 |
Drug Target Insights 2021; 15: 1-4 ISSN 1177-3928 | DOI: 10.33393/dti.2021.2197 REVIEW |
 |
Corticosteroid treatment reduces headache in eosinophilic meningitis: a systematic review
ABSTRACT
Background: Eosinophilic meningitis (EOM) is an emerging parasitic disease that can be found worldwide, of which acute severe headache is a presenting symptom. Although such headaches may persist for up to 2 months, studies have found corticosteroid to be effective in reducing this symptom. As the most recent systematic review was published in 2015, the aim of this study was to provide a more up-to-date examination of the role of corticosteroids in EOM.
Methods: We included randomized controlled trials of corticosteroid treatment for EOM regardless of comparators. Research articles published in five databases were searched and evaluated. The primary outcome was headache, which was compared among various treatment regimens.
Results: We found a total of 257 articles after duplication removal. Of those, two met the study criteria. According to these studies, oral prednisolone alone or in a combination of albendazole resulted in fewer patients with headache after a 2-week course of treatment compared with placebo (maximum of 9.1% vs. 45.5%). The duration of headache was also shorter in the prednisolone arm vs. placebo (maximum of 5 vs. 13 days). There were no serious side effects reported.
Conclusion: A 2-week course of treatment with oral corticosteroid with or without albendazole reduced headaches in patients with EOM.
Keywords: Angiostrongylus cantonensis, Headache, Prednisolone
Received: October 28, 2020
Accepted: February 4, 2021
Published online: March 8, 2021
This article includes supplementary materials
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2021 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Background
Eosinophilic meningitis (EOM) is defined by a cerebrospinal fluid (CSF) eosinophil count of less than 10% of total CSF white blood cells(1). The main cause of EOM is Angiostrongylus cantonensis infection. Humans become infected mainly through the consumption of or contact with contaminated food or drink(2). Patients with EOM usually present with acute severe headache(1). As fever and neck stiffness are uncommon in EOM, it may be misdiagnosed if physicians are not sufficiently aware of the condition(1). History of consuming raw freshwater snails or contaminated food is a crucial clue for EOM diagnosis (1,2).
EOM is an emerging and often neglected parasitic disease that can be found worldwide(3). A review published in 2008 found 2,827 cases of EOM reported from 30 countries(4). In addition, there are an estimated 0.3-2 infected people per 100,000 population per year with occasional outbreaks(5). From 1997 to 2008, there were 13 outbreaks in mainland China alone(6). If EOM is left untreated or undiagnosed, individuals are at increased risk of several severe neurological complications such as encephalitis or radiculomyelitis (7-9). The risk of encephalitis in untreated or undetected cases of EOM is 26% per day(7).
If treated with analgesic alone, the severe headaches caused by EOM can last up to 49 days(10). However, this duration can be reduced with corticosteroid administration(11), as it decreases inflammation in the meninges and brain. The most recent systematic review on corticosteroids in EOM was a Cochrane review published in 2015(12). It included one randomized controlled trial, which compared corticosteroids with placebo. It is recommended that systematic reviews be updated every 2 years to include any newly published articles(13). We thus updated and examined studies of corticosteroid treatment in EOM regardless of comparators.
Materials and methods
This study is a systematic review focused on the effects of corticosteroids in human EOM. We included randomized controlled trials of corticosteroid treatment for EOM regardless of comparators. The types of interventions were corticosteroids vs. placebo or other active treatments. The participants in the studies examined were EOM patients aged 15 years or older(12). Those who had already taken corticosteroids prior to study participation were excluded.
We searched five databases in this review: PubMed, Central database, Scopus, CINAHL Plus, and Web of Science. The search terms used were “meningit*, eosinophil*”, “Angiostrongylus cantonensis”, and “randomized controlled trials”. The full list of search terms are shown in Appendix 1 (See supplementary material). The final search was performed on November 22, 2019.
After duplication removal, initial screening was carried out for nonrelevant articles. Studies were considered relevant if they had been conducted to evaluate the role of corticosteroids in any form of headache caused by EOM. The full-text reports were subsequently reviewed by two independent authors (SK, KS). Of these, any randomized controlled trials were included in the final analysis. The primary outcomes were number of patients with persistent headache at 2 weeks after treatment and duration of headache by corticosteroid treatment regimen. Other outcomes included number of patients who underwent repeated lumbar puncture and side effects of treatment. We presented frequency with percentage for categorical variables. Continuous variables were described using median and range. Risk ratios (RR) with 95% confidence intervals (CI) were used to compare to compare a risk between two groups from randomized controlled trials (RCTs).
Biases of eligible studies were evaluated across six domains (sequence generation, allocation concealment, blinding of participants/personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias) by two authors independently (SK, KS). Biases were categorized as low risk, high risk, or unclear according to the guidelines specified in the Cochrane Handbook for Systematic Reviews of Interventions(12). Disagreements were reviewed and reported by a third reviewer (CN).
Results
We found a total of 288 articles in the following five databases: Scopus (254 articles), PubMed (21 articles), Web of Science (9 articles), Central database (3 articles), and CINAHL Plus (1 article). Two hundred fifty-seven remained after duplication removal (Fig. 1), of which 252 were excluded as nonrelevant. In total, five articles were selected for full-text review (11,14-17). Of these, only two met the study criteria. With regard to the excluded studies, one did not evaluate outcomes or headache, and the other two were not randomized controlled trials.
The first of the remaining studies was published in 2000 and enrolled 110 EOM patients(11). This study found that treatment with prednisolone resulted in fewer patients experiencing headaches at 2 weeks after treatment and shorter duration of headache when compared with placebo (9.1% vs 45.5%; RR 0.20 with 95% CI: 0.08 to 0.48 and 5 days vs. 13 days, respectively). The number of cases of repeated lumbar puncture was also significantly higher in the placebo group than in the prednisolone group (22 times vs. 7 times; RR 0.32 with 95% CI: 0.15 to 0.68), as shown in Table I.
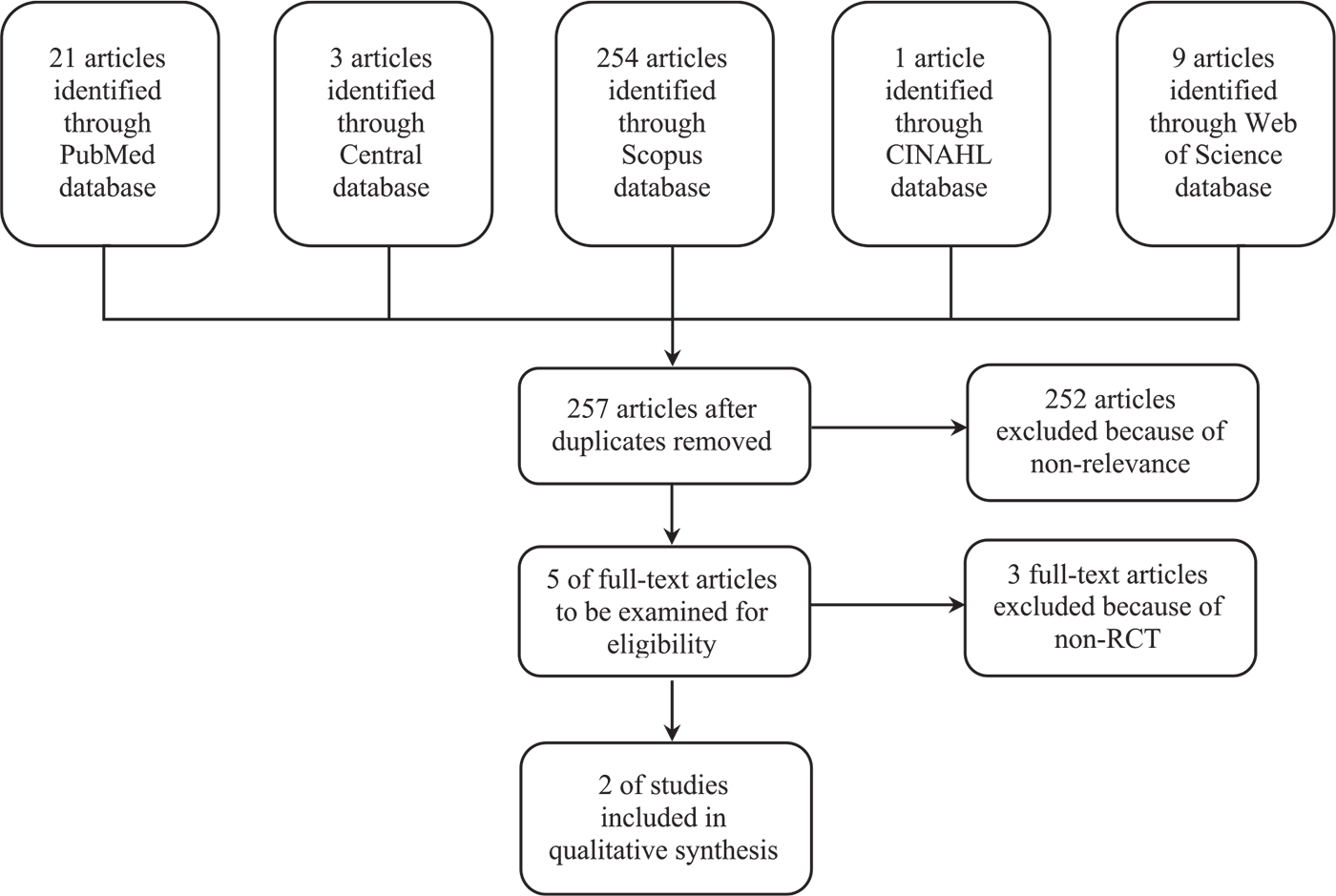
Fig. 1- Study flow of the systematic review on corticosteroid treatment in eosinophilic meningitis. RCT = randomized controlled trial.
| Factors | Study 1: 2000 | Study 2: 2009 | ||
|---|---|---|---|---|
| Active | Control | Active | Control | |
| Treatment | Pred | Placebo | Pred + albendazole | Pred |
| No. of patients | 55 | 55 | 53 | 51 |
| No. of patients with headache at 2 weeks (n, %) | 5 (9.1%) | 25 (45.5%) | 0 | 1 (2.0%) |
| Median (range) duration of headache, days | 5 (1-60) | 13 (1-56) | 3 (1-14) | 3 (1-15) |
| No. of patients with repeated LP (n, %) | 7 (12.8%) | 22 (40.0%) | NA | NA |
LP = lumbar puncture, NA = not available, Pred: prednisolone.
The second study was published in 2009 and conducted in 104 EOM patients(17). It compared prednisolone plus albendazole with prednisolone alone and found no significant difference in terms of the primary outcome. No data on repeated lumbar puncture were reported (Tab. I).
Both studies were conducted in Thailand, used only oral prednisolone, and reported no serious side effects from either prednisolone or prednisolone plus albendazole. Allocation, concealment, and stratification were not reported in either study. The second study was not blinded (Fig. 2).
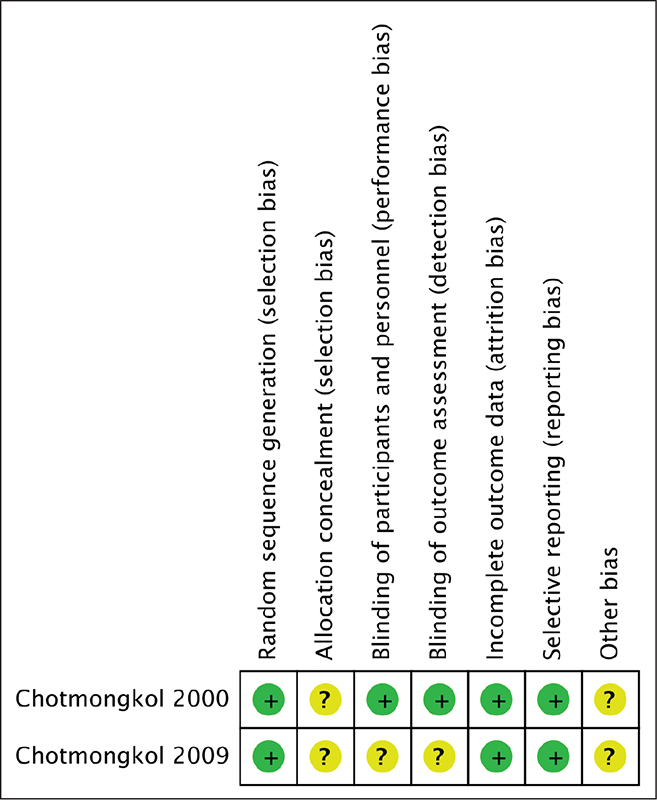
Fig. 2 - Biases of the included studies in the systematic review on corticosteroid treatment in eosinophilic meningitis.
| Factors | Study 1: 2004 | Study 2: 2004 | Study 3: 2006 |
|---|---|---|---|
| Treatment | Prednisolone 1 week | Prednisolone + albendazole | Prednisolone + mebendazole |
| No. of patients | 52 | 26 | 41 |
| No. of patients with headache at 2 weeks (n, %) | 6 (11.6%) | 3 (11.6%) | 4 (9.8%) |
| Median (range) duration of headache, days | NA | 4 (1-17) | 3 (1-20) |
| No. of patients with repeated LP (n, %) | 1 (2.0%) | 0 | 3 (7.4%) |
LP = lumbar puncture, NA = not available.
Discussion
Although there were two eligible studies, we were unable to perform a conventional meta-analysis or network meta-analysis due to the inconsistent treatment arms and incomplete cycle, respectively. As a result, this report was defined as a systematic review.
In both studies, oral prednisolone with or without albendazole yielded favorable outcomes in terms of both numbers of EOM patients with headache and duration of headache after 2 weeks of treatments. These findings were compatible with those of other single-arm studies examining the effects of prednisolone alone vs. prednisolone plus anthelmintics (15,18,19), as shown in Table II. The corticosteroid-based regimen with albendazole and that with mebendazole yielded comparable headache durations in the two studies in this review (3-4 days vs. 3-5 days), but the 1-week prednisolone treatment had higher numbers of patients with headache at 2 weeks (11.6%). There were no serious side effects of corticosteroid found in any of the five studies examined.
There were some limitations in these studies. First, both studies were conducted in northeast Thailand, an area endemic for EOM. Second, some information was not reported in the randomized controlled trials such as concealment or blinding. These items were reported as possible biases (Fig. 2). Finally, only oral prednisolone was administered for a duration of 2 weeks.
Conclusion
Two weeks of treatment with oral corticosteroid with or without albendazole reduced headaches in EOM patients.
Acknowledgments
The authors would like to thank the Division of Research Affairs at Khon Kaen University’s Faculty of Medicine (SY60201) and the North-Eastern Stroke Research Group (Khon Kaen University; Khon Kaen, Thailand).
Disclosures
Financial support: This study was supported by the Thailand Research Fund’s Distinguished Research Professor Grant (Grant no. DPG6280002) to Pewpan Maleewong Intapan and Wanchai Maleewong.
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1. Sawanyawisuth K, Chotmongkol V. Eosinophilic meningitis. Handb Clin Neurol. 2013;114:207-215. CrossRef PubMed
- 2. Khamsai S, Chindaprasirt J, Chotmongkol V, et al. Clinical features of eosinophilic meningitis caused by Angiostrongylus cantonensis in Thailand: a systematic review. Asia Pac J Sci Technol. 2020;25(2):APST-25-02-09. Online
- 3. Ansdell V, Wattanagoon Y. Angiostrongylus cantonensis in travelers: clinical manifestations, diagnosis, and treatment. Curr Opin Infect Dis. 2018;31(5):399-408. CrossRef PubMed
- 4. Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8(10):621-630. CrossRef PubMed
- 5. Barratt J, Chan D, Sandaradura I, et al. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology. 2016;143(9):1087-1118. CrossRef PubMed
- 6. Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis. 2012;31(4):389-395. CrossRef PubMed
- 7. Sawanyawisuth K, Takahashi K, Hoshuyama T, et al. Clinical factors predictive of encephalitis caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 2009;81(4):698-701. CrossRef PubMed
- 8. Maretić T, Perović M, Vince A, Lukas D, Dekumyoy P, Begovac J. Meningitis and radiculomyelitis caused by Angiostrongylus cantonensis. Emerg Infect Dis. 2009;15(6):996-998. CrossRef PubMed
- 9. Al Hammoud R, Nayes SL, Murphy JR, Heresi GP, Butler IJ, Pérez N. Angiostrongylus cantonensis meningitis and myelitis, Texas, USA. Emerg Infect Dis. 2017;23(6):1037-1038. CrossRef PubMed
- 10. Sawanyawisuth K, Sawanyawisuth K, Senthong V, et al. Clinical features and course of Angiostrongylus cantonensis eosinophilic meningitis in patients receiving supportive therapy. Food Waterborne Parasitol. 2020;21:e00095. CrossRef PubMed
- 11. Chotmongkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroid treatment of eosinophilic meningitis. Clin Infect Dis. 2000;31(3):660-662. CrossRef PubMed
- 12. Thanaviratananich S, Thanaviratananich S, Ngamjarus C. Corticosteroids for parasitic eosinophilic meningitis. Cochrane Database Syst Rev. 2015;2015(2):CD009088. PubMed
- 13. Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007;4(3):e78. CrossRef PubMed
- 14. Punyagupta S, Juttijudata P, Bunnag T. Eosinophilic meningitis in Thailand. Clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 1975;24(6 Pt 1):921-931. CrossRef PubMed
- 15. Sawanyawisuth K, Limpawattana P, Busaracome P, et al. A 1-week course of corticosteroids in the treatment of eosinophilic meningitis. Am J Med. 2004;117(10):802-803. CrossRef PubMed
- 16. Diao Z, Chen X, Yin C, Wang J, Qi H, Ji A. Angiostrongylus cantonensis: effect of combination therapy with albendazole and dexamethasone on Th cytokine gene expression in PBMC from patients with eosinophilic meningitis. Exp Parasitol. 2009;123(1):1-5. CrossRef PubMed
- 17. Chotmongkol V, Kittimongkolma S, Niwattayakul K, Intapan PM, Thavornpitak Y. Comparison of prednisolone plus albendazole with prednisolone alone for treatment of patients with eosinophilic meningitis. Am J Trop Med Hyg. 2009;81(3):443-445. CrossRef PubMed
- 18. Chotmongkol V, Sawadpanitch K, Sawanyawisuth K, Louhawilai S, Limpawattana P. Treatment of eosinophilic meningitis with a combination of prednisolone and mebendazole. Am J Trop Med Hyg. 2006;74(6):1122-1124. CrossRef PubMed
- 19. Chotmongkol V, Wongjitrat C, Sawadpanit K, Sawanyawisuth K. Treatment of eosinophilic meningitis with a combination of albendazole and corticosteroid. Southeast Asian J Trop Med Public Health. 2004;35(1):172-174. PubMed