 |
Drug Target Insights 2021; 15: 5-12 ISSN 1177-3928 | DOI: 10.33393/dti.2021.2192 REVIEW |
 |
Tamarind (Tamarindus indica L.) Seed a Candidate Protein Source with Potential for Combating SARS-CoV-2 Infection in Obesity
ABSTRACT
Introduction: Obesity and coronavirus disease (COVID)-19 are overlapping pandemics, and one might worsen the other.
Methods: This narrative review discusses one of the primary mechanisms to initiate acute respiratory distress syndrome, uncontrolled systemic inflammation in COVID-19, and presents a potential candidate for adjuvant treatment. Blocking the S protein binding to angiotensin-converting enzyme 2 (ACE-2) and the 3C-like protease (3CL pro) is an effective strategy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Results: Host proteases such as FURIN, trypsin, and transmembrane serine protease 2 (TMPRSS) act in S protein activation. Tamarind trypsin inhibitor (TTI) shows several beneficial effects on the reduction of inflammatory markers (tumor necrosis factor α [TNF-α], leptin) and biochemical parameters (fasting glycemia, triglycerides, and very low-density lipoprotein [VLDL]), in addition to improving pancreatic function and mucosal integrity in an obesity model. TTI may inhibit the action of proteases that collaborate with SARS-CoV-2 infection and the neutrophil activity characteristic of lung injury promoted by the virus.
Conclusion: Thus, TTI may contribute to combating two severe overlapping problems with high cost and social complex implications, obesity and COVID-19.
Keywords: 3CLpro, ACE-2, COVID-19, FURIN, HNE, Inflammation, TMPRSS
Received: October 20, 2020
Accepted: March 11, 2021
Published online: April 2, 2021
Drug Target Insights - ISSN 1177-3928 - www.aboutscience.eu/dti
© 2021 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
The relation between obesity and severe COVID-19
Coronavirus disease (COVID)-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) coronavirus, is a serious threat to health systems around the world. It overlaps with obesity, another global pandemic(1-3). SARS-CoV-2 infection started in December 2019 and spread rapidly because its transmission occurs through the airways.
The consequences of SARS-CoV-2 infection range from self-limited flu to fulminant pneumonia, respiratory failure, and death(4,5). Although the new coronavirus has mutations, it is still unclear whether they are related to its virulence (6), which might soon be answered through ongoing studies (7).
Advanced age is a risk factor that leads to the worsening of the clinical condition of the disease, placing the elderly as a vulnerable population with a high risk of death. However, severe cases also occur in middle-aged or younger people, and one of the possible contributing factors for this is the already known relationship between nutritional status and the prognosis of viral infections, which can contribute to the improvement or worsening of the disease(8-10).
According to Butler et al (11) and Muscogiuri et al (12) broader access to a healthy and balanced diet rich in vitamins, minerals, bioactive compounds, and antioxidants is essential to assist in reducing susceptibility to SARS-CoV-2 infection, in addition to the complications that can occur in the long term. The regional councils of the four United Nations Agencies (Food and Agriculture Organization of the United Nations—FAO, United Nations Children’s Fund—UNICEF, World Health Organization—WHO, and World Food Program—WFA) issued a joint statement on nutrition in the context of the COVID-19 pandemic in Asia and the Pacific. The recommendations emphasized the need for continuous adoption of advice and strategies ensuring nutritional surveillance, food quality, and food security.
On the other hand, the current moment demands the isolation of the world population as a strategy to contain the infection spread. Isolation can increases consumers’ preference for industrialized and ultra-processed foods. This preference occurs due to the practicality, hygienic safety, longer shelf life, of industrialized and ultra-processed foods compared to fresh and unprocessed products (13). This possible trend may increase food insecurity and directly impact weight gain(13-16), increasing the risk of overweight and obesity, which can persist for an extended period (17).
Diet composition and obesity have a fundamental role in the coregulation of adaptive immunity (18), influencing the modulation of the immune response and, consequently, affecting the severity of respiratory diseases and other infections (9). In general, adiposity can impair the ventilation of the base of the lungs, resulting in less oxygen saturation in the blood (19). Thus, in COVID-19, the need for treatment in intensive care units (ICUs) increases, and intubations are technically more challenging in obese patients, in addition to the difficulty in obtaining a diagnosis by imaging techniques.
According to the review study by Johnson et al (20), obesity damages various organs, which increases the susceptibility to several diseases, such as metabolic disorders, cardiovascular diseases, cancer, and viral infections such as influenza and COVID-19(1,21).
There is an intrinsic relationship between fat distribution and metabolic health status. The location of fat deposition in the body is determinant for health, and the ectopic deposition of triglycerides in the abdominal region, mainly visceral fat, causes a phenotype of high cardiometabolic risk—even for individuals who have a normal body mass index (BMI) but with a high abdominal circumference (AC)—due to unregulated cytokine secretion pathways. In addition, inflammation and increased release of circulating fatty acids is associated with visceral fat. Thus, the fat distribution phenotype contributes to metabolism, leading to metabolically unhealthy individuals (22).
Thus, visceral obesity can be an important risk factor to increase the severity of COVID-19 (23). Studies with patients affected by this disease in Germany and Italy have shown that as the area of visceral adipose tissue increased, there was a greater need for intensive therapy, regardless of other factors, such as age, sex, and associated diseases(24,25). Another study performed in China with COVID-19 patients showed that higher visceral and subcutaneous adipose tissue were independent risk factors for critical illness (26). In addition, visceral adipose tissue positively regulates the expression of plasminogen activator inhibitor 1, thus generating an increased risk of developing thrombosis in patients with visceral obesity affected by COVID-19 (27). Thus, understanding the metabolically unhealthy individual is essential, carefully assessing the response to SARS-CoV vaccination (23), as obesity and impaired metabolic health generate a potentially reduced immune response, which can negatively affect the vaccine’s effectiveness(28-31).
Obesity is characterized by a state of low-grade chronic inflammation related to changes in immune cells, including the number and types of cells present in the inflamed tissue. At the beginning of weight gain, immune cells infiltrate adipose tissue, contributing to persistent adipose inflammation and insulin resistance. Macrophages are classified into two subtypes of phenotypes, M1 proinflammatory and M2 anti-inflammatory. In eutrophic individuals, the M2 subtype is distributed throughout adipose tissue, producing interleukin (IL)-10 and expressing arginase-1 for collagen synthesis, which is important for promoting tissue repair. During the progression of weight gain to obesity, the M1 subtype becomes dominant, spreading inflammation through the production of mediators, such as tumor necrosis factor α (TNF-α), IL-1β, monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor 1 (PAI-1) and reactive oxygen species (ROS). As a result, M1 macrophages interrupt insulin sensitivity in adipose tissue and the liver (20).
The hormone leptin overlaps when referring to the link between obesity and inflammation, since leptin synthesis is directly proportional to the amount of adipose tissue, and inflammatory cytokines stimulate this synthesis. These inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and interferon gamma (IFN-γ), coagulation factors (fibrinogen and PAI-1), acute-phase protein (C-reactive protein and amyloid serum A [SAA]), white blood cell count, and chemokines are markers of inflammation. In patients with obesity, these markers are commonly elevated, and with the reduction of excess weight, there is a reduction in their plasma concentrations (32). There are also anti-inflammatory adipokines, such as IL-4, IL-5, and IL-10, which can be observed in obesity at low concentrations. Thus, an imbalance between these anti- and inflammatory cytokines can induce the inflammatory response (33).
Considering that obesity negatively affects the immune system, there is, therefore, a clear relationship with the higher susceptibility to the more severe COVID-19. This close relationship occurs due to the increased release of inflammatory cytokines by disrupting the integrity of tissues (adipose and lymphoid tissue, intestines, and lungs), which alters the activation of leukocytes, impairing the action of the immune system. As a result, there is a direct impact on the healing process, which prolongs recovery and increases the risk of evolution from respiratory infection to severe diseases with a high risk of death (34).
It is important to highlight that SARS-CoV is responsible for coding 3C-like protease (3CL pro), a cysteine protease (35), formally known as C30 endopeptidase that is chymotrypsin-like (36). It is considered a key component in polyprotein processing (37), which synthesizes nonstructural proteins and structural proteins, such as spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins (38). Thus, this protease plays an important role in the replication and transcription of viral ribonucleic acid (RNA) (39).
Recently, Hoffmann et al (40) demonstrated that the new coronavirus enters human cells through glycoprotein S, found on the surface of the virus. This glycoprotein can bind to the angiotensin-converting enzyme 2 (ACE-2) located in human cells. ACE-2 receptors are expressed in the intestine, kidneys, lungs, and blood vessels, becoming targets for SARS-CoV-2 infection. Hoffmann et al (40) also observed that the cellular transmembrane serine protease 2 (TMPRSS2) is needed for SARS-CoV-2 entrance in host cells. Xu et al (41) investigated the possible routes of SARS-CoV-2 infection in the oral cavity mucosa, exploring the expression of ACE-2 and the proportion and composition of the cells responsible for this function based on RNA-seq profiles and cell transcript-independent data. The results showed that ACE-2 could be expressed in the epithelial cells of the oral cavity, mainly in the tongue, oral and gingival tissues, indicating that the oral cavity mucosa can be a potential route for infection.
In the respiratory system, ACE-2 hydrolyzes angiotensin II to angiotensin 1-7, with an essential role in regulating the system. When ACE-1 activity is increased and ACE-2 is inhibited, intact angiotensin II acts through the angiotensin 1 (AT1) or 2 (AT2) receptor to exert proinflammatory responses and stimulate aldosterone secretion. As a result, these effects increase blood pressure and potentially cause hypokalemia, in addition to intensifying local vascular permeability, increasing the risk of respiratory distress syndrome. On the other hand, angiotensin 1-7 acts on the receptor pathway, leading to anti-inflammatory and antifibrotic responses that would be favorable to the recovery of patients with COVID-19 (42). Studies also describe the importance of the renin-angiotensin system (RAS) in the regulation of metabolism and the development of cardiovascular and inflammatory diseases(43-45). This system also modulates the endocrine and metabolic functions of adipocytes, hypertrophy, and hyperplasticity in obesity(46,47).
Obesity and its related comorbidities facilitate viral replication, increasing the risk of severe complications in SARS-CoV-2 infections. This increased risk occurs in obesity because the enlarged adipose tissue expresses higher levels of ACE-2 and, consequently, serves as a reservoir for the virus (48). The expression of ACE-2 in target tissues (lung, liver, and heart) is increased in diabetes (49), commonly seen in obese individuals. Hyperglycemia and type 2 diabetes also trigger the release of inflammatory cytokines, which in cases of COVID-19 can lead to higher release of cytokines (cytokine storm), generating an immune dysregulation that can lead to multiple organ failure and death (50). Obesity also induces deregulated lipogenesis that promotes the high expression of ACE-2 in the lungs (51). The increased leptin synthesis stimulated by inflammatory cytokines (33) can also worsen the clinical condition of obese patients with COVID-19.
Therefore, drugs that mediate metabolic responses targeting ACE-2 have been considered promising in the modulation of glucose metabolism and blood pressure control. These drugs may also prevent the entry of the new coronavirus through competitive pathways of ACE (9).
Results
Can the Kunitz trypsin inhibitor from tamarind seeds combat SAS-CoV-2 Infection in Obesity?
Considering these dysfunctions in patients with COVID-19, several studies and tests with drugs, traditionally used to control these changes, whether endocrine or metabolic, appear. Some adjuvant therapies for COVID-19 deserve special mention, such as immunomodulatory agents, immunoglobulin therapy, corticosteroids, and anticytokines used to control endocrine and metabolic changes(9,52,53).
A new mechanism related to the inhibition of the transmembrane protease serine 2, encoded by the TMPRSS2 gene, is an additional target for drugs for research. As already discussed, researchers have demonstrated that SARS-CoV-2 uses the SARS-CoV ACE-2 receptor to enter cells. The serine protease TMPRSS2 is also necessary for the initiation of protein S. A TMPRSS2 inhibitor, Camostat mesylate—a synthetic serine protease inhibitor (trypsin), approved in Japan for clinical use, blocked entry and could be another option treatment in COVID-19(9,40). Other studies also highlight Camostat mesylate for the treatment of pancreatitis(9,52) currently in phase 2 clinical trial in COVID-19.
Furthermore, inhibition of 3CL pro can block protein S synthesis and coronavirus proliferation (39). Most studies have focused mainly on small-molecule compounds from virtual screening based on a 3CL pro structure (54). Several inhibitors, which can be classified as peptoids and non-peptidomimetics, have shown good inhibitory activity of this protease (39).
Protease inhibitors have been widely studied. One reason is that they are present in multiple forms in plants, animals, and microorganisms. In addition, protease inhibitors are natural protease regulators that are intrinsically involved in biological processes such as digestion, healing, viral replication, and the blood clotting cascade, among others, and that need precise regulation(55,56).
Thus, the prospect of peptides for use in biotechnology has led to a number of molecules’ discovery. Its use in experimental models has exposed mammals to risks that have not yet been evaluated. Although the benefits of proteins and peptides substantially outweigh the potential harmful effects, their use is not without risks. There is a balance between the ability of peptides to induce desirable effects, that is, their bioactivity, and the potential toxic effects associated with their cell-penetrating properties (57).
Among protease inhibitors, trypsin inhibitors are widely extracted from seeds to be purified and characterized for application in various studies (58). These molecules have shown, in recent studies, performance in different mechanisms involving the control of obesity and its related effects, such as the production of hormones related to satiety, affecting the central nervous system and the small intestine; reduction of food consumption and weight gain; improvement of the lipid profile; and reduction of the inflammatory process associated with obesity, regardless of weight loss (59).
A trypsin inhibitor extracted from seeds of the tamarind fruit, Tamarindus indica L., belonging to the legume family, occurring in all regions of Brazil (60) has been extensively studied by our research group.
The first study to evaluate the potential of the tamarind trypsin inhibitor (TTI) to reduce weight gain was developed by Ribeiro et al (61). In this study, male eutrophic Wistar rats were fed a standard diet and received the isolated TTI by gavage for 11 days at a dose of 25 mg/kg. TTI administration reduced food intake in these animals. To better understand this reduction in consumption, another experiment using the same experimental model evaluated food consumption 1 h, 2 h, and 16 h after gavage with TTI, in addition to serum cholecystokinin (CCK), using doses of 25 and 50 mg/kg. TTI reduced food consumption 16 h after its administration dose-dependently (61). In this study, TTI did not cause changes in the liver enzymes and serum proteins of Wistar rats, in addition to not affecting the histological aspects of the liver, stomach, intestine, and pancreas. In addition, TTI did not cause classical deleterious effects on protein digestion and, consequently, malnutrition at the doses tested, as demonstrated by the measurement of serum proteins.
Obesity is a condition that leads to numerous physiological changes, and the behavior of TTI in this condition needs to be assessed. For this, Carvalho et al (62) used Wistar rats with diet-induced obesity, assessing food consumption and other biochemical parameters, using the 25 mg/kg dose of TTI, as proposed by Ribeiro et al (61). In animals with obesity, TTI reduced food consumption without inducing weight loss. TTI reduced plasma TNF-α to undetectable concentrations, showing that the isolated inhibitor also influenced other aspects related to obesity, such as low-grade chronic inflammation (62).
In the same experimental model of obesity, Costa et al (63) observed that animals with obesity treated with TTI decreased food intake in a similar way to eutrophic animals. The animals with obesity treated with TTI showed a slight reduction in the Lee index, which, although not significant, was important because of the consumption of a diet rich in ultra-processed foods called High Glycemic Index and Glycemic Load (HGLI) (64). Although TTI did not alter the plasma CCK, it decreased the expression of the CCK-1R gene and plasma leptin in animals with obesity compared to the group with obesity without treatment (63).
TTI is characterized as a protein isolate with high inhibitory activity for trypsin, obtained in a process that generates enrichment of this protein, but not its purification (61). Therefore, it is possible that other molecules can influence the biochemical and bioactive characteristics of TTI.
Due to the many biological functions of TTI in the context of obesity, new technologies that could potentialize these functions were assessed. Nanotechnology, through nanoencapsulation, can promote the protection of bioactive substances in oral administration, which exposes these molecules to digestive processes, compromising active sites essential to biological activity. In addition, nanoencapsulation provides controlled release at a specific target and further intensification of the biological effect (65).
Thus, TTI was nanoencapsulated to increase the efficiency and stability of antitrypsin activity. The isolated and conjugated effects of chitosan and isolated whey protein on incorporation, antitrypsin activity, and TTI stability at different temperatures and pH conditions were investigated. The combination of chitosan and isolated whey protein (ECW) formed nanoparticles (109 nm), promoted a reduction in the half maximal inhibitory concentration (IC50; 0.05 mg) compared to pure TTI (0.21 mg), and preserved antitrypsin activity up to 80°C (35.0% [3.74]) compared to isolated agents and TTI, which have no inhibitory activity. Besides, the nanoparticles showed stability under different pH conditions. Thus, ECW proved to be an essential strategy to improve the function and stability of TTI (66).
To assess the safety of administration by gavage in addition to maintaining the inhibitory activity, the encapsulated TTI (ECW) was evaluated in a preclinical obesity study. Costa (67) also assessed cytotoxicity using the Caco-2 and CCD-18Co strains (human intestinal cells). The cytotoxicity assay exceeded 70% cell viability for Caco-2 and CCD-18Co when exposed to different concentrations of ECW. For the subacute blood toxicity of the bioactive dose of ECW, through a complete blood count, liver, and kidney function, there was an absence of subacute blood toxicity, demonstrated by the lack of toxic effects on the biochemical parameters evaluated.
Considering that ECW proved to be safe in the face of the parameters evaluated, its biological activity was tested after encapsulation (68). The nanoencapsulated TTI was also offered by gavage in a preclinical obesity model to test whether the isolated inhibitor would maintain its modulating properties on the altered biochemical parameters of the experimental model used. The biological activity was maintained, with emphasis on ECW, which significantly reduced blood glucose, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), and raised high-density lipoprotein cholesterol (HDL-c), in addition to possibly favoring greater protection to pancreatic tissue.
The purification of these inhibitors is a necessary and critical step to define their structural characteristics and specificity of binding to other molecules. Isolating these proteins from all other proteins that are present in the same biological source is difficult because trypsin inhibitors have great molecular diversity (69). Medeiros et al (70) described the TTI purification process, obtaining a molecule with 100% inhibition for trypsin (pTTI), heat resistant and with a partially identified amino acid sequence (currently complete and with structural modeling—unpublished data), which had high homology with other trypsin inhibitors of the Kunitz family. pTTI also did not affect plasma CCK concentrations but reduced circulating leptin concentrations in animals with obesity at a dose of 730 μg/kg. Leptin is an important hormone in the energy balance, which is elevated in individuals with obesity, leading to the development of a resistance condition (70).
The effect of the purified trypsin inhibitor tamarind was also evaluated in a model of metabolic changes in Wistar rats with obesity and dyslipidemia. Obesity was induced using the HGLI diet (64). The animals treated with pTTI at a dose of 730 μg/kg had significantly lower food intake than the untreated group. However, the groups did not show differences in weight gain. pTTI showed great anti-inflammatory potential, reducing the relative expression of TNF-α messenger RNA and positive immunostaining in adipocyte immunohistochemical analysis of obese animals, as well as plasma cytokine concentrations (71). The anti-inflammatory effects of the isolated or purified inhibitor on adipose tissue were observed regardless of weight changes, which may suggest a direct beneficial effect on this tissue that may alter its structure.
This result shows the potential of this molecule since obesity is classically considered a disease that induces a low grade of chronic inflammation, causing changes in tissues, notably the intestinal mucosa (72) and adipose tissue (73). Due to these promising biological effects and safety demonstrated in preclinical studies with partially purified TTI, this inhibitor could be explored in alternative obesity therapies.
The purification process can enhance the functional properties of a molecule, making it necessary to reassess the safety of its use. At a dose approximately thirty times lower, pTTI performed the same biological activities as TTI. This result emphasizes the need to assess whether its use generated harmful effects on the liver, a target tissue in toxicity studies; pancreas, a classically affected organ by trypsin inhibitors; adipose tissue, where pTTI, in previous studies, has shown anti-inflammatory effects; and intestine, a potential site of action that has not yet been studied.
Trypsin inhibitors have well-reported deleterious effects. Evidence has shown impaired growth due to poor digestion and absorption, metabolic changes in the pancreas, such as increased enzyme secretion, hypertrophy and hyperplasia, and metabolic disturbance in the use of amino acids (74).
Thus, pTTI was evaluated for possible toxic effects, focusing on the histopathological and stereological characteristics of organs involved in its metabolism, processing, and biological activity (liver and pancreas) and the tissues most affected by the obesity model (small intestine and visceral adipose tissue). pTTI at its bioactive dose did not cause signs or symptoms of general toxicity or potential damage to the liver and pancreatic tissue of obese Wistar rats. pTTI also promoted a protective effect on the intestines of these animals, reducing the loss of intestinal villi, a well-characterized damage in obesity models. pTTI reduced the presence of inflammatory infiltrates in perirenal (visceral) adipose tissue. Therefore, its use in the tested models is safe and presents anti-inflammatory effects (unpublished data).
Besides the effects mentioned above, TTI is a promising molecule concerning the mechanisms associated with the inhibition of genes involved in the production of ACE-2, such as TMPRSS2 and FURIN (Fig. 1). These genes probably act by changing the organism’s epigenetic system responsible for the increase in ACE-2 expression, a potential target for antiviral intervention by SARS-CoV-2.
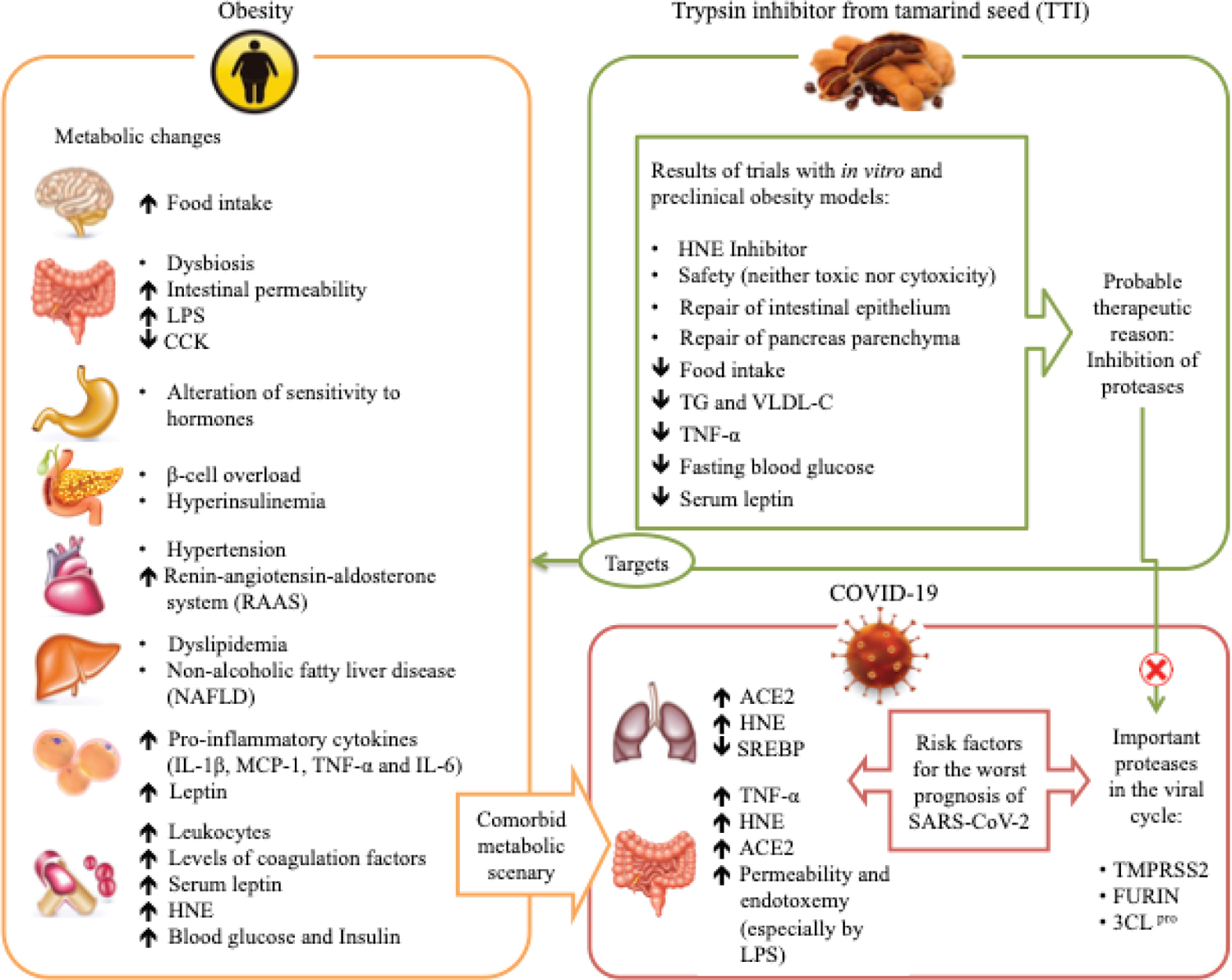
Fig. 1 - Obesity and associated comorbidities as risk factors for complications from SARS-CoV-2 infection, and hypothesis of the TTI mechanism of action. Obesity affects several organs, which have several responses, such as increased inflammation, changes in sensitivity and the action of hormones, dyslipidemia, and others. This metabolic deregulation favors increased expression of ACE-2, which is cleaved in the C-terminal segment by proteases such as TMPRSS2 and FURIN, and there is activation of the spike glycoprotein, so this process facilitates the entry of SARS-CoV-2 into the cells, causing viral infection. Also, 3CL pro is considered a key component in polyprotein processing and plays an important role in the replication and transcription of viral RNA. The TTI effects in in vitro and preclinical studies show several antiobesity and anti-inflammatory effects and appear to be possible inhibitors of the proteases TMPRSS2, FURIN, and 3CLpro.
ACE-2 = angiotensin-converting enzyme 2; CCK = cholecystokinin; FURIN = member of the mammalian prohormone-protein convertases family; HNE = human neutrophil elastase; IL-1β = interleukin 1-β; LPS = lipopolysaccharide; MCP-1 = monocyte chemoattractant protein 1; SREBP = sterol regulatory element-binding proteins; TG = triglyceride; TMPRSS2 = transmembrane serine protease 2; TNF-α = tumor necrosis factor α; TTI = trypsin inhibitor from tamarind; VLDL-c = very-low-density lipoprotein cholesterol; 3CL pro = 3C-like protease.
Adipokines secreted by adipose tissue can also affect airway function. Leptin is involved in neonatal lung development, surfactant production(75,76), and regulation of ventilatory impulse(76,77). Studies have consistently demonstrated the association of high concentrations of leptin and asthma(78,79). The leptin concentration was reduced with the use of TTI in animal models(63,70).
Several studies with trypsin inhibitors were related to obesity and its complications (59). According to Fook et al. (80), TTI showed selective activity, being highly effective against serine proteinases, especially against bovine trypsin and neutrophil elastase isolated from humans. The IC50 value was determined to be 55.96 μg/mL. The inhibitor also showed no cytotoxic or hemolytic activity in human blood cells. In addition, it exhibited different inhibition of the release of elastase by platelet-activating factor (PAF; 44.6%) and release by N-formyl-L-methionyl-L-leucyl-phenylalanine (fMLP; 28.4%), preferentially affecting elastase release by PAF stimuli. This may indicate selective inhibition in the receptors of the PAF (80). The same research group in 2010 conducted another study and demonstrated that the soy inhibitor (SKTI) reduced lipopolysaccharide (LPS)-induced acute lung injury in a preclinical model, significantly suppressing the inflammatory effects caused by elastase in a dose-dependent manner, suggesting the route of inhibition of human neutrophil elastase as a promoter of the improvement (81).
Several computational molecular docking studies have been carried out with some compounds to model binding interactions of various 3CL pro inhibitors and other proteases, such as TMPRSS2 (37,82-84). pTTI-derived peptides are also shown to be strong candidates for blocking these proteases since TTI is known to inhibit serine proteases such as trypsin and chymotrypsin, as previously demonstrated.
Conclusions
Therefore, trypsin inhibitors are promising alternatives, in addition to others already discussed in the scientific community, which can be used as adjuvants in COVID-19, especially in obese patients. Thus, the tamarind seed trypsin inhibitor may also be a preventive or adjuvant drug in the context of COVID-19, especially in worsened inflammatory conditions, such as obesity.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Finance Code 001.
Acknowledgments
The authors thank Professor Dr. Elizeu Antunes dos Santos for figure editing and the Federal University of Rio Grande do Norte (UFRN), especially the Pro-Rectory of Postgraduate and the Pro-Rectory of Research, for all efforts dedicated to supporting the research in our institution.
Disclosures
Conflict of Interest: The authors declare no conflicts of interest.
Financial support: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Finance Code 001.
References
- 1. Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 Mortality. Obesity (Silver Spring). 2020;28(6):1005. CrossRef PubMed
- 2. Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368(March):m1198. CrossRef PubMed
- 3. National Academies of Sciences and Medicine E, National Academies of Sciences, Engineering and M. Current Status and Response to the Global Obesity Pandemic: Proceedings of a Workshop—in Brief. (Callahan EA, ed.). The National Academies Press; 2019. CrossRef
- 4. Del Rio C, Malani PN. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020;323(14):1339-1340. CrossRef PubMed
- 5. Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14(3):211-212. CrossRef PubMed
- 6. Pachetti M, Marini B, Benedetti F, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18(1):179. CrossRef PubMed
- 7. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome main point: hydroxychloroquine was found to be more potent than chloroquine at inhibiting SARS-CoV-2 in vit. Clin Infect Dis. 2020;2:1-25.
- 8. Calder PC, Waitzberg DL, Klek S, Martindale RG. Lipids in parenteral nutrition: biological aspects. JPEN J Parenter Enteral Nutr. 2020;44(S1)(suppl 1):S21-S27. CrossRef PubMed
- 9. Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16(6):297-298. CrossRef PubMed
- 10. Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the patient with obesity—the editors speak out. Obesity (Silver Spring). 2020;28(5):847. CrossRef PubMed
- 11. Butler CC, van der Velden AW, Bongard E, et al. Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial. Lancet. 2020;395(10217):42-52. CrossRef PubMed
- 12. Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. Published online 2020:10-11. CrossRef
- 13. United Nations System Standing Committee on Nutrition—UNSCN. Food Environments in the COVID-19 Pandemic. UNSCN. Published 2020. Online. Accessed April 29, 2020.
- 14. Rundle AG, Park Y, Herbstman JB, Kinsey EW, Wang YC. COVID-19-related school closings and risk of weight gain among children. Obesity (Silver Spring). 2020;28(6):1008-1009. CrossRef PubMed
- 15. Berger ZD, Evans NG, Phelan AL, Silverman RD. Covid-19: control measures must be equitable and inclusive. BMJ. 2020;368(Sept 2001):m1141. CrossRef
- 16. Farrell P, Thow AM, Abimbola S, Faruqui N, Negin J. How food insecurity could lead to obesity in LMICs: when not enough is too much: a realist review of how food insecurity could lead to obesity in low- and middle-income countries. Health Promot Int. 2018;33(5):812-826. CrossRef PubMed
- 17. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;5793(February 2019):eabb5793. CrossRef
- 18. Pindjakova J, Sartini C, Lo Re O, et al. Gut dysbiosis and adaptive immune response in diet-induced obesity vs. systemic inflammation. Front Microbiol. 2017;8(JUN):1157. CrossRef PubMed
- 19. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755-767. CrossRef PubMed
- 20. Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218-238. CrossRef PubMed
- 21. Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034. CrossRef PubMed
- 22. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616-627. CrossRef PubMed
- 23. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135-149. CrossRef PubMed
- 24. Petersen A, Bressem K, Albrecht J, et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110(January):154317. CrossRef PubMed
- 25. Watanabe M, Caruso D, Tuccinardi D, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. CrossRef PubMed
- 26. Yang Y, Ding L, Zou X, et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity (Silver Spring). 2020;28(11):2040-2048. CrossRef PubMed
- 27. Shimomura I, Funahashi T, Takahashi M, et al. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2(7):800-803. CrossRef PubMed
- 28. Hazeldine J, Lord JM. Immunesenescence: a predisposing risk factor for the development of COVID-19? Front Immunol. 2020;11(Oct):573662. CrossRef PubMed
- 29. Kumar H. Healthy immunity: it’s all about immune regulation. Int Rev Immunol. 2020;39(6):245-246. CrossRef PubMed
- 30. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. CrossRef PubMed
- 31. Ledford H. How obesity could create problems for a COVID vaccine. Nature. 2020;586(7830):488-489. CrossRef PubMed
- 32. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141-150. CrossRef PubMed
- 33. do Prado WL, Lofrano MC, Oyama LM, et al. Obesity and inflammatory adipokines: practical implications for exercise prescription. Rev Bras Med Esporte. 2009;15(5):378-383. CrossRef
- 34. Morais AH de A, Aquino J de S, Silva-Maia JK da, Vale SH de L, Maciel BLL, Passos TS. Nutritional status, diet and viral respiratory infections: perspectives for SARS-CoV-2. Br J Nutr. Published online August 26, 2020:1-32. CrossRef
- 35. Pillaiyar T, Meenakshisundaram S, Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov Today. 2020;25(4):668-688. CrossRef PubMed
- 36. Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394-1399. CrossRef
- 37. Yu R, Chen L, Lan R, Shen R, Li P. Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. Int J Antimicrob Agents. 2020;56(2):106012. CrossRef PubMed
- 38. Forni D, Cagliani R, Clerici M, Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25(1):35-48. CrossRef PubMed
- 39. Liu Y, Liang C, Xin L, et al. The development of coronavirus 3C-like protease (3CLpro) inhibitors from 2010 to 2020. Eur J Med Chem. 2020;206:112711. CrossRef PubMed
- 40. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. Published online 2020:1-10. CrossRef
- 41. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368(Jan):m606. CrossRef PubMed
- 42. Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169(3):477-492. CrossRef PubMed
- 43. Santos RAS, Ferreira AJ, Simões E, Silva AC, Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93(5):519-527. CrossRef PubMed
- 44. Ferreira AJ, Santos RAS, Bradford CN, et al. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55(2):207-213. CrossRef PubMed
- 45. Santos PCJL, Krieger JE, Pereira AC. Renin-angiotensin system, hypertension, and chronic kidney disease: pharmacogenetic implications. J Pharmacol Sci. 2012;120(2):77-88. CrossRef PubMed
- 46. Shinozaki K, Ayajiki K, Nishio Y, Sugaya T, Kashiwagi A, Okamura T. Evidence for a causal role of the renin-angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension. 2004;43(2 I):255-262. CrossRef
- 47. Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35(6):1270-1277. CrossRef PubMed
- 48. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. CrossRef PubMed
- 49. Roca-Ho H, Riera M, Palau V, Pascual J, Soler MJ. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci. 2017;18(3):E563. CrossRef PubMed
- 50. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. CrossRef PubMed
- 51. Al Heialy S, Hachim MY, Senok A, et al. Regulation of angiotensin converting enzyme 2 (ACE2) in obesity: implications for COVID-19. bioRxiv. 2020;2:2020.04.17.046938. CrossRef
- 52. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824-1836. CrossRef PubMed
- 53. Mamber S, Krakowka S, Osborn J, et al. Could unconventional immunomodulatory agents help alleviate COVID-19 symptoms and severity? Preprints. 2020;(April). CrossRef
- 54. He J, Hu L, Huang X, et al. Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: insights from structures of protease and inhibitors. Int J Antimicrob Agents. 2020;56(2):106055. CrossRef PubMed
- 55. Farady CJ, Craik CS. Mechanisms of macromolecular protease inhibitors. Clin Lymphoma. 2010;11(17):19-222341-222346. CrossRef
- 56. Laskowski M Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49(1):593-626. CrossRef PubMed
- 57. Barkia I, Ketata Bouaziz H, Sellami Boudawara T, Aleya L, Gargouri AF, Saari N. Acute oral toxicity study on Wistar rats fed microalgal protein hydrolysates from Bellerochea malleus. Environ Sci Pollut Res Int. 2020;27(16):19087-19094. CrossRef PubMed
- 58. Souza DD, Brandão-Costa RMP, Albuquerque WWC, Porto ALF. Partial purification and characterization of a trypsin inhibitor isolated from Adenanthera pavonina L. seeds. S Afr J Bot. 2016;104:30-34. CrossRef
- 59. Cristina Oliveira de Lima V, Piuvezam G, Leal Lima Maciel B, Heloneida de Araújo Morais A. Trypsin inhibitors: promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J Enzyme Inhib Med Chem. 2019;34(1):405-419. CrossRef PubMed
- 60. Lewis GP. Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. CrossRef
- 61. Ribeiro JA, Serquiz AC, Silva PF, et al. Trypsin inhibitor from Tamarindus indica L. seeds reduces weight gain and food consumption and increases plasmatic cholecystokinin levels. Clinics (São Paulo). 2015;70(2):136-143. CrossRef PubMed
- 62. Carvalho FMCC, Lima VCOO, Costa IS, et al. A trypsin inhibitor from tamarind reduces food intake and improves inflammatory status in rats with metabolic syndrome regardless of weight loss. Nutrients. 2016;8(10):1-14. CrossRef PubMed
- 63. Costa IS, Medeiros AF, Carvalho FMC, et al. Satietogenic protein from tamarind seeds decreases food intake, leptin plasma and CCK-1r gene expression in obese wistar rats. Obes Facts. 2018;11(6):440-453. CrossRef PubMed
- 64. Luz ABS, Dos Santos Figueredo JB, Salviano BDPD, et al. Adipocytes and intestinal epithelium dysfunctions linking obesity to inflammation induced by high glycemic index pellet-diet in Wistar rats. Biosci Rep. 2018;38(3):1-15. CrossRef PubMed
- 65. Li S, Liu L, He G, Wu J. Molecular targets and mechanisms of bioactive peptides against metabolic syndromes. Food Funct. 2018;9(1):42-52. CrossRef PubMed
- 66. De Queiroz JLC, De Araújo Costa RO, Rodrigues Matias LL, et al. Chitosan-whey protein nanoparticles improve encapsulation efficiency and stability of a trypsin inhibitor isolated from Tamarindus indica L. Food Hydrocoll. 2018;84:247-256. CrossRef
- 67. Costa ROA. Identification of safety and potential clinical application of nanoparticles loaded with a trypsin inhibitor isolated from tamarind seeds (Tamarindus indica L.). Dissertation. Published online 2019. CrossRef
- 68. Matias LLR, Costa ROA, Passos TS, et al. Tamarind trypsin inhibitor in chitosan-whey protein nanoparticles reduces fasting blood glucose levels without compromising insulinemia: a preclinical study. Nutrients. 2019;11(11):2770. CrossRef PubMed
- 69. Santos EA, Oliveira AS, Arajo Rablo LM, Ferreira A, Arajo Morais AH. Affinity chromatography as a key tool to purify protein protease inhibitors from plants. In: Affinity Chromatography. InTech; 2012:35. CrossRef
- 70. Medeiros AF, Costa IS, Carvalho FMC, et al. Biochemical characterisation of a Kunitz-type inhibitor from Tamarindus indica L. seeds and its efficacy in reducing plasma leptin in an experimental model of obesity. J Enzyme Inhib Med Chem. 2018;33(1):334-348. CrossRef PubMed
- 71. Carvalho FMC, Lima VCO, Costa IS, et al. Anti-TNF-α agent tamarind kunitz trypsin inhibitor improves lipid profile of wistar rats presenting dyslipidemia and diet-induced obesity regardless of PPAR-γ induction. Nutrients. 2019;11(3):E512. CrossRef PubMed
- 72. Winer DA, Luck H, Tsai S, Winer S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23(3):413-426. CrossRef PubMed
- 73. Maurizi G, Della Guardia L, Maurizi A, Poloni A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J Cell Physiol. 2018;233(1):88-97. CrossRef PubMed
- 74. Adeyemo SM, Onilude AA. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger Food J. 2013;31(2):84-90. CrossRef
- 75. De Blasio MJ, Boije M, Kempster SL, et al. Leptin matures aspects of lung structure and function in the ovine fetus. Endocrinology. 2016;157(1):395-404. CrossRef PubMed
- 76. Torday JS, Powell FL, Farmer CG, Orgeig S, Nielsen HC, Hall AJ. Leptin integrates vertebrate evolution: from oxygen to the blood-gas barrier. Respir Physiol Neurobiol. 2010;173(1)(suppl):S37-S42. CrossRef PubMed
- 77. Bassi M, Furuya WI, Menani JV, et al. Leptin into the ventrolateral medulla facilitates chemorespiratory response in leptin-deficient (ob/ob) mice. Acta Physiol (Oxf). 2014;211(1):240-248. CrossRef PubMed
- 78. Sideleva O, Dixon AE. The many faces of asthma in obesity. J Cell Biochem. 2014;115(3):421-426. CrossRef PubMed
- 79. Sood A, Ford ES, Camargo CA Jr. Association between leptin and asthma in adults. Thorax. 2006;61(4):300-305. CrossRef PubMed
- 80. Fook JMSLL, Macedo LLP, Moura GEDD, et al. A serine proteinase inhibitor isolated from Tamarindus indica seeds and its effects on the release of human neutrophil elastase. Life Sci. 2005;76(25):2881-2891. CrossRef PubMed
- 81. Ribeiro JKC, Cunha DDS, Fook JMSLL, Sales MP. New properties of the soybean trypsin inhibitor: inhibition of human neutrophil elastase and its effect on acute pulmonary injury. Eur J Pharmacol. 2010;644(1-3):238-244. CrossRef PubMed
- 82. Thanigaimalai P, Konno S, Yamamoto T, et al. Development of potent dipeptide-type SARS-CoV 3CL protease inhibitors with novel P3 scaffolds: design, synthesis, biological evaluation, and docking studies. Eur J Med Chem. 2013;68:372-384. CrossRef PubMed
- 83. Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253(February):117592. CrossRef PubMed
- 84. Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766-788. CrossRef PubMed