sinusitis are at an increased risk of transmitting MRSA in hospital settings and have been implicated in several MRSA outbreaks (3,24). Presently, the MRSA universal screening policy is controversial because of the lack of robust evidence for the effectiveness of such a costly measure. Recommendations for this infection control policy are suggested to be made by healthcare professionals based on their specific contexts (25). We believe that in low-resource settings with a high burden of MRSA like Vietnam, an infection prevention and control program needs to be designed to actively screen for MRSA among HCWs with these comorbidities for prompt interventions.
Among MRSA hand and nasal carriers, the distinction between persistent and intermittent carriage is important because persistent carriage is associated with a significantly higher bacterial load than intermittent carriage, resulting in an increased risk of transmitting MRSA to others (3,26). For MRSA hand carriage in our study, persistent carriage was not recorded, but 3.6% (95% CI: 1%-12.3%) of participants were found to be intermittent carriers. This prevalence was not different compared with the MRSA hand carriage rate in HCWs from other regions, including North America (8.3%, 95% CI: 3.5%-14.5%), Asia (4%, 95% CI: 2.1%-6.3%), and Europe (2.5%, 95% CI: 1%-4.5%) (27). Staphylococcal hand carriage in HCWs is usually transient, which means it is detectable after a working shift and gone before the next shift (3). The lack of persistent hand carriage in our study is a reassuring result. Moreover, intermittent carriage is often self-limiting and requires no treatment in healthy people (28). However, the contaminated hands of HCWs who are persistent or intermittent carriers are the main MRSA transmission route in hospitals, which can be prevented by effective hand hygiene (3,12). The overall prevalence of MRSA nasal carriage in our study was 30.1% (95% CI: 19.5%-43.5%), which was not different from the rate reported from Gaza Strip (25.5%, 95% CI: 20%-32%) (29). However, our rate was higher than that reported from Ethiopia (5.8%, 95% CI: 3.5%-9.5%) (30) and Nigeria (8%, 95% CI: 4.6%-13.5%) (31). Furthermore, the prevalence of persistent carriage of 24.5% was 4.5 times higher than the rate of intermittent carriage (5.6%) in our study. Unlike MRSA hand carriage, persistent nasal carriage cannot be managed by hand hygiene (32), but nasal mupirocin has been demonstrated to be efficacious in decolonizing MRSA in HCWs (3). Nasal mupirocin also provides a cost-effective adjunct to other infection control measures, including the screening and isolation strategies in controlling MRSA (33,34). However, nasal decolonization using mupirocin has not yet been implemented in Vietnam. A localized infection control guideline with detailed instructions on how to prevent and control MRSA nasal carriage with a focus on nasal decolonization will provide long-term benefits to both HCWs and patients.
In our study, S. aureus isolates were highly resistant to penicillin (96.1%), erythromycin (71.9%), and clindamycin (70.3%). We also found a moderate resistance rate with ciprofloxacin (34.4%). All S. aureus strains were fully sensitive to sulfamethoxazole-trimethoprim and rifampicin. Our results are consistent with a study reported from Ethiopia, in which 93.1% (27/29) of S. aureus isolates colonizing in HCWs showed resistance to penicillin followed by erythromycin (62.1%) and ciprofloxacin (37.9%) (30). Although a lower resistance rate was documented with clindamycin (17.2%) in this report, a higher resistance rate of 51.7% was recorded for co-trimoxazole compared to our findings (30). The resistance rates with erythromycin (29.1%), clindamycin (11.2%), and ciprofloxacin (9.6%) of the 62 S. aureus isolates colonizing in HCWs in Gaza Strip were also lower than ours (29). Surprisingly, 14.5% of S. aureus isolates were found to be resistant to vancomycin in the Gaza Strip study, while this strain was not detected in our study (29). Higher resistance to the aforementioned antibiotics in our study could be due to excessive use, misuse, and irrational prescriptions of these medications in both hospitals and community in Vietnam (35,36). Therefore, there is an urgent need for robust antimicrobial stewardship programs in combination with adherence to infection control measures to tackle the growing threat of antibiotic resistance in S. aureus. The results of this antimicrobial stewardship would help tailor the MRSA infection prevention and control program to meet the local needs.
There are some limitations in our study. Our sample size was small, and thus may not be able to statistically detect the differences between study groups. However, our sample represented almost all staff (92%, 55/60) in the ICU of a leading tertiary hospital in Vietnam. In addition, it has been found that MRSA counts (colony-forming units/mL) may decrease over time among subjects exposed to a source of MRSA (37). Hence, MRSA counts can provide more insights into the clearance of MRSA among our participants. However, information on HCWs’ MRSA counts was not available in our study. We also did not use genotyping methods to identify the resistance gene mecA, which is commonly used to examine MRSA due to limited financial resources. However, our study was set up to prospectively screen for MRSA carriage among HCWs for 8 consecutive weeks, and an antimicrobial susceptibility profile was performed for all cultured S. aureus isolates. The multiple testing times for antibiotic susceptibility of S. aureus in our study helped increase the possibility of detection of MRSA carriage and reduce the inconsistency of phenotyping resistance compared to the genotyping method. Given that there is no similar study in Vietnam, our study is the first attempt to examine the burden of MRSA among local HCWs. Therefore, we may have missed some possible risk factors of MRSA carriage in HCWs in Vietnam.
In conclusion, our data suggest that southern Vietnam may be an emerging MRSA hotspot, where the study was conducted . HCWs, especially nursing assistants, with comorbidities tended to be MRSA carriers. Infection control education programs should be tailored to meet the different knowledge levels of all HCWs. In addition to strengthening hand hygiene practice and antimicrobial stewardship, infection control guidelines need to be designed to actively screen for MRSA among HCWs with comorbidities for prompt interventions.
Acknowledgments
The authors would like to acknowledge the kind support of all staff of the adult intensive care unit of the Hospital for Tropical Diseases for their voluntary participation in the study.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Jarvis WR. The epidemiology of colonization. Infect Control Hosp Epidemiol. 1996;17(1):47-52. CrossRef PubMed
- 2. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol. 2010;31(6):584-591. CrossRef PubMed
- 3. Albrich WC, Harbarth S, Bern H. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8(5):289-301. CrossRef PubMed
- 4. Joachim A, Moyo SJ, Nkinda L, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus among health care workers in tertiary and regional hospitals in Dar es Salam, Tanzania. Int J Microbiol. 2018;2018:5058390. CrossRef PubMed
- 5. Hawkins G, Stewart S, Blatchford O, Reilly J. Should healthcare workers be screened routinely for methicillin-resistant Staphylococcus aureus? A review of the evidence. J Hosp Infect. 2011;77(4):285-289. CrossRef PubMed
- 6. Thuy DB, Campbell J, Hoang NVM, et al. A one-year prospective study of colonization with antimicrobial-resistant organisms on admission to a Vietnamese intensive care unit. PLoS One. 2017;12(9):e0184847. CrossRef PubMed
- 7. Huang YC, Su LH, Wu TL, Lin TY. Methicillin-resistant Staphylococcus aureus nasal carriage in international medical conference attendees. J Microbiol Immunol Infect. 2019;52(2):242-247. CrossRef PubMed
- 8. Van Nguyen K, Zhang T, Thi Vu BN, et al. Staphylococcus aureus nasopharyngeal carriage in rural and urban northern Vietnam. Trans R Soc Trop Med Hyg. 2014;108(12):783-790. Online CrossRef PubMed
- 9. Tang CT, Nguyen DT, Ngo TH, et al. An outbreak of severe infections with community-acquired MRSA carrying the Panton-Valentine leukocidin following vaccination. PLoS One. 2007;2(9):e822. CrossRef PubMed
- 10. Thuy DB, Campbell J, Nhat LTH, et al. Hospital-acquired colonization and infections in a Vietnamese intensive care unit. PLoS One. 2018;13(9):e0203600. CrossRef PubMed
- 11. Faibis F, Laporte C, Fiacre A, et al. An outbreak of methicillin-resistant Staphylococcus aureus surgical-site infections initiated by a healthcare worker with chronic sinusitis. Infect Control Hosp Epidemiol. 2005;26(2):213-215. CrossRef PubMed
- 12. World Health Organization. Guidelines on hand hygiene in health care. 2009. Online. Accessed October 2022.
- 13. Thuy DB, Campbell J, Thuy CT, et al. Colonization with Staphylococcus aureus and Klebsiella pneumoniae causes infections in a Vietnamese intensive care unit. Microb Genom. 2021;7(2):1-14. CrossRef PubMed
- 14. Fernandes CJ, Fernandes LA, Collignon P; Australian Group on Antimicrobial Resistance. Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2005;55(4):506-510. CrossRef PubMed
- 15. Clark AE, Kaleta EJ, Arora A, Wolk DM. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev. 2013;26(3):547-603. CrossRef PubMed
- 16. Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6(791):791. CrossRef PubMed
- 17. Dulon M, Peters C, Schablon A, Nienhaus A. MRSA carriage among healthcare workers in non-outbreak settings in Europe and the United States: a systematic review. BMC Infect Dis. 2014;14(1):363. CrossRef PubMed
- 18. Sassmannshausen R, Deurenberg RH, Köck R, et al. MRSA prevalence and associated risk factors among health-care workers in non-outbreak situations in the Dutch-German EUREGIO. Front Microbiol. 2016;7:1273. CrossRef PubMed
- 19. Bhatta DR, Koirala S, Baral A, et al. Methicillin-resistant Staphylococcus aureus contamination of frequently touched objects in intensive care units: potential threat of nosocomial infections. Can J Infect Dis Med Microbiol. 2022:1023241. CrossRef PubMed
- 20. Salmon S, McLaws ML. Qualitative findings from focus group discussions on hand hygiene compliance among health care workers in Vietnam. Am J Infect Control. 2015;43(10):1086-1091. CrossRef PubMed
- 21. Duong MC, McLaws ML. Dangerous practices in a hemodialysis unit in Vietnam identify from mixed methods. BMC Infect Dis. 2017;17(1):181. CrossRef PubMed
- 22. Lien TQ, Chuc NTK, Hoa NQ, et al. Knowledge and self-reported practices of infection control among various occupational groups in a rural and an urban hospital in Vietnam. Sci Rep. 2018;8(1):5119. CrossRef PubMed
- 23. Zimmerman PA, Yeatman H, Jones M, Murdoch H. Success in the South Pacific: a case study of successful diffusion of an infection prevention and control program. Healthc Infect. 2015;20(2):54-61. CrossRef PubMed
- 24. Boyce JM, Opal SM, Potter-Bynoe G, Medeiros AA. Spread of methicillin-resistant Staphylococcus aureus in a hospital after exposure to a health care worker with chronic sinusitis. Clin Infect Dis. 1993;17(3):496-504. CrossRef PubMed
- 25. Bonten MJM, Weinstein RA. Making sense of universal screening for MRSA. Lancet Infect Dis. 2016;16(3):272-273. CrossRef PubMed
- 26. Nouwen JL, Ott A, Kluytmans-vandenbergh MFQ, Van Belkum A, Verbrugh HA. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule”. CID; 2004:39.
- 27. Montoya A, Schildhouse R, Goyal A, et al. How often are health care personnel hands colonized with multidrug-resistant organisms? A systematic review and meta-analysis. Am J Infect Control. 2019;47(6):693-703. CrossRef PubMed
- 28. Nouwen J, Boelens H, van Belkum A, Verbrugh H. Human factor in Staphylococcus aureus nasal carriage. Infect Immun. 2004;72(11):6685-6688. CrossRef PubMed
- 29. El Aila NA, Al Laham NA, Ayesh BM. Nasal carriage of methicillin resistant Staphylococcus aureus among health care workers at Al Shifa hospital in Gaza Strip. BMC Infect Dis. 2017;17(1):28. CrossRef PubMed
- 30. Legese H, Kahsay AG, Kahsay A, et al. Nasal carriage, risk factors and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus among healthcare workers in Adigrat and Wukro hospitals, Tigray, Northern Ethiopia. BMC Res Notes. 2018;11(1):250. CrossRef PubMed
- 31. Malini J, Harle SA, Padmavathy M, et al. Methicillin-resistant Staphylococcus aureus carriage among the health care workers in a tertiary care hospital. J Clin Diagn Res. 2012;6(5):791-793.
- 32. Gurieva TV, Bootsma MCJ, Bonten MJM. Decolonization of patients and health care workers to control nosocomial spread of methicillin-resistant Staphylococcus aureus: a simulation study. BMC Infect Dis. 2012;12(302):302. CrossRef PubMed
- 33. Robotham JV, Graves N, Cookson BD, et al. Screening, isolation, and decolonisation strategies in the control of methicillin resistant Staphylococcus aureus in intensive care units: cost effectiveness evaluation. BMJ. 2011;343(7827):d5694. CrossRef PubMed
- 34. Wassenberg MWM, de Wit GA, Bonten MJM. Cost-effectiveness of preoperative screening and eradication of Staphylococcus aureus carriage. PLoS One. 2011;6(5):e14815. CrossRef PubMed
- 35. Nguyen KV, Thi Do NT, Chandna A, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health. 2013;13(1):1158. Online CrossRef PubMed
- 36. Nga TT, Chuc NTK, Hoa NP, et al. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacol Toxicol. 2014;15(1):6. Online CrossRef PubMed
- 37. Angen Ø, Feld L, Larsen J, et al. Transmission of methicillin-resistant Staphylococcus aureus to human volunteers visiting a swine farm. Appl Environ Microbiol. 2017;83(23):1-10. CrossRef PubMed