 |
Drug Target Insights 2022; 16: 6-11 ISSN 1177-3928 | DOI: 10.33393/dti.2022.2343 ORIGINAL RESEARCH ARTICLE |
 |
Anatomical and functional responses to single brolucizumab injection in neovascular age-related macular degeneration patients not responding to antiangiogenics: a case series
ABSTRACT
Introduction: Neovascular age-related macular degeneration (nAMD) is treated with antivascular endothelial growth factor (anti-VEGF) drugs. However, resistance to anti-VEGF therapy is observed in some patients. Brolucizumab is a new-generation anti-VEGF drug for the treatment of nAMD, with proven efficacy in fluid resolution and long-lasting effects.
Methods: We report here a case series of nAMD patients not responding to previous anti-VEGF therapy showing anatomical and functional response to a single intravitreal injection of brolucizumab.
Results: Nine patients with nAMD, undergoing treatment with anti-VEGF therapy (aflibercept, bevacizumab, or ranibizumab) but with either fluid persistence or frequent fluid recurrences in retinal compartments, were switched to intravitreal brolucizumab and examined 4 weeks postinjection. No signs of active disease were observed in all but one patient, with complete retinal fluid resolution in seven patients. Central macular thickness and visual acuity significantly improved, and changes were sustained for up to 12 weeks in a subset of three patients. No adverse reactions were observed.
Conclusions: This new anti-VEGF drug showed great efficacy since the first week from the injection with a significative reduction of subretinal fluid and rapid improvement of visual acuity. In conclusion, brolucizumab administered intravitreally appears to be an effective treatment in nAMD patients, leading to both early anatomical and functional improvements.
Keywords: Anti-VEGF, Brolucizumab, Case series, Neovascular age-related macular degeneration (nAMD)
Received: September 24, 2021
Accepted: March 16, 2022
Published online: March 24, 2022
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2022 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Neovascular age-related macular degeneration (nAMD) is a chronic degenerative disease characterized by the pathological growth of vessels, which leak blood and fluids in the various retinal compartments, leading to loss of visual acuity (VA) (1,2). Fluids accumulating in retinal compartments (i.e., intraretinal fluid [IRF], subretinal fluid [SRF], or fluid accumulating in the subretinal pigment epithelium [sub-RPE] space) are used as biomarkers for disease activity. Treatment of nAMD is based on the use of antivascular endothelial growth factor (anti-VEGF) drugs, with the objective of controlling the exudation from the vessels to minimize disease activity and consequently avoid VA loss (3,4). Appropriate treatment intervals maintain and sometimes improve patients’ vision in the long term (3,5-9).
Macular neovascularization secondary to nAMD requires continuous treatment because it remains active for years (10). Randomized controlled trials showed that SRF and/or IRF is still present after two years of treatment in around 40%-50% of patients (3,11,12). Long-term retrospective observational studies have also reported around 40% of patients with detectable active disease at the end of the study, after 3 and 10 years, respectively (9,13). The activity pattern of nAMD is often unpredictable and patients must be continuously monitored and treated to avoid permanent VA loss (14-16), thus representing an important burden for both patients and clinicians (4,17).
In clinical practice, patients are treated in a personalized way with the aim of controlling recurrences, but over time anti-VEGF therapies may experience reduced effectiveness: after 5 and 10 years of treatment, 41% and 42% of patients, respectively, made at least one switch to other anti-VEGFs (9,18). The poor response (with either fluid persistence or recurrences) may be due to tachyphylaxis, changes in the neovascular membrane characteristics (e.g., increased fibrosis acting like a resorption barrier), chronic changes in vessel walls, changes in the type of lesion evolving over time, or an inadequate treatment frequency (19,20). As a consequence, switching treatment becomes necessary and it is important to re-establish control of the disease. While VA is not usually regained, treatment switch is consistently associated with anatomical improvement (20,21). In patients showing fluid persistence despite at least three-monthly injections with bevacizumab, switching to either aflibercept or ranibizumab showed comparable efficacy, with anatomical improvement noticeable after a single injection (22).
Brolucizumab 6 mg (Beovu®, Novartis AG, Basel, Switzerland), a monoclonal single-chain variable domain antibody fragment, is a new-generation anti-VEGF drug approved for the treatment of nAMD, with proven efficacy in fluid resolution and long-lasting effects (23). Brolucizumab shows great promise in reducing the risk of undertreatment and permanent VA loss. Results from the HAWK and HARRIER studies (24) showed that patients treated with brolucizumab (administered at 8- and 12-week intervals after three-monthly loading doses) had a significantly higher reduction in retinal central subfield thickness (CST) and absence of SRF/IRF at 48 and 95 weeks postinjection, compared with those treated with aflibercept (23). These data also showed that disease activity was controlled in a shorter timeframe after diagnosis when using brolucizumab compared with aflibercept (24). The use of brolucizumab in patients showing no response to other anti-VEGFs offers a valuable therapeutic option to maintain disease control and VA while reducing treatment burden.
Preliminary observations on patients switched to brolucizumab have recently been published (25,26), confirming its efficacy in controlling fluid accumulation. However, more data are needed to further characterize brolucizumab efficacy and rapidity in obtaining complete fluid resolution and the impact on functional outcomes.
We report here the anatomical and functional responses observed in a group of nAMD patients with persistent or recurrent fluids 1 month after a single injection of brolucizumab, as assessed retrospectively in a center in Italy.
Materials and methods
Study design
We retrospectively analyzed data from nine patients with nAMD who were switched to brolucizumab 6 mg because they were not responsive to other anti-VEGFs. All recruited patients were followed and treated at Villa Donatello Hospital, Florence, Italy. The use of brolucizumab for treating nAMD was officially introduced in October 2020. Patient demographic and clinical information collected at baseline (i.e., before the brolucizumab injection) included: age; gender; treatment prior to brolucizumab injection (anti-VEGF used, treatment duration, number of injections, last interval between injections, time from last injection to the first brolucizumab injection); VA; CST; type of lesion; and the presence of IRF, SRF, or sub-RPE fluid, fibrosis, subretinal fibrosis, and subretinal hyperreflective material (SHRM).
Following the first brolucizumab injection, patients were examined at two time points (T1 = 1 ± 1 weeks and T2 = 4 ± 1 weeks) to determine the presence of anatomical and functional changes using slit-lamp ophthalmoscopy, spectral domain optical coherence tomography (SD-OCT), fundus examination, and VA assessment (ETDRS scoring). Three patients had further assessments at a longer follow-up.
Due to the possibility of intraocular inflammation (IOI), vasculitis, or retinal vascular occlusion, all patients were informed and instructed to report, within 24 hours of the injection, any of the following symptoms: redness, reduction of viscous, floaters, eye pain or pressure, scotoma, or blurred vision. A notification letter was sent to the local Ethical Committee, according to Italian regulations. All patients provided written informed consent prior to receiving the brolucizumab injection.
Patient inclusion and exclusion criteria
The study only included patients with nAMD not responsive anymore to other anti-VEGFs (i.e., showing either fluid persistence or frequent fluid recurrences), who were first injected with brolucizumab by December 2020, and had a visit 1 month (T2 range 4±1 weeks) after the injection. Patients with a maculopathy other than nAMD or who received brolucizumab injection after December 2020 were excluded.
Study outcomes
The main outcome measured was the proportion of patients without SRF and IRF 1 month after brolucizumab injection. Secondary outcomes were the proportion of patients with reduced (or absent) SRF/IRF 1 month after injection, the proportion of sub-RPE fluid resolution 1 month after injection in patients with sub-RPE fluid at baseline, the proportion of patients without active disease 1 month after injection, the mean time to first disease inactivation, and the mean difference of CST and VA between baseline and 1-month postinjection.
Statistical analysis
Descriptive statistics were used to summarize demographic and clinical data at baseline and successive time points, as appropriate. Continuous data were described using the mean, standard deviation (SD), and range, while categorical data were expressed as percentages. The difference in CST and VA between baseline and the assessment at 1 month was tested using the nonparametric Wilcoxon signed-rank test, with statistical significance set at p < 0.05. The time to first disease inactivation was determined using Kaplan-Meier survival analysis using the two available observation time points, T1 and T2. Analyses were performed using IBM® SPSS® version 26.
Results
Patient characteristics at baseline
A total of nine patients were eligible and included in the study. None of the patients had signs of IOI at baseline or study end. Patient demographics and clinical and treatment history at baseline are summarized in Table I.
Before switching to brolucizumab, six patients were treated with a single anti-VEGF: aflibercept (n = 2) or bevacizumab (n = 2) or ranibizumab (n = 2). The remaining patients switched from aflibercept to bevacizumab (n = 1), from bevacizumab to aflibercept (n = 1), and from bevacizumab to ranibizumab to aflibercept (n = 1).
| Characteristic | Data |
|---|---|
| Age (years), mean (SD; range) | 77.2 (11.6; 58-90) |
| Female gender, no. (%) | 7 (77.8) |
| Clinical profile, mean (SD) | |
| VA (letters) | 54.4 (20.1) |
| CST (μm) | 386.4 (97.5) |
| Angiographic lesion type, no. (%) | |
| 1 | 4 (44.4) |
| 2 | 5 (55.6) |
| With IRF | 6 (66.7) |
| With SRF | 7 (77.8) |
| With sub-RPE fluid | 3 (33.3) |
| With fibrosis | 3 (33.3) |
| With subretinal fibrosis | 1 (11.1) |
| With SHRM | 1 (11.1) |
| Anti-VEGF treatment prior to brolucizumab injection, mean (SD; min-max) | |
| Treatment length (months) | 9.9 (5.8; 3-18) |
| No. of injections | 5.7 (3.5; 2-12) |
| Last administration interval (months) | 2 (0.7; 1-3) |
| Months from last injection to brolucizumab switch | 3.1 (1.2; 2-6) |
| Reason for switching to brolucizumab, no. (%) | |
| Fluid persistence | 4 (44.4) |
| Frequent fluid recurrences | 5 (55.6) |
µm = micrometers; CST = retinal central subfield thickness; IRF = intraretinal fluid; max = maximum; min = minimum; no. = number; RPE = retinal pigment epithelium; SD = standard deviation; SHRM = subretinal hyperreflective material; SRF = subretinal fluid; VA = visual acuity; VEGF = vascular endothelial growth factor.
Anatomical and functional outcomes at 1-month postinjection
Patients were assessed within 1 week and 1 month (mean 29.1 ± 4.6 days, range 24-35) after the first brolucizumab injection. No signs of active disease were observed in eight (88.9%) patients: in seven patients (77.8%) SRF and IRF were absent, while in one patient (11.1%) fluid was only partially reabsorbed. All three patients presenting with sub-RPE fluid at baseline showed complete resolution.
The CST showed a significant reduction from 398.4 (97.5 SD) μm at baseline to 258.3 (32.4 SD) μm at 1 month postinjection (mean difference 131.1 μm, 95% confidence interval [CI] 46.4-215.8; related-samples Wilcoxon signed-rank test p = 0.007).
In parallel, VA improved significantly from 54.4 (20.1 SD) letters at baseline to 72.8 (16 SD) letters at 1 month postinjection (mean difference 18.3 letters, 95% CI 6.64-30.02; related-samples Wilcoxon signed-rank test p = 0.011).
The estimated mean and median time to disease inactivation were 14.2 (range 5-23.4) days and 3 (range 2.03-3.97) days, respectively (shown in Fig. 1).
In the three patients who were further examined at 60, 62, and 92 days, respectively, from the injection, no sign of disease reactivation or fluid recurrence was observed, and the VA remained stable; thus, there was no need for additional injections. As a case example, the retinal SD-OCT B-scans of one of the patients at different time points following brolucizumab injection are shown in Figure 2.
IOI, vasculitis, or retinal vascular occlusion was not reported for any patient in the follow-up period under consideration.
Discussion
Significant anatomical improvements were observed in patients switching to brolucizumab, as expected according to the available data (25,27,28). These included a significant reduction in CST and almost complete resolution of fluids in most patients.
Although the most frequent timepoint used to evaluate the postinjection anatomical response is approximately 1 month, in the case of no response it would be useful to have an earlier assessment since there is a correlation between CST reduction and the time passed from the last injection (29,30). In the group of patients we described, the response to brolucizumab treatment was rapid according to anatomical improvement, which occurred on average 2 weeks after the injection.
Furthermore, in the subgroup of patients followed for more than 4 weeks, the improvements were stable for at least 8 to 12 weeks after injection. Despite these results being obtained in non-treatment-naïve patients, they seem to be in line with those from the HAWK and HARRIER studies, which showed that nAMD was controlled for at least 12 weeks in more than 50% of naïve patients injected with brolucizumab (24). Such long-term effects suggest patients may be stabilized with an interval of 12 weeks between injections, thus constituting a significant improvement of treatment burden. This is also clearer considering that retinal fluid was not successfully controlled with injections every 8 weeks before the switch to brolucizumab.
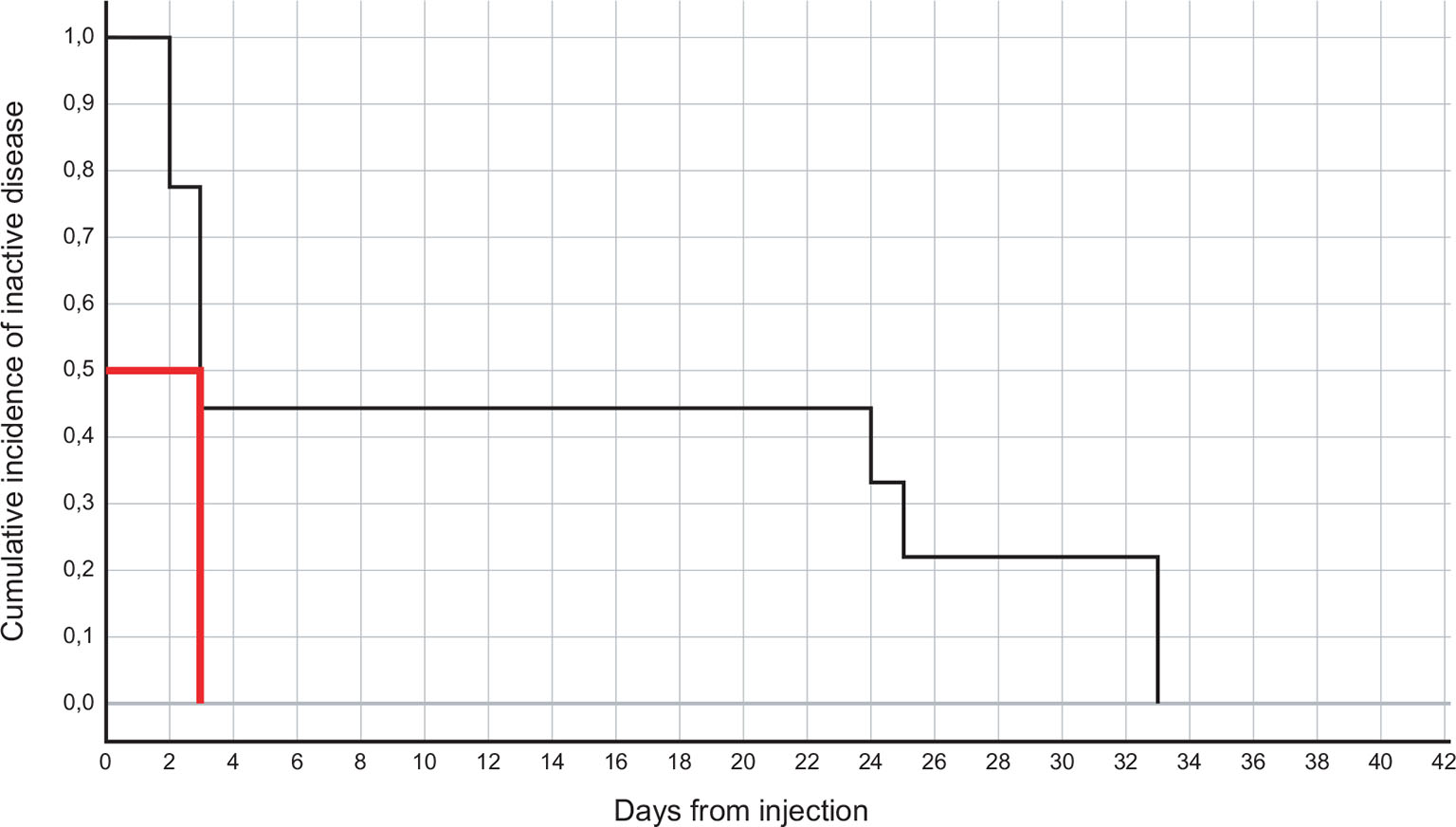
Fig. 1 - Kaplan-Meier plot for time to disease inactivation in the nine patients. The median (50th percentile) is indicated in red.
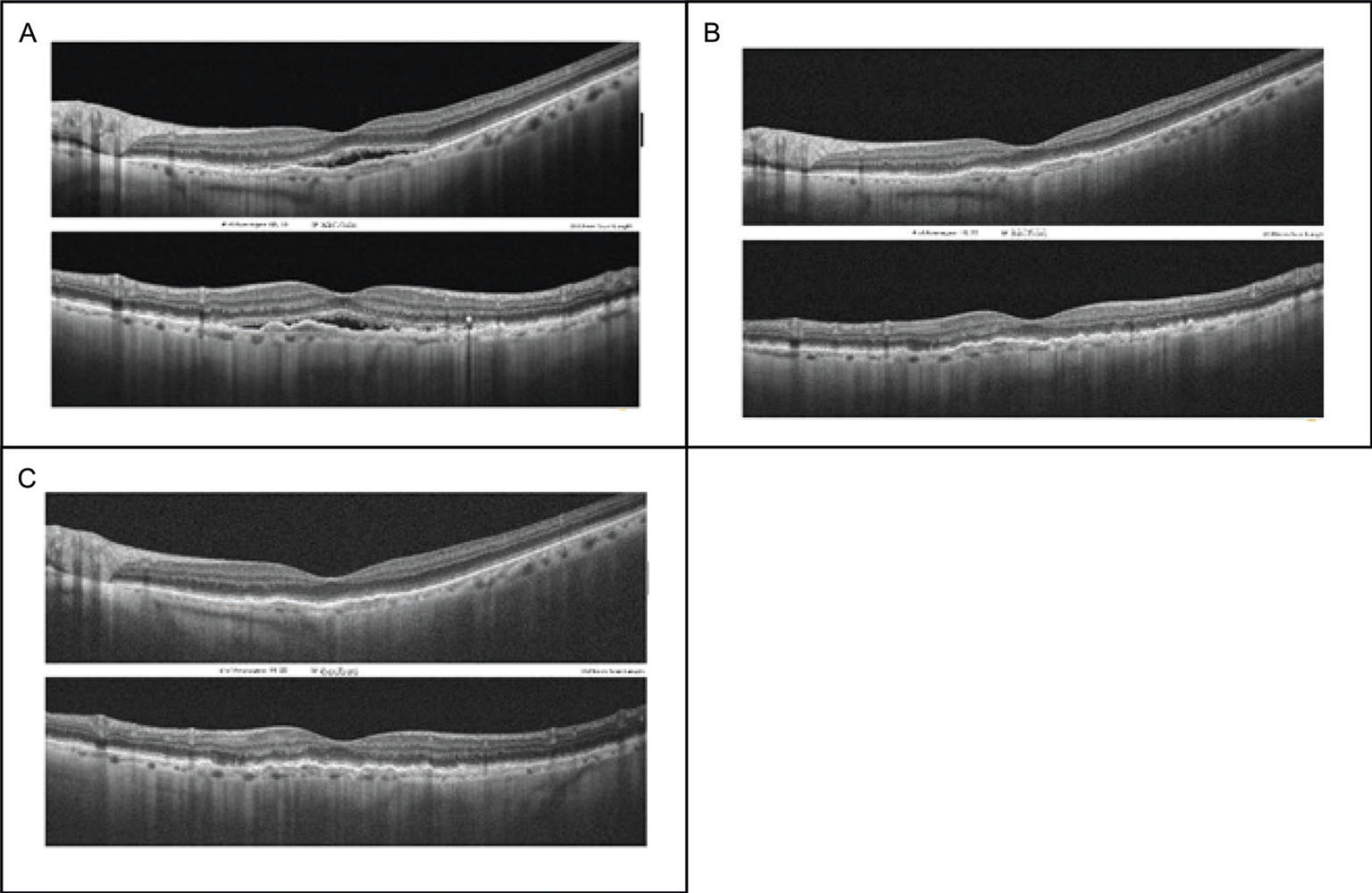
Fig. 2 - Representative case of a woman, 90 years old, treated with three intravitreal injections of aflibercept during the previous 9 months. Spectral domain optical coherence tomography (SD-OCT) B-scans at different time points: A) Baseline (before brolucizumab injection). B) Follow-up 1 month after injection. C) Follow-up 2 months after injection.
In our group of patients, we also observed a significant improvement in terms of VA. This appears to contradict a recent case series of six patients who did not show any increase in VA (26), and a retrospective analysis of 172 eyes, in which there was no improvement in VA after one to three brolucizumab injections (28). There are two possible explanations for our observation: the low number of cases in this case series, and the fact that our patients were treated for a shorter follow-up compared with the other studies (9.9 months and at least 2 years, respectively).
No inflammatory adverse events were reported in our patients, which may also be due to the small sample size and short follow-up. An independent Safety Review Committee, analyzing inflammatory ocular adverse events in the HAWK and HARRIER trials, observed an incidence of retinal vasculitis and retinal vascular occlusion of 3% and 2%, respectively, with a risk of severe visual loss in approximately 1 in every 200 patients (31).
In this context, it is important to consider the balance between the risk of adverse events and the benefits. In our case series, the decision to initiate brolucizumab treatment was made for patients who were not responding anymore to other anti-VEGFs despite regular treatment. Notwithstanding, close contact with the patient is needed during follow-up, as it is expected that adverse inflammatory events can be successfully managed if they are recognized and treated promptly.
This retrospective case series has some limitations. Despite the important improvements observed, the sample size was small, and the measured changes had wide ranges. To better characterize the long-term efficacy and safety profile of brolucizumab in clinical practice, patients undergoing a treatment switch to brolucizumab should be followed for longer. Furthermore, naïve patients not undergoing treatment with other anti-VEGFs should be recruited to verify the anatomical superiority of brolucizumab and to optimize the treatment of nAMD by reducing the treatment burden for both patients and clinicians (24,25).
Conclusion
In conclusion, a single injection of brolucizumab was shown to be rapid and effective in fluid resolution, thus representing a valuable therapeutic option for controlling disease activity in nAMD patients. Indeed, the first month after the injection was shown to be very important to predict the response to the anti-VEGF drug of the single patient and brolucizumab showed a rapid and intense response.
Acknowledgments
We thank Gabriele Massimetti, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, for the statistical support. Medical writing support was provided by Corrado Minetti on behalf of Health Publishing & Services Srl, which was funded by Novartis Farma SpA.
Disclosures
Statement of ethics: This retrospective study was conducted in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its subsequent amendments. A notification letter was sent to the local Ethical Committee (Comitato Etico Area Vasta Centro at the Careggi University Hospital, Florence, Italy), as required by Italian regulations. Written informed consent for both medical treatment and publications of their data was obtained from all participants.
Conflict of interest: The authors have no conflicts of interest to declare.
Funding sources: This manuscript did not receive any funding.
Author contributions: Study design: Silvio Zuccarini. Data collection: Fabrizio Puce, Alessandro Crisà. All authors contributed to writing, reviewing, and editing the manuscript.
Data availability statement: Data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137(3):496-503. CrossRef PubMed
- 2. Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology. 2020;127(5):616-636. CrossRef PubMed
- 3. Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1-24. CrossRef PubMed
- 4. Wykoff CC, Clark WL, Nielsen JS, Brill JV, Greene LS, Heggen CL. Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J Manag Care Spec Pharm. 2018 Feb;24(2-a Suppl):S3-S15. CrossRef PubMed
- 5. Rasmussen A, Brandi S, Fuchs J, et al. Visual outcomes in relation to time to treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2015;93(7):616-620. CrossRef PubMed
- 6. Real JP, Granero GE, De Santis MO, et al. Rate of vision loss in neovascular age-related macular degeneration explored. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1859-1865. CrossRef PubMed
- 7. Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration—a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016;16(1):31. CrossRef PubMed
- 8. Lanzetta P, Loewenstein A; Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259-1273. CrossRef PubMed
- 9. Gillies M, Arnold J, Bhandari S, et al. Ten-year treatment outcomes of neovascular age-related macular degeneration from two regions. Am J Ophthalmol. 2020;210:116-124. CrossRef PubMed
- 10. Chandra S, Arpa C, Menon D, et al. Ten-year outcomes of antivascular endothelial growth factor therapy in neovascular age-related macular degeneration. Eye (Lond). 2020;34(10):1888-1896. CrossRef PubMed
- 11. Heier JS, Brown DM, Chong V, et al; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. CrossRef PubMed
- 12. Guymer RH, Markey CM, McAllister IL, et al; FLUID Investigators. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723-734. CrossRef PubMed
- 13. Bhandari S, Nguyen V, Arnold J, et al. Treatment outcomes of ranibizumab versus aflibercept for neovascular age-related macular degeneration: data from the Fight Retinal Blindness! Registry. Ophthalmology. 2020;127(3):369-376. CrossRef PubMed
- 14. Essex RW, Nguyen V, Walton R, et al; Fight Retinal Blindness Study Group. Treatment patterns and visual outcomes during the maintenance phase of treat-and-extend therapy for age-related macular degeneration. Ophthalmology. 2016;123(11):2393-2400. CrossRef PubMed
- 15. Nguyen V, Vaze A, Fraser-Bell S, et al; Fight Retinal Blindness! Study Group. Outcomes of suspending VEGF inhibitors for neovascular age-related macular degeneration when lesions have been inactive for 3 months. Ophthalmol Retina. 2019;3(8):623-628. CrossRef PubMed
- 16. Rusakevich AM, Zhou B, Wong TP, Wykoff CC. Quarterly anti-vascular endothelial growth factor dosing for neovascular age-related macular degeneration: real-world clinical outcomes. Ophthalmic Surg Lasers Imaging Retina. 2019;50(9):e250-e256. CrossRef PubMed
- 17. Monés J, Singh RP, Bandello F, Souied E, Liu X, Gale R. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of mindset. Ophthalmologica. 2020;243(1):1-8. CrossRef PubMed
- 18. Gillies MC, Campain A, Barthelmes D, et al; Fight Retinal Blindness Study Group. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837-1845. CrossRef PubMed
- 19. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012;96(1):1-2. CrossRef PubMed
- 20. Chakravarthy U, Bezlyak V, Sagkriotis A, et al. Effectiveness of continued ranibizumab therapy in neovascular age-related macular degeneration versus switch to aflibercept: real world evidence. Ophthalmol Retina. 2019;3(1):8-15.e1. CrossRef PubMed
- 21. Nguyen CL, Gillies MC, Nguyen V, et al; Fight Retinal Blindness! Study Group. Characterization of poor visual outcomes of neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor agents. Ophthalmology. 2019;126(5):735-742. CrossRef PubMed
- 22. Salcedo-Villanueva G, Feria-Anzaldo E, Romo-Aguas JC, et al. Anti-VEGF treatment switch in neovascular age-related macular degeneration: a comparison of aflibercept versus ranibizumab after a single-dose switch. Int Ophthalmol. 2019;39(9):2023-2031. CrossRef PubMed
- 23. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: Ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89-99. CrossRef PubMed
- 24. Dugel PU, Koh A, Ogura Y, et al; HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. CrossRef PubMed
- 25. Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-early real-world experience: BREW study. Eye (Lond). 2021;35(4):1045-1047. CrossRef PubMed
- 26. Avaylon J, Lee S, Gallemore RP. Case series on initial responses to intravitreal brolucizumab in patients with recalcitrant chronic wet age-related macular degeneration. Int Med Case Rep J. 2020;13:145-152. CrossRef PubMed
- 27. Seguin-Greenstein S, Lightman S, Tomkins-Netzer O. A meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age-related macular degeneration following switching to treatment with aflibercept. J Ophthalmol. 2016;2016:4095852. CrossRef PubMed
- 28. Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441-448. CrossRef PubMed
- 29. Bontzos G, Bagheri S, Ioanidi L, et al. Nonresponders to ranibizumab anti-VEGF treatment are actually short-term responders: a prospective spectral-domain OCT study. Ophthalmol Retina. 2020;4(12):1138-1145. CrossRef PubMed
- 30. Zola M, D’Alessandro E, Sherif M, et al. Refractory neovascular age-related macular degeneration: time-dependent changes of central retinal thickness with anti-VEGF treatment. Graefes Arch Clin Exp Ophthalmol. 2021 Jun;259(6):1477-1486 CrossRef PubMed
- 31. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021 Jul;128(7):1050-1059. CrossRef PubMed