 |
Arch Physioter 2025; 15: 32-41 ISSN 2057-0082 | DOI: 10.33393/aop.2025.3323 ORIGINAL RESEARCH ARTICLE |
 |
Comparisons of the effects of psychologically-informed and usual physiotherapy on pain sensitivity in chronic low back pain: an exploratory randomized controlled trial
ABSTRACT
Introduction: The presence of altered central pain processing and modulation, as well as negative psychological factors, have been suggested to impede recovery in chronic low back pain (CLBP). Psychologically-informed physiotherapy (PiP) aims to specifically address the latter factors—in addition to physical factors—to improve treatment effects. This study aims to determine if the effect of PiP is superior to usual physiotherapy (UP) on pain sensitivity and modulation in participants with CLBP and if changes in these variables were associated with changes in clinical outcomes.
Methods: Forty participants with CLBP were randomly allocated to PiP or UP. Seven physiotherapy sessions over 6 weeks plus a booster session at an 11-week follow-up were delivered. Pressure pain threshold (PPT), temporal summation of pain (TSP), and exercise-induced hypoalgesia were assessed on lumbar, upper, and lower limb sites at baseline and after 6 weeks. Linear mixed models tested if PiP was superior to UP on pain sensitivity/modulation. Linear regressions tested if pain sensitivity/modulation changes were associated with changes in clinical outcomes (pain intensity, physical functioning, symptoms of central sensitization).
Results: PiP was not superior to UP to modulate pain sensitivity/modulation variables. All PPTs increased after 6 weeks regardless of the approach. Lumbar PPT and lumbar and lower limb TSP changes were associated with physical functioning changes.
Conclusion: Although our study suggests that neither approach has a superiority to impact on pain sensitivity, both approaches elicited widespread hypoalgesia. Future powered trials should verify if pain sensitivity can be a mediator of physical functioning improvement, as suggested by our results.
Keywords: Exercise-induced hypoalgesia, Pressure pain threshold, Psychologically informed physiotherapy, Quantitative sensory testing, Temporal summation of pain, Usual physiotherapy
Received: September 30, 2024
Accepted: January 27, 2025
Published online: February 17, 2025
Clinical Trial Protocol Number
NCT04979403
Corresponding author:
Hugo Massé-Alarie
email: hugo.masse-alarie@fmed.ulaval.ca
Archives of Physiotherapy - ISSN 2057-0082 - www.archivesofphysiotherapy.com
© 2025 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
What is already known about this topic
- Psychologically-informed Physiotherapy (PiP) consists of identifying and addressing both physical (including sensitization) and psychological factors contributing to pain. This approach is deemed superior to usual physiotherapy (UP) to manage chronic low back pain (CLBP).
What does the study add?
- PiP has been claimed to influence pain sensitivity, modulation, and psychological factors. In this exploratory clinical trial, we observed that both physiotherapy approaches reduced pressure pain sensitivity.
Introduction
Chronic low back pain (CLBP) is highly prevalent in the general population (1) and represents the leading cause of disability worldwide (2). Considering that the specific cause of CLBP is elusive in most patients (90%), the term non-specific CLBP is commonly used (3). This may be explained by the multidimensional nature of CLBP. Indeed, several factors such as psychological, social, biophysical, genetics, and comorbidities may modulate both central pain processing and nociceptive inputs, giving rise to an individual’s pain experience (4). For example, it has been demonstrated that participants with CLBP had reduced exercise-induced hypoalgesia (EIH) and increased pain during a muscle contraction remote from the lower back area, suggesting an alteration in central mechanisms for pain control (5). This is coherent with meta-analyses reporting higher pain sensitivity in CLBP compared to controls, although pain modulation (frequently measured using conditioned pain modulation paradigm) was altered only in some studies (6). This could reflect the presence of nociplastic pain mechanisms (7-9), which is suggested to contribute to chronicity and long-term disability. Some authors proposed, although not all agree (8-10), that patients with predominant nociplastic CLBP phenotype often present with negative psychological factors (e.g., pain catastrophizing, fear) that are predictors of poor prognosis (11). Consequently, most contemporary treatments to manage CLBP consider the multiple factors impacting the pain experience, for example, by targeting (i) nociplastic pain mechanisms and (ii) psychological factors associated with poor prognosis through psychologically-informed approaches (1213-14).
Psychologically informed approaches integrate physical, behavioral, and psychological interventions to improve a patient’s quality of life (15). When delivered by physiotherapists, it is referred to as psychologically-informed physiotherapy (PiP). PiP includes usual physiotherapy (UP) interventions such as manual therapy and exercises along with strategies based on cognitive-behavioral principles and pain neuroscience education (12). Although a meta-analysis demonstrated that interventions similar to PiP (i.e., combining physical and psychological interventions) are more effective in improving pain and physical functioning than usual care (16), the impact of PiP on central pain mechanisms (e.g., pain sensitivity and modulation alteration) has been scarcely studied. PiP may have an impact on pain sensitivity and modulation via different mechanisms. First, it has been suggested that targeting negative emotional and cognitive factors could influence the pain modulation system (17) through the corticolimbic system (18). For example, studies have demonstrated associations between pain catastrophizing and pain sensitivity (19). Second, pain neuroscience education—which also targets psychological factors and pain conceptualization—has been suggested to “desensitize” the central nervous system, thus influencing pain sensitivity and modulation variables and, ultimately, pain-related outcomes (20,21). Emerging evidence may support these hypotheses. A randomized controlled trial demonstrated that the combination of pain neuroscience education and exercises reduced pain sensitivity of the lower back area more than exercise alone (21). Nonetheless, interventions commonly used by physiotherapists may also influence pain sensitivity. Specifically, meta-analyses reported a reduction in pain sensitivity following manipulation (22) and exercises (23). It remains unclear whether PiP has a greater impact on pain sensitivity and modulation variables than UP. Moreover, despite the fact that some groups proposed that PiP may “desensitize” the central nervous system (20,21) and consequently affect pain-related outcomes, it is not known whether changes in pain sensitivity and modulation are associated with changes in pain and disability.
The objectives of this exploratory study are (i) to compare the effects of PiP and UP on pain sensitivity and modulation variables and (ii) to determine if changes in pain sensitivity and modulation are associated with changes in clinical outcomes. We hypothesized that (i) PiP will have a greater influence on pain sensitivity and modulation variables than UP, and (ii) changes in pain sensitivity/modulation variables will be associated with changes in patient-reported outcomes.
Methods
Study design
The complete methodological details and results (feasibility and clinical effect of physiotherapy) of this pilot and exploratory randomized controlled trial were published (24), and the protocol was prospectively registered in ClinicalTrials (NCT04979403). A brief overview of the methods is provided in the following sections.
A two-arm parallel pilot randomized clinical trial (RCT) with a 6-month follow-up was conducted. Quantitative sensory testing (QST) measures were collected at Cirris (research center) at baseline and at a 6-week follow-up. Patient-reported outcome measures (PROM) were assessed at baseline, 6 weeks, 12 weeks, and 24 weeks after randomization using Research Electronic Data Capture (REDCap) (25). Considering the PROMs have been published, only PROMs collected at baseline and 6-week follow-up are reported in this study to fulfill the second objective of the study.
All participants were randomly allocated to one of the two groups (PiP, UP) and underwent their assigned interventions. Each intervention consisted of eight sessions spread over 11 weeks. Precisely, a 60-minute assessment meeting and a follow-up session were delivered during the 1st week, followed by weekly sessions until the 6th week, and a final follow-up session (i.e., “booster” session) in the 11th week (between 30-45 mins per follow-up session).
Participants
Forty participants with CLBP were recruited according to the following inclusion criteria: (i) adults between 18 and 65 years, (ii) non-specific CLBP (>3 months), and (iii) classified at high risk of poor prognosis based on the STarT Back Screening Tool (i.e., psychosocial subscore ≥4/5) (26). Exclusion criteria were any cause of specific LBP (e.g., fracture, cancer) (3), litigation, and neuropathic pain [>4 on the Douleur Neuropathique 4 (DN4) questionnaire (27)]. The presence of leg pain per se was not an exclusion criterion in the absence of signs and symptoms of neuropathic pain. Considering this study was planned as a feasibility and pilot RCT, no a priori sample size was calculated as recommended for this type of study (28). Nonetheless, considering α = 0.05 and β = 0.20, an effect size of d = 0.91 (objective 1) and a coefficient of correlation of r = 0.41 (objective 2) can be detected based on the study’s sample size.
The study was approved by the Ethics Research Committee of the Centre Intégré Universitaire de Santé et de Services Sociaux de la Capitale-Nationale (Project: # 2021-2227) in accordance with the Declaration of Helsinki. All participants provided their written informed consent prior to their inclusion in the clinical trial.
Randomization/Blinding
A random allocation sequence (1:1) was generated with the random function on an Excel file, stratified by sex and physical functioning (Oswestry Disability Index [ODI] cutoff = 20) in blocks of 4. A researcher not involved in the assessment and interventions (CCP) carried out concealed allocation, assigned participants to an intervention group, and contacted each participant after the baseline evaluation to inform them of the location of the clinics to receive treatment. The evaluator of QST measures was blinded to participants’ allocation (AD). Participants were instructed not to discuss with the evaluator about the intervention received, and the physiotherapist and the clinic consulted.
Interventions
Physiotherapists in each arm worked at two different sites of a private physiotherapy clinic (PhysioInteractive group, Quebec City, Canada—urban setting) to limit contamination. Physiotherapists from one site provided UP, while physiotherapists from the other site provided PiP. UP included all interventions that a physiotherapist can provide in the province of Quebec (e.g., education, advice to stay active, exercises, manual therapy). PiP included UP along with a psychologically informed approach (12). Management strategies such as establishing common goals with the patient and therapeutic alliance, behavior change model strategies, motivational interviews, education on pain neurophysiology, gradual exposure, and stress management techniques were used in PiP to mitigate psychosocial factors/barriers. Interventions are fully detailed elsewhere (24).
Outcomes
Quantitative sensory testing (QST)
Static and dynamic pain measurements were tested using standardized verbal instructions according to the recommendations from the German Research Network on Neuropathic Pain (29). Static pain sensitivity was measured using the pressure pain threshold (PPT) (30). PPTs were measured ipsilateral to the side of worst pain for the lower back and upper limb but contralateral to pain for the lower limb. If pain was central, it was considered as a right LBP. Three sites were tested: lumbar erector spinae (lumbar) muscles on the muscle bulk at 2-3 cm lateral to the L4-L5 interspinous space, tibialis anterior (lower limb) at one-third of the distance between the upper part of the head of the fibula and the lower part of the medial malleolus, and wrist flexors (upper limb) at one-third of the distance between the medial epicondyle and the styloid process of the radius. Participants were positioned supine for upper and lower limb measurements and prone for lumbar measurements. All anatomical landmarks were marked before measurement. The evaluator used a handheld digital algometer (1-cm2 probe—FPIX, Wagner Instruments, Greenwich, CT, USA) to apply pressure at a rate of ~0.5 kg/cm2/s. A one-minute break was taken between trials. The testing order was randomized. An average of three trials was used. The algometer can measure up to 11 kg/cm2. Out of 720 measurements, 43 (5.97%) were above this limit and considered as 11 kg/cm2.
Dynamic pain sensitivity was measured using temporal summation of pain (TSP) (31) and EIH (5, 32). TSP is considered a proxy of the wind-up phenomenon and would represent the change in excitability of the spinal neurons (29). For TSP, three sites were tested, namely the L4-L5 interspinous space (lumbar), the dorsal part of the first cuneiform (lower limb), and the dorsal part of the capitate (upper limb). Participants were positioned in crooked lying for wrist and leg measurements and prone for lumbar measurements. The evaluator used a pinprick stimulator (256 mN, MRC Systems GmbH, Heidelberg, Germany) for a series of ten punctuate stimuli at 1 Hz. The evaluator used a visual metronome out of the participant’s sight to respect the frequency of 1 Hz. A one-minute break was taken between the three trials. The average of the three trials was used. TSP was the difference between the pain rated on the numerical pain rating scale (NPRS—from 0 [no pain] to 10 [worst imaginable pain]) after ten stimuli and that after a single stimulus. TSP and PPT are reliable measurements (33,34).
EIH is characterized by a reduction in pain sensitivity following an acute bout of exercise (35,36) and is mediated by opioids and non-opioid mechanisms (e.g., endocannabinoid, serotoninergic) (36,37). To measure EIH, all PPT measurements were repeated after the performance of an isometric wrist contraction. Electromyography was used to standardize the level of muscle activation during wrist contraction by providing feedback to participants to ensure the consistency of contraction. An electromyographic wireless sensor (TrignoTM Wireless EMG System, Delsys, USA) was placed on the PPT landmark of the wrist flexors following SENIAM recommendations (38). The participant was seated, elbow at 90°, the forearm was supinated and the wrist placed under the edge of a table to perform an isometric wrist flexion. Maximal voluntary contractions (MVC) of wrist flexion were measured three times with one-minute breaks between each trial. The highest trial, measured as peak-to-peak, was retained as MVC. Then, an isometric wrist flexion contraction was maintained for 4 minutes at 25% MVC, and visual feedback was provided on a monitor. Two 5-s breaks were allowed for all participants if pain or fatigue was present (32,39). The percentage of change from post- to pre-contraction PPT measurements was calculated and represent EIH.
Patient-reported outcome measures
As reported in the published article (24), many PROMs (e.g., pain intensity, physical functioning, pain catastrophizing) were measured to explore the effects of the intervention. Pain intensity and physical functioning, which are the most widely used clinical outcomes, were used to represent clinical changes of participants with CLBP using the average pain intensity as measured with the NPRS in the last week and the Oswestry Disability Index (ODI) (40). For objective 2, the two latter measures were considered the primary outcomes. We also tested the association between symptoms of central sensitization [measured using Central Sensitization Inventory [CSI] (41)] and QST measures, considering they have been hypothesized to represent overlapping, although different, constructs (42). Other PROMs were used to characterize the study sample and as covariates/confounders in statistical analyses.
Statistical analysis
Sociodemographic data were compared with independent samples t-tests for interval variables, with Mann-Whitney tests for ordinal variables, and with Chi-square tests for proportion variables.
For objective 1, we used linear mixed models using Group (PiP, UP), Time (baseline, 6 weeks), and Group × Time as fixed factors, participants’ intercept as a random factor, and age and sex as covariates to explore the effect of the PiP compared to UP on QST outcomes (PPT, EIH, TSP). A compound symmetry covariance matrix was used, and Sidak’s corrections for multiple comparisons were applied. Intention-to-treat analyses were used, i.e., each participant was analyzed in their allocated group.
Because analyses of the PROMs were published (24), no further statistical analyses on these outcomes are reported in this paper. Means and mean differences between 6-week follow-up and baseline were reported for pain intensity, physical functioning, and symptoms of central sensitization.
For objective 2, linear regressions were computed to determine if differences in QST were associated with differences in clinical profile by pooling the results from both groups. Differences between the 6-week follow-up and baseline data were measured for QST (ΔPPT, ΔEIH, ΔTSP) and clinical variables (ΔPain, ΔODI, ΔCSI). Outcomes (dependent variables) of linear regressions were ΔPain, ΔODI, ΔCSI. Independent variables were ΔPPT, ΔEIH, and ΔTSP at the different sites. Linear regressions were adjusted for potential confounders as self-reported age, sex, baseline pain catastrophizing scale and baseline symptoms of central sensitization. Potential confounders were selected based on potential causal influences with dependent and independent variables (43).
The mean and standard deviation are presented in the manuscript unless otherwise specified. A value of p < 0.05 was considered statistically significant.
Results
Figure 1 depicts the CONSORT flow diagram of the study. Of the 291 potential participants assessed for eligibility, 40 were randomly allocated to one of the two groups (PiP or UP). In addition to the five participants who were lost at the 6-week follow-up, baseline, follow-up data for EIH (low back and lower limb) for one participant was not available since it reached the limit of the algometer (11 kg²/cm) at each trial, impeding the measurement of PPT change.
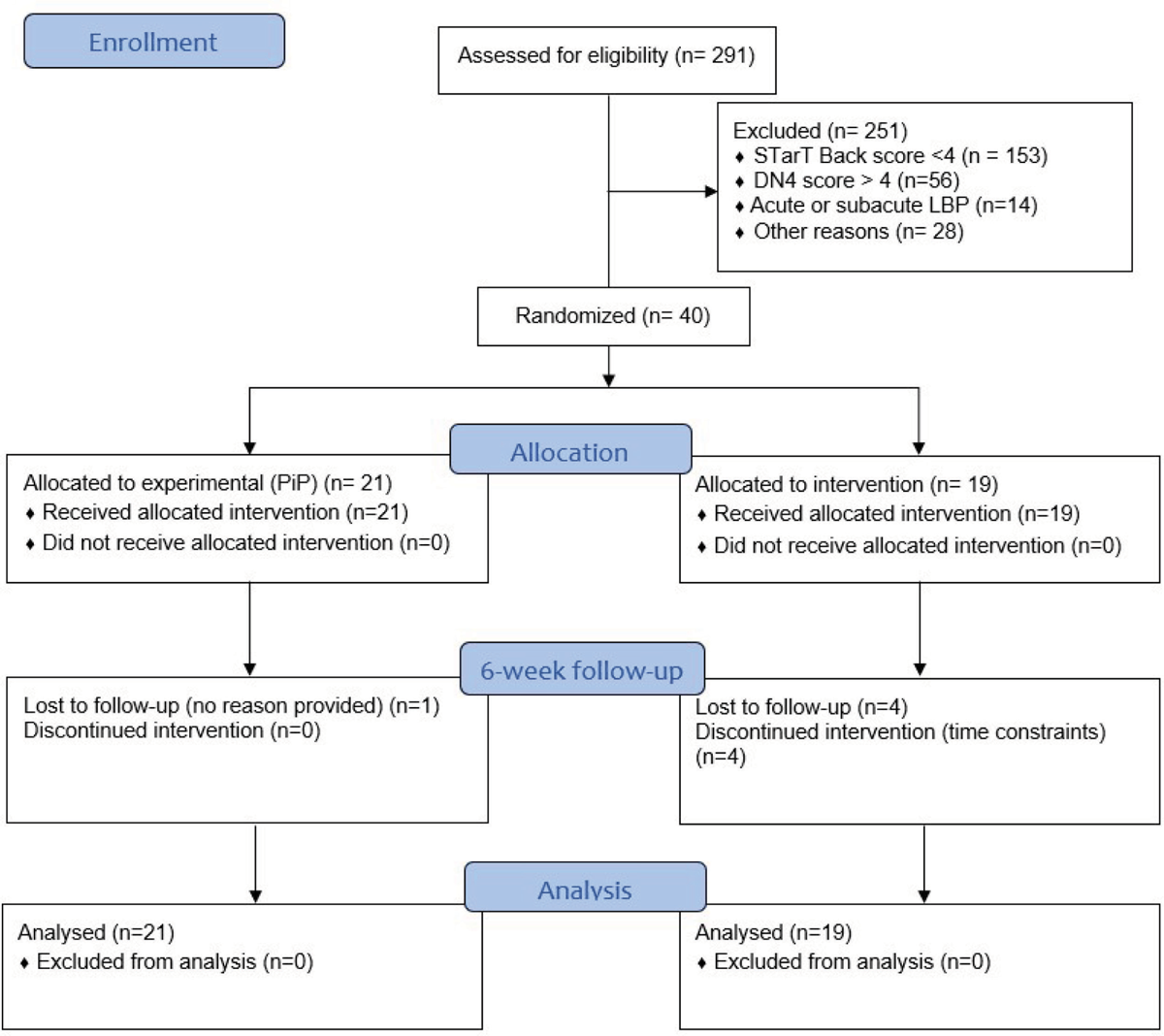
FIGURE 1- CONSORT flowchart depicting the pilot randomized trial.
Sociodemographic and baseline data of participants
Sociodemographic variables and scores measured by self-reported questionnaires at baseline were compared between groups and presented in Table 1. Both groups were comparable on all variables (p < 0.05), except for pain catastrophizing, which was significantly higher for UP.
Quantitative sensory testing
Table 2 reports the estimated means and 95% confidence intervals extracted from the linear mixed models for all quantitative sensory testing outcomes, in addition to the within-sample difference (p-values of the effect of time) and Group × Time interaction (p-values). Specifically, a Group × Time interaction was observed for TSP of the wrist flexors (F(1, 33.95) = 4.84; p = 0.04). However, pairwise comparisons did not detect differences between groups at baseline (0.06 [−0.89, 1.02]; p = 0.90) and at 6 weeks (−0.74 [−1.73; 0.25]; p = 0.14), and within-group for PiP (0.5 [−0.0, 1.0]; p = 0.051) or UP (−0.3 [−0.2, 0.9]; p = 0.26). No other interaction was detected (p > 0.10—Table 2). Main effects of time were observed for PPT of lumbar (F(1, 33.731) = 5.81; p = 0.02), lower limb (F(1, 34.61) = 8.85; p = 0.01), and upper limb (F(1, 35.19) = 6.36; p = 0.02) sites, meaning that PPT at all sites increased over time (reduced pain sensitivity) regardless of the group. No other main effect of time was detected for EIH (p > 0.48) or TSP (p > 0.47). Figure 2 displays the individual changes between baseline and 6-week follow-up of all the QST outcomes.
Patient-reported outcomes measures
Table 3 describes changes in PROMs between baseline and 6-week follow-up for physical functioning, pain intensity, and symptoms of central sensitization. Although no additional analyses were undertaken in the current manuscript, significant effects of time were observed without significant Group × Time interaction; the detailed analyses are reported elsewhere (24).
Linear regressions between QST and pain-related outcomes
Table 4 reports the results of the linear regressions between changes in pain sensitivity/modulation and changes in pain-related outcomes. Out of the 27 analyses, three were statistically significant. Specifically, there was a significant negative association between the change in lumbar PPT and the change in physical functioning, meaning that each increase of 1 kg/cm² for lumbar PPT was associated with a reduction of 2.36% on the ODI score (β = −2.36 [−4.25, −0.46]; p = 0.02). Also, significant positive associations were detected between the lumbar and lower limb TSP and changes in physical functioning; a reduction of 1 point on TSP resulted in a reduction of 3.50% and 3.10% on ODI score, respectively (lumbar TSP: β = 3.50 [1.01, 6.00]; p = 0.01; foot TSP: β = 3.10 (0.38, 5.82); p = 0.03). No other significant associations were observed.
| UP (n = 19) | PiP (n = 21) | p-value | |
|---|---|---|---|
| Age | 33.1 (8.8) | 35.8 (11.0) | 0.41 |
| Male, n (%) | 10 (52.6) | 12 (57.1) | 1.00 |
| LBP duration, n (%) | 0.10 | ||
| 3-5 months | 2 (11) | 0 (0) | |
| 6-11 months | 4 (21) | 4 (19) | |
| 1-5 years | 3 (16) | 10 (48) | |
| More than 5 years | 10 (53) | 7 (33) | |
| Pain intensity (average last 7 days) | 5.7 (2.0) | 5.0 (1.9) | 0.31 |
| Physical functioning (ODI – range: 0-100) | 27.1 (12.2) | 23.9 (11.8) | 0.41 |
| Kinesiophobia (TSK-11 – range 11-44) | 28.7 (8.1) | 25.9 (7.4) | 0.25 |
| Pain catastrophizing (PCS – range: 0-52) | 25.7 (8.5) | 18.3 (10.8) | 0.02 |
| Symptoms of central sensitization (9-item CSI – 0-36) | 20.7 (6.2) | 17.6 (5.0) | 0.09 |
*Data are presented as mean (SD) unless stated otherwise.
UP: Usual physiotherapy; PiP: psychologically-informed physiotherapy; LBP: low back pain; ODI; Oswestry Disability Index; TSK: Tampa Scale for Kinesiophobia;
PCS: Pain Catastrophizing Scale; CSI: Central Sensitization Inventory.
| PiP | UP | ΔUP – ΔPiP* | Interaction
(p-value) |
Within-sample difference ¥ | Time effect
(p-value) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | ΔPiP | Baseline | 6 weeks | ΔUP | |||||
| PPT (kg/cm²) | ||||||||||
| Lumbar | 5.0
[3.9, 6.1] |
5.3
[4.2, 6.4] |
0.3
[-0.5, 1.1] |
3.6
[2.5, 4.8] |
5.0
[3.9, 6.1] |
1.3
[0.2, 2.0] |
1.0 | 0.16 | 0.7
[0.1, 1.3] |
0.02 |
| Lower limb | 4.8
[3.9, 5.7] |
5.4
[4.5, 6.3] |
0.6
[-0.1, 1.3] |
4.2
[3.2, 5.1] |
5.0
[4.0, 6.0] |
0.9
[0.1, 1.6] |
0.3 | 0.60 | 0.7
[0.2, 1.2] |
0.01 |
| Upper limb | 3.2
[2.5, 4.0] |
3.4
[2.7, 4.2] |
0.2
[-0.4, 0.8] |
2.5
[1.7, 3.3] |
3.4
[2.6, 4.3] |
0.9
[0.3, 1.6] |
0.7 | 0.10 | 0.6
[0.1, 1.0] |
0.02 |
| EIH (% of change) | ||||||||||
| Lumbar | 3.8
[–7.9, 15.4] |
1.6
[–10.3, 13.6] |
–2.1
[–16.1, 11.9] |
14.3
[2.3, 26.3] |
15.3
[2.0, 28.6] |
1.0
[–14.2, 16.3] |
3.1 | 0.76 | –0.5
[–10.9, 9.8] |
0.92 |
| Lower limb | 7.4
[–3.0, 17.8] |
–3.1
[–13.7, 7.6] |
–10.5
[–24.2, 3.1] |
4.6
[–6.1, 15.3] |
8.1
[–3.9, 20.0] |
3.5
[–11.3, 18.3] |
14.0 | 0.17 | –3.5
[–13.6, 6.6] |
0.48 |
| Upper limb | 8.2
[–3.3, 19.7] |
5.7
[–6.1, 17.4] |
–2.5
[–18.3, 13.2] |
9.2
[–2.8, 21.3] |
5.4
[–8.2, 19.0] |
–3.9
[–21.3, 13.6] |
1.4 | 0.91 | –3.2
[–15.0, 8.6] |
0.59 |
| TSP | ||||||||||
| Lumbar | 2.0
[1.4, 2.7] |
2.3
[1.6, 2.9] |
0.2
[–0.5, 0.8] |
2.4
[1.4, 2.7] |
1.9
[1.1, 2.6] |
–0.6
[–1.3, 0.2] |
–0.8 | 0.13 | –0.2
[–0.7, 0.3] |
0.47 |
| Lower limb | 2.5
[1.8, 3.2] |
2.5
[1.8, 3.2] |
0.0
[–0.7, 0.7] |
2.0
[1.2, 2.7] |
1.9
[1.1, 2.7] |
–0.0
[–0.8, 0.7] |
–0.1 | 0.90 | –0.0
[–0.5, 0.5] |
0.95 |
| Upper limb | 1.9
[1.2, 2.5] |
2.4
[1.7, 3.0] |
0.5
[–0.0, 1.0] |
1.9
[1.2, 2.6] |
1.6
[09, 2.3] |
–0.3
[–0.2, 0.9] |
–0.8 | 0.04 | 0.1
[–0.3, 0.5] |
0.65 |
*ΔUP – ΔPiP: Between-group difference at 6 weeks; ¥ Within-group difference of the whole sample (Post – pre).
PiP: psychologically-informed physiotherapy; UP: usual physiotherapy; ΔPiP: Within-group difference (Post-pre) for PiP; ΔUP: Within-group difference (Post-pre) for PiP; PPT: Pain pressure threshold; EIH: Exercise-induced hypoalgesia; TSP: Temporal summation of pain.
Bold indicates significant p-values.
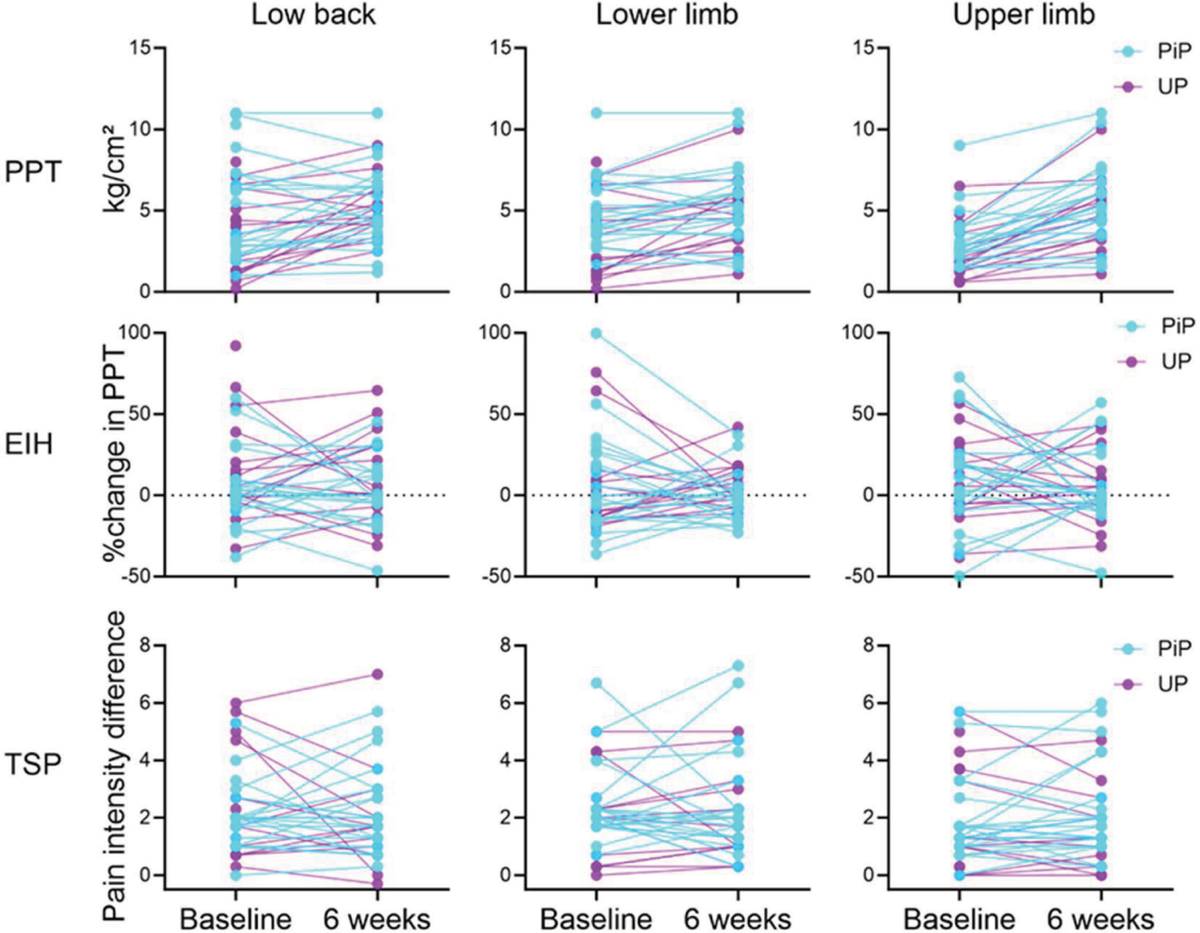
FIGURE 2 - Individual pain sensitivity (PPT) and modulation (TSP, EIH) variable changes (baseline to 6 weeks) at the low back, lower limb, and upper limb sites for both physiotherapy approaches. Note the increase in PPTs detected in both groups. PPT: pressure pain threshold; EIH: exercise-induced hypoalgesia; TSP: temporal pain summation; PiP: psychologically-informed physiotherapy; UP: usual physiotherapy.
| PiP | UP | Within sample difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | ΔPiP | Baseline | 6 weeks | ΔUP | ||
| Physical functioning | 23.9 (11.8) | 16.8 (10.4) | –7.1 (9.6) | 27.1 (12.2) | 21.9 (14.8) | –8.4 (9.4) | –6.3 (10) |
| Pain intensity | 5.0 (1.9) | 3.1 (2.0) | –2.1 (2.1) | 5.7 (2.0) | 3.7 (2.1) | –1.8 (2.2) | –2.0 (2.1) |
| Symptoms of central sensitization | 17.6 (5.0) | 15.2 (6.4) | –2.3 (5.7) | 20.7 (6.2) | 15.7 (7.2) | –4.3 (6.1) | –3.2 (5.9) |
PiP: Psychologically-informed physiotherapy; UP: Usual physiotherapy; ΔPiP: Within-group difference (Post-pre) for PiP; ΔUP: Within-group difference (Post-pre) for PiP; SD: standard deviation.
Note that all scores were significantly different at 6 weeks compared to baseline (see Desgagnés et al., 2024).
| ΔPain | ΔODI | ΔCSI | |
|---|---|---|---|
| ΔPPT | |||
| Lumbar | β = –0.31 (–0.73, 0.11); p = 0.15 | β = –2.36 (–4.25, –0.46); p = 0.02 | β = –0.09 (–1.15, 1.32); p = 0.88 |
| Lower limb | β = 0.06 (–0.48, 0.60); p = 0.83 | β = –1.05 (–3.52, 1.43); p = 0.39 | β = –0.39 (–1.85, 1.08); p = 0.59 |
| Upper limb | β = –0.27 (–0.85, 0.31); p = 0.35 | β = –2.46 (–5.08, 0.16); p = 0.07 | β = –0.35 (–1.98, 1.29); p = 0.67 |
| ΔEIH | |||
| Lumbar | β = 0.01 (–0.020, 0.036); p = 0.58 | β = –0.01 (–0.14, 0.12); p = 0.90 | β = –0.02 (–0.09, 0.06); p = 0.69 |
| Lower limb | β = 0.01 (–0.016, 0.037); p = 0.41 | β = 0.05 (–0.08, 0.17); p = 0.44 | β = 0.03 (–0.04, 0.11); p = 0.35 |
| Upper limb | β = –0.01 (–0.030, 0.017); p = 0.56 | β = 0.02 (–0.10, 0.13); p = 0.74 | β = 0.002 (–0.07, 0.07); p = 0.95 |
| ΔTSP | |||
| Lumbar | β = 0.53 (–0.02, 1.07); p = 0.06 | β = 3.50 (1.01, 6.00); p = 0.01 | β = 1.15 (–0.45, 2.76); p = 0.15 |
| Lower limb | β = 0.26 (–0.31, 0.83); p = 0.36 | β = 3.10 (0.38, 5.82); p = 0.03 | β = 0.05 (–1.70, 1.79); p = 0.96 |
| Upper limb | β = –0.11 (–0.83, 0.62); p = 0.76 | β = 0.01 (–3.62, 3.64); p = 1.00 | β = –0.85 (–2.96, 1.25); p = 0.41 |
CSI: Central sensitization inventory; PCS: Pain catastrophizing scale; ODI: Oswestry disability index; PPT: Pressure pain threshold; EIH: Exercise-induced hypoalgesia; TSP: Temporal summation of pain; Δ: score at 6 weeks minus score at baseline.
Bold and italic: p < 0.05.
Discussion
The first objective of this study was to explore the effects of PiP and UP on pain sensitivity and modulation variables using PPT, EIH, and TSP. We observed an increase in PPTs at 6 weeks regardless of the interventions, suggesting a widespread induced hypoalgesia without superiority of a physiotherapy approach. These findings refute our hypothesis that PiP would have a larger influence on pain sensitivity and modulation variables. The second objective was to determine if changes in pain sensitivity and modulation were associated with changes in pain and physical functioning. Significant associations between differences in physical functioning and (i) PPT in the lumbar region, (ii) TSP in the lumbar region, and (iii) TSP at the lower limb were observed. However, no association with changes in pain intensity or symptoms of central sensitization was detected. These findings tend to dispute the hypothesis that changes in sensitivity/modulation variables are associated with clinical changes for most variables collected, with the exception of physical functioning. Nonetheless, this is important to consider that this is an exploratory study with a small sample size, and findings need to be interpreted with caution and replicated in future research.
The lack of PiP superiority over UP to influence pain sensitivity and modulation may appear surprising, considering that PiP—including pain neuroscience education—suggests a “desensitization” effect in chronic pain (17,20). Nonetheless, the literature specific to CLBP is scarce and only a few clinical trials reported the effects of PiP on pain sensitivity. Bodes Pardo et al., (2018) (2018) (21) reported an increase in PPT in favor of pain neuroscience education combined with therapeutic exercise compared to therapeutic exercise alone in participants with CLBP. Pain neuroscience education aims to modify beliefs through pain reconceptualization (44) and is commonly included in PiP. Furthermore, Cognitive Functional Therapy in patients with severe CLBP increased back PPT in a single-arm clinical trial (45). Cognitive Functional Therapy is an approach consistent with PiP (14). However, due to the absence of a control group, it is not possible to determine if the increase was caused by the intervention (45). Another RCT, but in participants with knee osteoarthritis (46), reported no superiority of pain neuroscience education over biomedical education plus knee mobilization; both interventions increased PPTs and did not influence other QST measurements. Although the literature seems to support an increase in PPTs following PiP, other approaches also increase them. Indeed, both manual therapy and exercises, which were the core of the UP group, are known to reduce pain sensitivity. Meta-analyses observed that exercise (23) and spinal manipulation (22) significantly increase PPTs. Thus, discrepancies between our results and those from Bodes Pardo et al., (2018) (21) could be explained by the presence of both manual and exercise therapies in UP. Different mechanisms may explain this intervention-induced hypoalgesia (i.e., an increase in PPTs). For example, exercises may (i) normalize the alteration in areas of the central nervous system contributing to pain processing via neuroplasticity, (ii) reduce nociception related to spine microtrauma via improved motor control, or (iii) indirectly modify psychological risk factors (e.g., reduction in fear of movement) (23). Similarly, spinal manipulation was suggested to activate pain modulation mechanisms at both spinal and supraspinal levels (22). Considering that both physiotherapy approaches comprised (i) manual and exercise therapies and (ii) may have influenced psychological factors directly (PiP) or indirectly (UP), it is possible that overlapping mechanisms may have contributed to the increase in PPTs. Overall, our results suggest that both physiotherapy approaches may reduce pressure pain sensitivity. Nonetheless, the exploratory nature of the study and the few numbers of studies published in the area preclude any clinical recommendations on the most appropriate intervention to influence pain sensitivity. If future powered RCTs confirm our findings, it may suggest that different physiotherapy approaches (exercise, manual therapy, pain education, etc.) may be used to reduce pain sensitivity if this is a specific therapeutic objective.
Despite the fact that PPTs were increased following both interventions, no effect was observed for TSP or EIH. Although PPT is the QST measurement that is most often used as a pain sensitivity proxy, some studies tested the effects of various interventions on other QST measurements. In contrast to our results, a systematic review suggested that physiotherapy interventions may influence various types of QST measurements (e.g., TSP, conditioned pain modulation) (47). For example, spinal manipulation reduced TSP more than stationary bicycle in patients with CLBP (48). These discrepancies could be explained by the different types of paradigms used. For example, most studies included in the systematic review used a suprathreshold heat response consisting of rating pain intensity following repeated heat stimuli (47). In contrast, we used a pinprick to measure TSP. Other reasons for discrepancies could be related to different clinical populations recruited or the time elapsed between measurements. EIH was not included as an outcome in the meta-analysis. In a recent study, we demonstrated that EIH was absent in participants with CLBP (5). In contrast, our sample of participants with CLBP seemed to present with “normal” EIH (i.e., hypoalgesia was present at baseline for most sites). This difference might be explained by the low reliability of EIH, which may, therefore, be less suitable for use as an outcome (49).
For the second objective, aiming to determine the associations between changes in PROMs and in pain sensitivity and modulation variables, we observed that changes in PPT and TSP were associated with changes in physical functioning but not in pain intensity. The absence of significant associations with pain remains intriguing. We could speculate that the reduction in pain sensitivity following both physiotherapy approaches allowed participants to re-engage in everyday life activity and then improve their physical functioning. For example, a study of participants with knee osteoarthritis observed significant correlations between TSP at the knee and sensitivity to physical activity (50). Although indirect evidence, it is possible that pain sensitivity variables such as TSP may be related to sensitivity during activity and influence physical functioning. To our knowledge, only a few studies tested the associations between changes in pain sensitivity and PROMs and did not identify significant associations. For instance, Nim et al. (2021) (50) reported no significant association between changes in QST variables and clinical improvement outcomes following spine manipulation in participants with LBP. Similarly, Palsson et al., 2021 (51) did not report any correlations between changes in PROMs and in QST measurements following a rehabilitation program, including exercises, even though pain and physical functioning both improved. Thus, high discrepancies are present, making it difficult to interpret our results. Our hypothesis explaining the influence of pain sensitivity on physical functioning underlies causal influence which cannot be ascertained with the current analyses. Also, the small proportion of significant associations between changes in clinical outcomes and QST can be explained by the large number of statistical analyses done (type I error). Further studies with larger sample sizes and additional analyses are necessary to confirm that change in pain sensitivity could be a mediator of the improvement in physical functioning.
Another interesting finding is the absence of a correlation between changes in pain sensitivity and modulation variables and changes in symptoms of “central sensitization.” This highlights the contrast in the constructs between QST and CSI; sensitization to nociceptive/somatosensory stimuli as measured with QST does not grasp the whole construct measured by the CSI. While certain aspects may appear analogous, CSI encompasses a wider range of symptoms typically observed in patients with features of “central sensitization” associated with a general hypersensitivity to various sensory stimuli (e.g., sound, odor), general health (e.g., gastro-intestinal, dermatology) and lifestyle behaviors (e.g., sleep). This is in line with a study demonstrating low or no association between CSI scores and PPT and conditioned pain modulation, respectively (42).
Limitations
The current study has limitations that must be considered when interpreting our results and their applicability to clinical settings. First, the small sample size limits the statistical power of our analyses, and our results should be interpreted cautiously. Second, the absence of a “placebo” or minimal intervention group precludes a definitive determination of whether the increased PPTs were attributable to the interventions received or the natural trajectory of pain sensitization in CLBP patients. For example, reliability analyses showed that PPT could increase at the second measurement within a single session (34), even though estimated PPT changes were superior to the minimal detectable changes (33).
Conclusions
Usual and psychologically informed physiotherapy approaches elicited widespread hypoalgesia after 6 weeks of treatment as measured by PPTs, but not for the TSP or EIH. In addition, changes in pain sensitivity (e.g., PPT and TSP) were associated with changes in physical functioning but not in pain intensity. Contrary to initial expectations, our study suggests that neither approach demonstrated a significant advantage over the other to impact pain sensitivity. Future studies with a larger sample size and including a “minimal intervention” group may help to determine if the hypoalgesia induced by physiotherapy approaches is caused by physiotherapy interventions or if it is associated with the natural evolution of pain sensitivity over time.
Disclosures
Conflict of interest: None of the authors have potential conflicts of interest to be disclosed.
Financial support: The project was funded by a grant from REPAR (rehabilitation network of the Fonds de recherche du Québec). HMA is supported by a research scholar from Fonds de recherche du Québec – Santé (281961). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Meucci RD, Fassa AG, Faria NM. Prevalence of chronic low back pain: systematic review. Rev Saude Publica. 2015;49(0):1. CrossRef PubMed
- 2. James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. CrossRef PubMed
- 3. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736-747. CrossRef PubMed
- 4. Hartvigsen J, Hancock MJ, Kongsted A, et al. Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-2367. CrossRef PubMed
- 5. Patricio P, Mailloux C, Wideman TH, et al. Assessment of exercise-induced hypoalgesia in chronic low back pain and potential associations with psychological factors and central sensitization symptoms: A case-control study. Pain Pract. 2023;23(3):264-276. CrossRef PubMed
- 6. den Bandt HL, Ickmans K, Leemans L, et al. Differences in quantitative sensory testing outcomes between patients with low back pain in primary care and pain-free controls. Clin J Pain. 2022;38(6):381-387. CrossRef PubMed
- 7. Nijs J, Kosek E, Chiarotto A, et al. Nociceptive, neuropathic, or nociplastic low back pain? The low back pain phenotyping (BACPAP) consortium’s international and multidisciplinary consensus recommendations. Lancet Rheumatol. 2024;6(3):e178-e188. CrossRef PubMed
- 8. Shraim MA, Massé-Alarie H, Hodges PW. Methods to discriminate between mechanism-based categories of pain experienced in the musculoskeletal system: a systematic review. Pain. 2021;162(4):1007-1037. CrossRef PubMed
- 9. Shraim MA, Sluka KA, Sterling M, et al. Features and methods to discriminate between mechanism-based categories of pain experienced in the musculoskeletal system: a Delphi expert consensus study. Pain. 2022;163(9):1812-1828. CrossRef PubMed
- 10. Smart KM, Blake C, Staines A, et al. Clinical indicators of ‘nociceptive,’ ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man Ther. 2010;15(1):80-87. CrossRef PubMed
- 11. da C Menezes Costa L, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. 2012;184(11):E613-E624. CrossRef PubMed
- 12. Ballengee LA, Zullig LL, George SZ. Implementation of psychologically informed physical therapy for low back pain: where do we stand, where do we go? J Pain Res. 2021;14:3747-3757. CrossRef PubMed
- 13. Hodges PW. Hybrid approach to treatment tailoring for low back pain: a proposed model of care. J Orthop Sports Phys Ther. 2019;49(6):453-463. CrossRef PubMed
- 14. O’Sullivan PB, Caneiro JP, O’Keeffe M, et al. Cognitive functional therapy: an integrated behavioral approach for the targeted management of disabling low back pain. Phys Ther. 2018;98(5):408-423. CrossRef PubMed
- 15. Main CJ, George SZ. Psychologically informed practice for management of low back pain: future directions in practice and research. Phys Ther. 2011;91(5):820-824. CrossRef PubMed
- 16. Ho EK, Chen L, Simic M, et al. Psychological interventions for chronic, non-specific low back pain: systematic review with network meta-analysis. BMJ. 2022;376:e067718. CrossRef PubMed
- 17. Van Oosterwijck J, Meeus M, Paul L, et al. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: a double-blind randomized controlled trial. Clin J Pain. 2013;29(10):873-882. CrossRef PubMed
- 18. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377-391. CrossRef PubMed
- 19. Meints SM, Mawla I, Napadow V, et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain. 2019;160(4):833-843. CrossRef PubMed
- 20. Van Oosterwijck J, Nijs J, Meeus M, et al. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. J Rehabil Res Dev. 2011;48(1):43-58. CrossRef PubMed
- 21. Bodes Pardo G, Lluch Girbés E, Roussel NA, et al. Pain neurophysiology education and therapeutic exercise for patients with chronic low back pain: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2018;99(2):338-347. CrossRef PubMed
- 22. Coronado RA, Gay CW, Bialosky JE, et al. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22(5):752-767. CrossRef PubMed
- 23. Belavy DL, Van Oosterwijck J, Clarkson M, et al. Pain sensitivity is reduced by exercise training: evidence from a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;120:100-108. CrossRef PubMed
- 24. Desgagnés A, Côté-Picard C, Gaumond A, et al. Efficacy of a psychologically-informed physiotherapy intervention in patients with chronic low back pain at high risk of poor prognosis: a pilot and feasibility randomized controlled trial. Physiother Can. 2024;76(2):163-174. CrossRef PubMed
- 25. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. CrossRef PubMed
- 26. Hill JC, Dunn KM, Lewis M, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632-641. CrossRef PubMed
- 27. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1-2):29-36. CrossRef PubMed
- 28. Abbott JH. The distinction between randomized clinical trials (RCTs) and preliminary feasibility and pilot studies: what they are and are not. J Orthop Sports Phys Ther. 2014;44(8):555-558. CrossRef PubMed
- 29. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231-243. CrossRef PubMed
- 30. Coronado RA, Bialosky JE, Robinson ME, et al. Pain sensitivity subgroups in individuals with spine pain: potential relevance to short-term clinical outcome. Phys Ther. 2014;94(8):1111-1122. CrossRef PubMed
- 31. McPhee ME, Vaegter HB, Graven-Nielsen T. Alterations in pronociceptive and antinociceptive mechanisms in patients with low back pain: a systematic review with meta-analysis. Pain. 2020;161(3):464-475. CrossRef PubMed
- 32. Mailloux C, Wideman TH, Massé-Alarie H. Wrist, but not back, isometric contraction induced widespread hypoalgesia in healthy participants. Front Pain Res (Lausanne). 2021;2:701830. CrossRef PubMed
- 33. Mailloux C, Beaulieu LD, Wideman TH, et al. Within-session test-retest reliability of pressure pain threshold and mechanical temporal summation in healthy subjects. PLoS One. 2021;16(1):e0245278. CrossRef PubMed
- 34. de Oliveira FCL, Cossette C, Mailloux C, et al. Within-session test-retest reliability of pressure pain threshold and mechanical temporal summation in chronic low back pain. Clin J Pain. 2023;39(5):217-225. CrossRef PubMed
- 35. Naugle KM, Fillingim RB, Riley JL III. A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13(12):1139-1150. CrossRef PubMed
- 36. Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain. 2019;20(11):1249-1266. CrossRef PubMed
- 37. Koltyn KF, Brellenthin AG, Cook DB, et al. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15(12):1294-1304. CrossRef PubMed
- 38. Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. Oct 2000;10(5):361-74. CrossRef
- 39. Chiarotto A, Boers M, Deyo RA, et al. Core outcome measurement instruments for clinical trials in non-specific low back pain. Pain. 2018;159(3):481-495. CrossRef PubMed
- 40. Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276-285. CrossRef PubMed
- 41. Kregel J, Schumacher C, Dolphens M, et al. Convergent validity of the dutch central sensitization inventory: associations with psychophysical pain measures, quality of life, disability, and pain cognitions in patients with chronic spinal pain. Pain Pract. 2018;18(6):777-787. CrossRef PubMed
- 42. McNamee R. Confounding and confounders. Occup Environ Med. 2003;60(3):227-234. CrossRef PubMed
- 43. Nijs J, Meeus M, Cagnie B, et al. A modern neuroscience approach to chronic spinal pain: combining pain neuroscience education with cognition-targeted motor control training. Phys Ther. 2014;94(5):730-738. CrossRef PubMed
- 44. Vaegter HB, Ussing K, Johansen JV, et al. Improvements in clinical pain and experimental pain sensitivity after cognitive functional therapy in patients with severe persistent low back pain. Pain Rep. 2019;5(1):e802. CrossRef PubMed
- 45. Lluch E, Dueñas L, Falla D, et al. Preoperative pain neuroscience education combined with knee joint mobilization for knee osteoarthritis: a randomized controlled trial. Clin J Pain. 2018;34(1):44-52. CrossRef PubMed
- 46. Arribas-Romano A, Fernández-Carnero J, Molina-Rueda F, et al. efficacy of physical therapy on nociceptive pain processing alterations in patients with chronic musculoskeletal pain: a systematic review and meta-analysis. Pain Med. 2020;21(10):2502-2517. CrossRef PubMed
- 47. Bialosky JE, Bishop MD, Robinson ME, et al. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89(12):1292-1303. CrossRef PubMed
- 48. Vaegter HB, Lyng KD, Yttereng FW, et al. Exercise-induced hypoalgesia after isometric wall squat exercise: a test-retest reliability study. Pain Med. 2019;20(1):129-137. CrossRef PubMed
- 49. Wideman TH, Finan PH, Edwards RR, et al. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain. 2014;155(4):703-711. CrossRef PubMed
- 50. Nim CG, Kawchuk GN, Schiøttz-Christensen B, et al. Changes in pain sensitivity and spinal stiffness in relation to responder status following spinal manipulative therapy in chronic low Back pain: a secondary explorative analysis of a randomized trial. BMC Musculoskelet Disord. 2021;22(1):23. CrossRef PubMed
- 51. Palsson TS, Christensen SWM, De Martino E, et al. Pain and disability in low back pain can be reduced despite no significant improvements in mechanistic pain biomarkers. Clin J Pain. 2021;37(5):330-338. CrossRef PubMed