 |
Arch Physioter 2024; 14: 1-10 ISSN 2057-0082 | DOI: 10.33393/aop.2024.2916 REVIEW |
 |
Pragmatism in manual therapy trials for knee osteoarthritis: a systematic review
ABSTRACT
Introduction: Manual therapy is an often-utilized intervention for the management of knee osteoarthritis (OA). The interpretation of results presented by these trials can be affected by how well the study designs align applicability to real-world clinical settings.
Aim: To examine the existing body of clinical trials investigating manual therapy for knee OA to determine where they fall on the efficacy-effectiveness spectrum.
Methods: This systematic review has been guided and informed by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Randomized controlled trials that investigated manual therapy treatments for adults with knee OA were retrieved via searches of multiple databases to identify trials published prior to April 2023. The Rating of Included Trials on the Efficacy-Effectiveness Spectrum (RITES) tool was used to objectively rate the efficacy-effectiveness nature of each trial design. The Cochrane Risk of Bias 2.0 assessment tool (RoB-2) was used to assess the risk of bias across five domains.
Results: Of the 34 trials, a higher percentage of trials had a greater emphasis on efficacy within all four domains: participant characteristics (76.5%), trial setting (82.4%), flexibility of intervention (61.8%), and clinical relevance of experimental and comparison intervention (50.0%). In addition, 14.8% of the trials had low risk of bias, 44.1% had high risk of bias, and 41.2% had some concerns regarding bias.
Conclusions: While many trials support manual therapy as effective for the management of knee OA, a greater focus on study designs with an emphasis on effectiveness would improve the applicability and generalizability of future trials.
Keywords: Effectiveness, Efficacy, Knee osteoarthritis, Manual therapy, Mobilization, Systematic review
Received: June 29, 2023
Accepted: January 9, 2024
Published online: February 26, 2024
This article includes supplementary information
Archives of Physiotherapy - ISSN 2057-0082 - www.archivesofphysiotherapy.com
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0).
Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
What’s already known about this topic?
- Despite clinical trials revealing substantial treatment effects favoring manual therapy for the management of knee osteoarthritis (OA), challenges still remain with implementation and translation of this work into clinical practice.
- One reason is that the majority of the research in this field is based on more explanatory or more pragmatic trial designs.
What does this study add?
- We conducted a systematic review of 34 trials that assessed treatment effects for manual therapy interventions to determine where the trials fall on the efficacy-effectiveness spectrum.
- Of the 34 trials, a majority had a greater emphasis on efficacy for all four domains: participant characteristics (76.5%), trial setting (82.4%), flexibility of interventions (61.8%), and clinical relevance of experimental and comparison intervention (50%).
Introduction
Knee osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability in older adults, with symptomatic OA continuing to rise partly due to the global obesity epidemic and aging population (1-4). Knee OA has become a significant burden to society because of its chronic nature and high cost of treatment, with estimated costs in the United States greater than $27 billion annually (5,6). Several nonpharmacological interventions have demonstrated effectiveness, the most promising being exercise therapy (7). Manual therapy has also proven effective for reducing pain and improving function in individuals with knee OA (5,8-12). Assessing the context in which these interventions are assessed is valuable to better understand their applicability and generalizability to real-world clinical practice. In addition to difficulty associated with blinding subjects, therapists, and assessors in these types of nonpharmacological trials, another challenge is that trials vary with respect to their study design, which can make it hard to determine their real-world clinical applicability (5,9,13,14).
The various components of a clinical trial design have characteristics that make them more explanatory or more pragmatic (15). Some trials are more explanatory in nature, meaning they are carried out under ideal and controlled circumstances to demonstrate if an intervention can achieve a desired result (16,17). When this occurs, a study is said to have high focus on efficacy and internal validity; however, the results may be less generalizable as the study parameters do not always reflect real-world practice (e.g., very selective inclusion criteria and no presence of comorbidities) (18). Other study designs are more pragmatic, with the goal of assessing the effectiveness of an intervention across various settings, people, and times in a way that would more closely reflect delivery in real-world settings (17). Trials with a pragmatic design tend to have higher external validity, leading to improved applicability in real-life situations (15). It is important to note that trials are rarely fully explanatory or pragmatic, but instead fall along a spectrum (15). These differences in design structure require readers to not only focus on the results of the study but also consider participant characteristics, trial setting, flexibility of interventions, and clinical relevance of experimental and comparison interventions in order to understand how applicable the results are for their clinical practice (19).
Several meta-analyses suggest manual therapy has value for the management of knee OA, at minimum in the short term (5,9,20). As an intervention that physical therapists continue to utilize and that patients perceive as beneficial (21), manual therapy may have the ability to provide a window of opportunity to enable active intervention approaches, such as exercise (21,22). To better understand their applicability and generalizability in real-world clinical practice, it is important to understand where manual therapy trials fall along the explanatory-pragmatic spectrum (5,9,23,24). The Rating of Included Trials on the Efficacy-Effectiveness Spectrum (RITES) tool was developed to enable the assessment of published trials along this spectrum (19), but has not yet been used to assess knee OA trials. The objective of this review was to determine where trials investigating manual therapy for knee OA fall on the explanatory-pragmatic spectrum in order to better understand optimal applicability, generalization, and implementation of this intervention.
Methods
The systemic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) (25,26). The review protocol was prospectively registered in the PROSPERO database (CRD42022327706). There were no patients involved in this review.
Search strategy
A literature search was performed using PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), and Embase to identify trials published prior to April 2023. In addition to these databases, the authors performed manual searches by cross-referencing trials included in related systematic reviews to capture all relevant studies in order to maximize the quality of this review. The systematic reviews that were examined consisted of any related to the eligibility criteria for this review.
Search strategies were developed using medical subject headings (MeSH) and keywords pertaining to the knee, OA, manual therapy, randomized controlled trials, and adult/young adult. Medical librarians assisted with the searches (Supplementary material, Appendix A). The primary search methods used were appropriate to each database, which included MeSH terms, CINAHL headings, subject headings, and keywords and their synonyms. Truncation and wildcards were used to account for different spellings and alternative words that may be used to describe our keywords (e.g., arthr* to identify arthritis or arthrosis). The Boolean operators “AND” and “OR” were used to combine search terms. Filters for the English language and the time frame of 1975 to April 2023 were used.
Study selection
Randomized clinical trials where the primary focus was assessing the effect of manual therapy interventions for adult patients with knee OA were included. Full text of all trials had to be available in the English language. Animal trials, trials that included subjects with diagnoses other than knee OA in any compartment, or trials where subjects had any surgery in the past 6 months or had undergone a knee arthroplasty in the involved knee at any time were excluded (Supplementary material, Appendix B).
Because the label of manual therapy can be broad and extensive (e.g., includes massage, lymphatic drainage, passive range of motion) (27), we deliberately limited the definition of manual therapy for this review as a treatment primarily consisting of joint mobilizations or manipulations performed by a healthcare provider, even if it was part of a multimodal intervention as long as the effect of the manual therapy intervention was being assessed. Trials including other forms of manual therapy (e.g., massage, soft tissue mobilization, lymphatic massage/drainage, cupping, dry needling, acupuncture, acupressure, and stretching) in the absence of joint mobilization or manipulation were excluded. Trials assessing manual therapy as part of a group of interventions where the effect of manual therapy was not assessed (e.g., a trial where everyone received manual therapy as part of standard care and the purpose of the trial was to assess the effect of other interventions, such as exercise, education, medications, etc.) were also excluded from the review. The eligibility requirements for this review were chosen to maximize the relevance and overall quality of this review.
Data management
Covidence data management software (Veritas Health Innovation Ltd, Melbourne, Australia) was used for study screening, full-text review, and data extraction (28).
Data extraction
Two reviewers independently screened all titles and abstracts to determine eligibility for full-text review. Any disagreements were discussed for resolution, and a third reviewer was consulted for final disposition, as necessary. Upon completion of title and abstract screenings, the remaining full-text trials were screened by the same two reviewers using the predetermined eligibility criteria. Reasons for exclusion were documented within Covidence (Supplementary material, Appendix C).
Data extracted included total number of subjects, mean age in years, mean body mass index, proportion of males and females, and the year the trial was published. In addition, the RITES tool was used to rate the efficacy-effectiveness nature of each study. Descriptors of maximal efficacy and maximal efficiency are provided in Table 1 (19). The RITES tool is used to rate the efficacy-effectiveness nature of trials by assessing four different domains (participant characteristics, trial settings, flexibility of interventions, and clinical relevance of experimental and comparison interventions) using a 5-point Likert scale, with 1 indicating a strong emphasis on efficacy (more explanatory), and 5 indicating a strong emphasis on effectiveness (more pragmatic) (19). The two reviewers independently scored each study using the RITES tool and consulted with a third reviewer when there was a lack of consensus.
Risk of bias assessment
The Cochrane Collaboration Risk of Bias 2.0 assessment tool (RoB-2) was used to assess the risk of bias across five separate domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results (29,30). Each domain was rated as having low risk, high risk, or some concerns regarding the risk of bias for that trial. The two reviewers independently scored each trial using RoB-2 to determine the potential risk for bias when looking at the results. In the event of a difference in opinion, consensus was reached by consulting with a third reviewer.
Efficacy-effectiveness spectrum
The RITES tool was used to assess where the components of each trial fell along the efficacy-effectiveness continuum (14). It was developed for post hoc assessment of trials in a systematic review based on efficacy-effectiveness continuum along four domains: participant characteristics, trial settings, flexibility of intervention(s), and clinical relevance of experimental and comparison intervention(s). A Likert scale ranging from 1 (strong emphasis on efficacy) to 5 (strong emphasis on effectiveness) is used in scoring. A rating of not applicable (N/A) may be given when information for a domain is unavailable. Trials typically cannot be completely categorized as explanatory or pragmatic as a whole, but instead rated along a continuum. In addition, different components of a trial design may fall in different places along the efficacy-effectiveness continuum. Thus, each domain is scored independently, without putting forth an overall score for a trial.
Data synthesis and analysis
Interrater reliability between reviewers was calculated for title and abstract and full-text screening using Cohen’s kappa. Levels of agreement were defined as <0 = no agreement, 0-0.20 = slight agreement, 0.21-0.40 = fair agreement, 0.41-0.60 = moderate agreement, 0.61-0.80 = substantial agreement, and 0.81-1.0 = almost perfect agreement (31). Descriptive statistics were calculated for RITES tool scores that included the count and percentage within each of the four domains. For each RITES domain, the results were separated into three different groups, those that had more emphasis on efficacy (scores of 1-2), those with more emphasis on effectiveness (scores of 4-5), and those that were balanced or neutral (scores of 3). In addition, the four domain scores from each trial were averaged together to determine if the individual trial design, with all domain scores considered together, leaned more toward efficacy or efficiency. For RoB-2, count data and percentage were calculated for all trials based on ratings of low risk, high risk, or some concerns regarding the risk of bias. Interrater reliability between reviewers was assessed for all four domains of the RITES tool and final RoB-2 scores. Finally, all trials were classified as being positive or null based on the primary outcome and then assessed for associations with trial design emphasis on efficacy vs. effectiveness using contingency tables and Fisher’s exact test. IBM SPSS Statistics (version 28; Chicago, IL) was used for all analyses.
Deviations from prospective protocol registration
There were no deviations from the prospective protocol registration.
Results
Search results
The initial search yielded 2,656 citations, and after removing 1,584 duplicates, 1,074 titles and abstracts required screening. After title and abstract screening and full-text review, 34 trials (13,14,32-63) were included in the final review (Fig. 1). Specific details regarding each trial that was excluded can be found in Supplementary material, Appendix C. Features of the 34 trials are included in Supplementary material, Appendices D and E.
| Domain | Descriptor for efficacy orientation | Example | Descriptor for effectiveness orientation | Example |
|---|---|---|---|---|
1. Participant characteristics |
Participants are a homogeneous population and are not similar to those seen in usual care. Inclusion/exclusion criteria are often intentionally chosen to increase chances of compliance or successful outcomes, as compared with treatments provided in usual care. | Inclusion criteria consisted of individuals with bilateral primary knee OA based on the clinical criteria of the American College of Rheumatology as long as knee radiographs revealed Stage II-III OA, as per the Kellgren and Lawrence staging scale (35). Individuals were excluded if they had vascular and cardiovascular disease, obesity, acute or chronic pain in the spine, hip, or ankle, and those using anti-inflammatory drugs beyond simple pain relievers (35,39). |
The participants are similar to those who would receive the experimental intervention as a part of usual care. This means that their age, severity of illness, and comorbidities are similar to those patients who would be candidates for the intervention in a usual care setting. | Inclusion criteria consisted of individuals with knee OA based on the clinical criteria of the American College of Rheumatology. In addition, individuals need to be able to walk in their daily living with or with aid, and need to be able to communicate and follow orders (46). Individuals were excluded if they had rheumatoid gout or other systemic joint disease; cerebrovascular conditions such as acute or recurrent myocardial infarction, unstable angina pectoris; neurological conditions such as Parkinson’s disease; undergone knee surgery, corticosteroid injection to the knee within previous 30 days; and an injury or accident at the back and lower limbs within previous 6 weeks (46). |
2. Trial setting |
The setting used maximizes the ability to carry out the trial and identify an intervention effect if there is one. This typically means that very few settings are used, and they may be more specialized than the setting in which the experimental intervention would be delivered in usual care. | Interventions were provided in the outpatient physical therapy department of a single military medical center (13). | Multiple settings are used, often consisting of subsettings that are typical for usual care for the studied intervention (e.g., primary care, specialized care). | Interventions were provided at clinical sites in Pittsburgh, Pennsylvania, Salt Lake City, Utah, and San Antonio, Texas (41). |
3. Flexibility of intervention(s) |
There is a strict protocol that limits the flexibility of experimental and comparison interventions that are delivered. This is done to achieve improved intervention adherence. Cointerventions are often prohibited. | All participants received a standardized treatment, with one group receiving a standardized joint mobilization intervention, one group receiving a different standardized joint mobilization intervention, and one group receiving a standardized exercise intervention (47). | Experimental and comparison interventions are provided with flexibility that is identical to that in usual care. Cointerventions are often permitted, as is the case in usual care. | All participants were randomized into one of four groups. The treatments they received consisted of various combinations of manual therapy, exercise, and/or booster sessions, which allowed for some flexibility of interventions, as per the patient presentation (32). |
4. Clinical relevance of experimental and comparison intervention(s) |
The experimental and/or comparison interventions are not a clinically relevant or best current treatment. Examples would be the use of a placebo, a no treatment control, the use of subclinical doses, or trial durations that are less than that seen in usual care. | All participants were assigned to a treatment. One group received manual therapy interventions consisting of joint mobilization, muscle stretching, and soft tissue mobilization. The other group received a placebo intervention consisting of the therapist placing both hands on the knee without any pressure for 10 minutes (39). | Both the experimental and comparison interventions are likely to be considered best practice, and the duration of interventions is similar to that in usual care. | All participants were assigned to a treatment. One group received manual therapy for the knee, lumbar spine, hip, and ankle, as required, and performed a standardized exercise routine in the clinic and at home. The other group received the standardized exercise routine in the clinic and at home. All treatments could be considered best practice (13). |
Based on a 5-point Likert scale the evidence deriving from each domain should be rated:
1 = strong emphasis on efficacy
2 = rather strong emphasis on efficacy
3 = balanced emphasis on both efficacy and effectiveness
4 = rather strong emphasis on effectiveness
5 = strong emphasis on effectiveness
OA = osteoarthritis; RITES = Rating of Included Trials on the Efficacy-Effectiveness Spectrum.
Table adapted from Wieland et al (19).
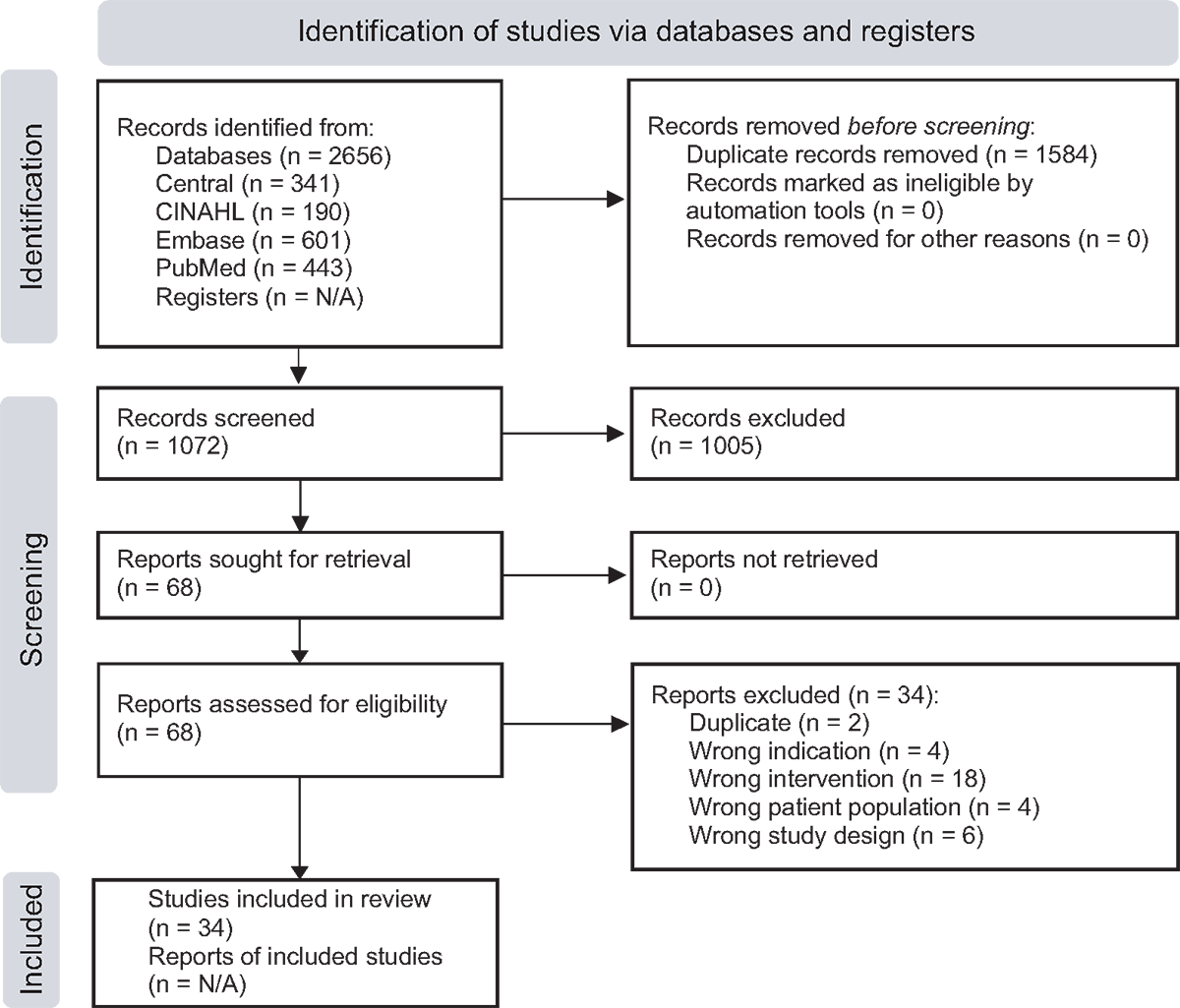
FIGURE 1 - PRISMA 2020 flow diagram for new systematic reviews that included searches of databases and registers only. PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analysis.
RITES domain scores
Overall RITES scores by domain are provided in Figure 2. A higher percentage of trials had a greater emphasis on efficacy within all four domains: participant characteristics (76.5%; n = 26) (13,34-41,43,44,47-49,51-60,62,63), trial setting (82.4%; n = 28) (13,14,32-36, 39,42-48,50-58,60-63), flexibility of intervention (61.8%; n = 20) (33-37,39,42,43,45-47,49-51,53,54,56,58,60,61,63), and clinical relevance of experimental and comparison intervention (50.0%; n = 17) (14,34-37,39,42,46,47,50,51, 53-55,59,60,62). In addition, when the RITES scores for all four domains of each trial were averaged, 29 trials were more oriented toward efficacy (mean [SD] of 2.2 [0.4] and range 1 to 3) (13,14,34-37,39,42-63), whereas three trials were more oriented toward effectiveness (mean [SD] of 3.6 [0.5] and range 3 to 5) (32,40,41). The remaining two trials had a mean score of 3.0 across the four domains, indicating a balanced emphasis between efficacy and effectiveness (33,38). Despite this overall emphasis on efficacy, 18 of the 34 trials had at least one domain with a score greater on the effectiveness spectrum (13,14,32,33, 37,38,40,41,43,45,46,48,50,55,58,59,61,63) (Tab. 2).
For the participant characteristics domain, 26 trials (76.5%) had scores that emphasized efficacy (13,34-41,43,44, 47-49,51-60,62), five trials (14.7%) emphasized effectiveness (32,33,46,50,61), and three trials (8.8%) had a balanced emphasis between efficacy and effectiveness (14,42,45). In the trial setting domain, 28 trials (82.4%) had scores that emphasized efficacy (13,14,32,36,39,42-48,50-58,60-63), five trials (14.7%) emphasized effectiveness (37,38,40,41,59), and one trial (2.9%) had a balanced emphasis between efficacy and effectiveness (49). The flexibility of intervention(s) domain had 21 trials (61.8%) that emphasized efficacy (33-37,39,42,43,45-47,49-51,53,54,56,58,60,61,63), seven trials (20.6%) emphasizing effectiveness (13,14,32,38,40,41,55), and six trials (17.6%) that exhibited a balanced emphasis between efficacy and effectiveness (44,48,52,57,59,62). Finally, the clinical relevance of experimental and comparison intervention(s) domain had 17 trials (50.0%) that emphasized efficacy (14,34-37,39,42,46,47,50,51,53-55,59,60,62), 11 trials (32.4%) emphasized effectiveness (13,32,33,40,41,43,45,48, 58,61,63), and six trials (17.6%) had a balanced emphasis between efficacy and effectiveness (38,44,49,52,56,57).
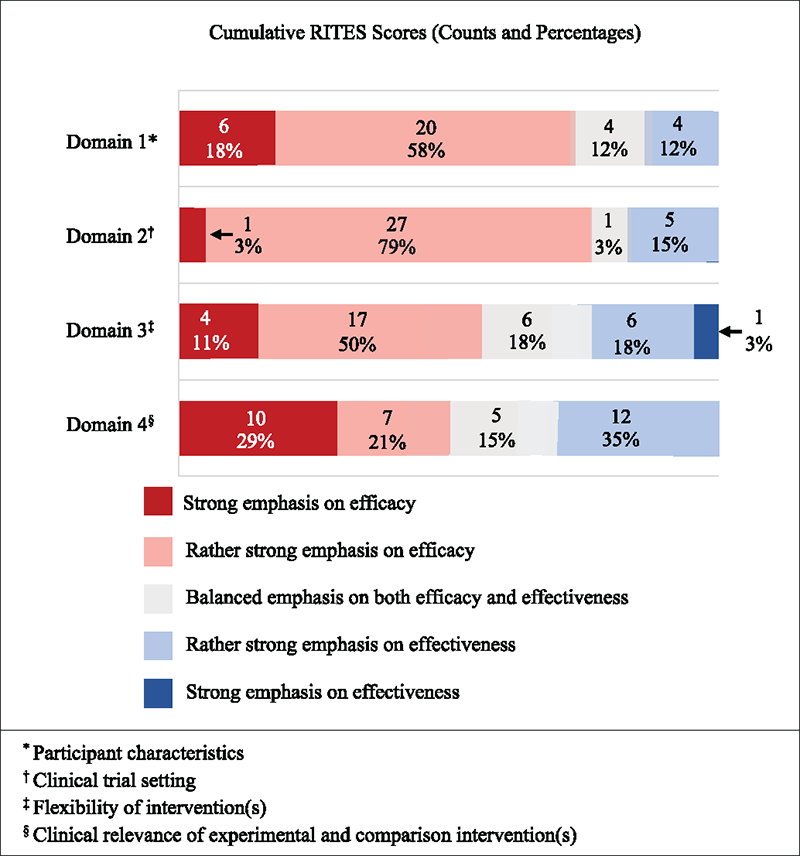
FIGURE 2 - Cumulative RITES scores (percentage and count). RITES = Rating of Included Trials on the Efficacy-Effectiveness Spectrum. *Participant characteristics; †Clinical trial setting; ‡Flexibility of intervention(s); §Clinical relevance of experimental and comparison intervention(s).
| Primary author and year of publication | RITES scores* | |||
|---|---|---|---|---|
| D1† | D2‡ | D3§ | D4|| | |
| Abbott et al (32) | 4 | 2 | 5 | 4 |
| Ali et al (33) | 4 | 2 | 2 | 4 |
| Alkhawajah and Alshami (34) | 2 | 2 | 2 | 1 |
| Altinbilek et al (35) | 1 | 2 | 2 | 2 |
| Bhagat et al (36) | 2 | 2 | 2 | 1 |
| Courtney et al (37) | 2 | 4 | 1 | 1 |
| Crossley et al (38) | 1 | 4 | 4 | 3 |
| Cruz-Montecinos et al (39) | 1 | 2 | 2 | 1 |
| Deyle et al (14) | 3 | 2 | 4 | 2 |
| Deyle et al (13) | 2 | 1 | 4 | 4 |
| Dwyer et al (40) | 2 | 4 | 4 | 4 |
| Fitzgerald et al (41) | 2 | 4 | 4 | 4 |
| Forestier et al (42) | 3 | 2 | 2 | 1 |
| Jeyakumar et al (43) | 2 | 2 | 2 | 4 |
| Jin et al (44) | 2 | 2 | 3 | 3 |
| Kaya Mutlu et al (45) | 3 | 2 | 2 | 4 |
| Kornkamon and Wanitcha (46) | 4 | 2 | 2 | 1 |
| Lalit et al (47) | 2 | 2 | 1 | 2 |
| Lizis et al (48) | 2 | 2 | 3 | 4 |
| Mahmooda et al (49) | 1 | 3 | 2 | 3 |
| Moss et al (50) | 4 | 2 | 2 | 1 |
| Narang and Ganvir (51) | 2 | 2 | 2 | 2 |
| Nigam et al (52) | 1 | 2 | 3 | 3 |
| Pollard et al (53) | 2 | 2 | 2 | 1 |
| Pozsgai et al (54) | 2 | 2 | 2 | 1 |
| Rao et al (55) | 2 | 2 | 4 | 2 |
| Razek and Shenouda (56) | 2 | 2 | 2 | 3 |
| Reza et al (57) | 2 | 2 | 3 | 3 |
| Sharma (58) | 2 | 2 | 2 | 4 |
| Sit et al (59) | 2 | 4 | 3 | 1 |
| Syed and Wani (60) | 2 | 2 | 2 | 2 |
| Taj et al (61) | 4 | 2 | 1 | 4 |
| Tucker et al (62) | 2 | 2 | 3 | 2 |
| Witwit et al (63) | 1 | 2 | 1 | 5 |
RITES, Rating of Included Trials on the Efficacy-Effectiveness Spectrum.
*RITES scoring, based on a 5-point Likert scale: 1 = strong emphasis on efficacy; 2 = rather strong emphasis on efficacy; 3 = balanced emphasis on both efficacy and effectiveness; 4 = rather strong emphasis on effectiveness; 5 = strong emphasis on effectiveness; N/A = information not available.
† RITES Domain 1: participant characteristics.
‡ RITES Domain 2: trial setting.
§ RITES Domain 3: flexibility of intervention(s).
|| RITES Domain 4: clinical relevance of experimental and comparison intervention(s).
Of the 34 trials, only seven had null findings (40,41,44,47, 61-63). Fisher’s exact test revealed no statistically significant relationship between where studies fell on the efficacy-effectiveness spectrum and a positive outcome of the primary outcome (p = 0.27).
Risk of bias for included trials
Five of the included trials (14.8%) had low risk of bias (13,36,53,57,63), 15 trials (44.1%) had high risk of bias (43-51, 54-56,60-62), and 14 trials (41.2%) had some concerns for risk of bias (14,32-35,37-42,52,58,59) (Supplementary material, Appendix F). The most common cause for bias included measurement of the outcome (43,44,46,48,50,51,54-56,61,62), and the least amount of bias was in the selection of reported outcome (13,14,32-63). When comparing risk of bias across the trials, all five of those with low risk of bias also had an emphasis on efficacy (13,36,53,57,63).
Rater agreement
Interrater reliability was к = 0.25 (fair agreement) for title and abstract screening and к = 0.31 (fair agreement) for full-text screening. Interrater reliability between reviewers for the participants’ characteristics domain was к = 0.45 (fair agreement), к = 0.39 (fair agreement) for trial settings, к = 0.34 (fair agreement) for flexibility of interventions, and к = 0.32 (fair agreement) for clinical relevance of experimental and comparison interventions. Interrater reliability between the reviewers for RoB-2 was к = 0.04 (slight agreement). These values were related to initial agreement when reviewing the trials. It is important to note that consensus was reached on all initial ratings, and a third reviewer needed to be consulted for only three trials (8.3%).
Discussion
This systematic review assessed existing manual therapy trials for knee OA to determine where the current body of evidence falls on the efficacy-effectiveness spectrum. The findings suggest that the majority of trials trend toward efficacy in all four domains of the RITES tool, especially for participant characteristics and clinical trial settings. While a previous systematic review has looked at a similar question in trials involving manual therapy for low back pain (64), this is the first known review assessing trials for knee OA.
Participant characteristics
A large percentage of trials (76.5%) were higher on the explanatory end of the spectrum in the participant characteristics domain, with the primary reason being related to their exclusion criteria (13,34-41,43,44,47-49,51-63). Patients were most commonly excluded from trials due to the presence of other diagnoses or comorbidities, and while this could confound treatment effect, it is a more accurate representation of patients seeking care for knee OA. For example, vascular and cardiovascular disease, obesity, acute or chronic pain in the spine, hip, or ankle, and those using anti-inflammatory drugs beyond simple pain relievers are common presentations for individuals with knee OA (35,39). Excluding these individuals could result in conclusions that may not be relevant to the types of patients seen in most clinics (16,18). To achieve a more pragmatic rating would have required a study population that included patients with diagnoses, comorbidities, symptom durations, and age ranges similar to common knee OA patients that seek care (16,18).
Trial setting
The majority of trials (82.4%) had an emphasis on efficacy in the trial setting domain due to the trials being carried out in settings that were dissimilar from common practice (13,14,32-36,39,42-48,50-58,60-63). These included specialized clinics, specialized trial or academic centers, and military clinics and settings, and also used a limited number of clinicians who were often specifically trained for the interventions being assessed. While this may enable researchers to better determine the effect of the interventions without compromising internal validity, it limits external validity (15,18). To achieve more pragmatic trial settings, researchers should strive to use a broad array of clinics and clinicians that better mimic typical medical providers and healthcare settings (15,16).
Flexibility of interventions
The flexibility of interventions domain had an emphasis on efficacy. The majority of clinical trials (61.8%) required strict manual therapy protocols with little flexibility or prohibited cointerventions (33-37,39,42,43,45-47,49-51,53,54,56,58,60,61,63). Some reasons for a strict protocol include the ability to better attribute the treatment effect to the intervention being assessed, rather than an influence from other confounders. Even efforts to control or improve intervention adherence may lead to different results than can be expected in real-world settings (15,16). On the other hand, seven of the trials had a more pragmatic emphasis (13,14,32,38,40,41,55), which was accomplished by allowing more flexibility with the interventions between the trial populations. This approach allowed clinicians to manage patients based on their perceived needs with greater flexibility.
Clinical relevance of experimental and comparison intervention
Clinical relevance of experimental and comparison of interventions slightly favored efficacy (50.0%) (14,34-37,39,42,46, 47,50,51,53-55,59,60,62) compared to those with designs more focused on effectiveness (32.4%) (13,32,33,40,41,43,45,48,58,61,63), and those that had a balanced emphasis on efficacy and effectiveness (17.6%) (38,44,49,52,56,57). Trials with a more explanatory design were less likely to have one of the treatment arms considered clinically relevant or best practice, such as using controls, placebo, or sham interventions, all of which provide a less than desirable comparison when considering generalizability to real-world settings (19). Treatment duration may also have been much shorter than the duration of treatments used in real-world practice (19). On the other hand, trials emphasizing a pragmatic approach use flexibility with interventions that mimic typical practice, and they use comparison groups that are often considered to represent best practice or usual care (19).
Outcomes relative to efficacy-effectiveness spectrum
When examining trial outcomes relative to where studies fell on the efficacy-effectiveness spectrum, there were no significant associations. This means that trial design has no bearing on whether a study showed a treatment effect. However, definitive conclusions cannot be made because there were only seven null trials out of the 34 trials (40,41,44,47,61-63).
Clinical implications
The majority of trials investigating manual therapy for knee OA were on the explanatory end of the spectrum across all four RITES domains. This is similar to what was reported by Maddox et al (64) for individuals with low back pain, except their review found a rather strong emphasis toward the pragmatic end of the spectrum with the domain related to clinical relevance of experimental and comparison interventions.
The role of explanatory trials is to analyze the mechanism of interventions under controlled circumstances (19). In this instance, explanatory trials help to determine if manual therapy is an effective treatment for knee OA. However, they lack the ability to generalize the results because the settings are often not representative of real-world clinical practice. That is where pragmatic trials provide their value by providing clinicians with the ability to know if manual therapy can be beneficial for patients with knee OA in real-world settings (15-17). The result of this review demonstrates lack of generalizability with the majority of studies examining manual therapy for knee OA.
Recommendation for future research
While the current body of literature demonstrates potential benefits when using manual therapy for individuals with knee OA, many of those recommendations come from trials that are more explanatory than pragmatic, making them less generalizable (5,9). Additional pragmatic studies examining manual therapy for knee OA in real-world scenarios and across a variety of settings and clinicians would help improve the applicability and implementation of these interventions. For example, the study design could include patient population with some comorbidities, especially those commonly associated with knee OA (diabetes and obesity); multiple/diverse trial settings or general clinical practice settings, not specialty treatment clinics; and flexibility of interventions, allowing cross-treatments whenever/if needed while ensuring that the methodology of interest is systematically and objectively directed toward best practice. Finally, it is worth noting that manual therapy may not be unique here, and these findings may be very similar to what is observed for trial designs of other interventions for knee OA.
Limitations
This review had the primary goal of assessing where this body of evidence falls on the efficacy-effectiveness spectrum, with no intention to examine the effectiveness of manual therapy for knee OA. Therefore, conclusions should not be inferred regarding pooled treatment effects or the value of manual therapy interventions for knee OA.
Conclusions
Thirty-four manual therapy trial designs for knee OA were assessed for their fit along the explanatory-pragmatic spectrum. The majority of trial designs were more explanatory, making the results less generalizable across patient populations, clinical settings, and compared to other commonly used interventions. When examining the effectiveness of manual therapy for the treatment of knee OA, more pragmatic study designs would help improve implementation and applicability of research results. This can be achieved by using a more diverse patient population, a larger number of clinics, intervention protocols that are more pragmatic, and comparison treatments that represent best practice or usual care. All of these will help improve the ability to generalize findings from manual therapy trials for knee OA.
Abbreviations
к, kappa; CINAHL, Cumulative Index to Nursing and Allied Health Literature; IL, Illinois; n, number; OA, osteoarthritis; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; RITES, Rating of Included Trials on the Efficacy-Effectiveness Spectrum; RoB-2, Cochrane Risk of Bias 2.0.
Acknowledgments
This is the final version of record of this article as stated in DOI: 10.33393/AOP.2024.3326 (Online)
We would like to thank Cindy Reinl (Bellin Health Systems librarian) and Kenneth L. Carriveau, Jr. and Rachel Blume (Baylor University librarians) for their assistance with performing thorough literature searches for this systematic review. We would also like to thank Mareli Klopper for her mentoring during the completion of this systematic review.
Disclosures
Conflict of interest: The authors declare that they have no competing interests.
Financial support: Authors have not received any financial support for this study.
Authors’ contributions: KRA contributed to the design of the systematic review, data collection and data analysis, and all drafts and revisions of the manuscript. AOF contributed to the design of the systematic review, data collection and data analysis, and all drafts and revisions of the manuscript. JLY contributed to the design of the systematic review, data collection and data analysis, to all drafts and revisions of the manuscript, and had final approval of the version to be published. CDM contributed to the design of the systematic review, contributed to data collection and data analysis, and contributed to drafts and revisions of the manuscript. DIR contributed to the design of the systematic review, data collection and data analysis, to all drafts and revisions of the manuscript, and had final approval of the version to be published.
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information section.
Disclaimer: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University, U.S. Department of Defense, or the U.S. Government.
References
- 1. Paradowski PT, Lohmander LS, Englund M. Osteoarthritis of the knee after meniscal resection: long term radiographic evaluation of disease progression. Osteoarthritis Cartilage. 2016;24(5):794-800. CrossRef PubMed
- 2. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-388. CrossRef PubMed
- 3. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189-2196. CrossRef PubMed
- 4. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355-369. CrossRef PubMed
- 5. Anwer S, Alghadir A, Zafar H, Brismée JM. Effects of orthopaedic manual therapy in knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy. 2018;104(3):264-276. CrossRef PubMed
- 6. Arthritis Foundation [Internet]. Arthritis by the numbers; 2020. Available from: Online (Accessed June 2023)
- 7. Mo L, Jiang B, Mei T, Zhou D. Exercise therapy for knee osteoarthritis: a systematic review and network meta-analysis. Orthop J Sports Med. 2023;11(5):23259671231172773. CrossRef PubMed
- 8. Deyle GD, Allen CS, Allison SC, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382(15):1420-1429. CrossRef PubMed
- 9. Tsokanos A, Livieratou E, Billis E, et al. The efficacy of manual therapy in patients with knee osteoarthritis: a systematic review. Medicina (Kaunas). 2021;57(7):696. CrossRef PubMed
- 10. Runge N, Aina A, May S. The benefits of adding manual therapy to exercise therapy for improving pain and function in patients with knee or hip osteoarthritis: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2022;52(10):675-A13. CrossRef PubMed
- 11. Feng T, Wang X, Jin Z, et al. Effectiveness and safety of manual therapy for knee osteoarthritis: an overview of systematic reviews and meta-analyses. Front Public Health. 2023;11:1081238. CrossRef PubMed
- 12. Rhon DI, Kim M, Asche CV, Allison SC, Allen CS, Deyle GD. Cost-effectiveness of physical therapy vs intra-articular glucocorticoid injection for knee osteoarthritis: a secondary analysis from a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2142709. CrossRef PubMed
- 13. Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132(3):173-181. CrossRef PubMed
- 14. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85(12):1301-1317. CrossRef PubMed
- 15. Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217-224. CrossRef PubMed
- 16. Merali Z, Wilson JR. Explanatory versus pragmatic trials: an essential concept in study design and interpretation. Clin Spine Surg. 2017;30(9):404-406. CrossRef PubMed
- 17. Khorsan R, Crawford C. How to assess the external validity and model validity of therapeutic trials: a conceptual approach to systematic review methodology. Evid Based Complement Alternat Med. 2014;2014:694804. CrossRef PubMed
- 18. Patino CM, Ferreira JC. Internal and external validity: can you apply research study results to your patients? J Bras Pneumol. 2018;44(3):183. CrossRef PubMed
- 19. Wieland LS, Berman BM, Altman DG, et al. Rating of Included Trials on the Efficacy-Effectiveness Spectrum: development of a new tool for systematic reviews. J Clin Epidemiol. 2017;84:95-104. CrossRef PubMed
- 20. Rhon DI, Flynn TW, Shepherd MH, Abbott JH. Leveraging the short-term benefits of manual therapy which includes exercise over exercise therapy alone appears justified for knee osteoarthritis. J Orthop Sports Phys Ther. 2023;53(1):49-50. CrossRef PubMed
- 21. Ferreira RM, Martins PN, Pimenta N, Gonçalves RS. Physical therapists’ choices, views and agreements regarding non-pharmacological and non-surgical interventions for knee osteoarthritis patients: a mixed-methods study. Mediterr J Rheumatol. 2023;34(2):188-219. CrossRef PubMed
- 22. Rhon DI, Deyle GD. Manual therapy: always a passive treatment? J Orthop Sports Phys Ther. 2021;51(10):474-477. CrossRef PubMed
- 23. Collins CK, Masaracchio M, Brismée JM. The future of orthopedic manual therapy: what are we missing? J Man Manip Ther. 2017;25(4):169-171. CrossRef PubMed
- 24. French HP, Brennan A, White B, Cusack T. Manual therapy for osteoarthritis of the hip or knee—a systematic review. Man Ther. 2011;16(2):109-117. CrossRef PubMed
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. CrossRef PubMed
- 26. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. Online CrossRef PubMed. Accessed October 15, 2022.
- 27. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2020;72(2):149-162. CrossRef PubMed
- 28. Covidence 2020. Better systematic review management. Online. Accessed June 2023.
- 29. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. Online CrossRef PubMed. Accessed March 28, 2022.
- 30. Higgins JPT, Altman DG, Gøtzsche PC, et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(Oct18 2):d5928. Online CrossRef PubMed. Accessed March 28, 2022.
- 31. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. CrossRef PubMed
- 32. Abbott JH, Chapple CM, Fitzgerald GK, et al. The incremental effects of manual therapy or booster sessions in addition to exercise therapy for knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther. 2015;45(12):975-983. CrossRef PubMed
- 33. Ali SS, Ahmed SI, Khan M, Soomro RR. Comparing the effects of manual therapy versus electrophysical agents in the management of knee osteoarthritis. Pak J Pharm Sci. 2014;27(4)(suppl):1103-1106. PubMed
- 34. Alkhawajah HA, Alshami AM. The effect of mobilization with movement on pain and function in patients with knee osteoarthritis: a randomized double-blind controlled trial. BMC Musculoskelet Disord. 2019;20(1):452. Online CrossRef PubMed
- 35. Altınbilek T, Murat S, Yumuşakhuylu Y, İçağasıoğlu A. Osteopathic manipulative treatment improves function and relieves pain in knee osteoarthritis: a single-blind, randomized-controlled trial. Turk J Phys Med Rehabil. 2018;64(2):114-120. CrossRef PubMed
- 36. Bhagat M, Neelapala YVR, Gangavelli R. Immediate effects of Mulligan’s techniques on pain and functional mobility in individuals with knee osteoarthritis: a randomized control trial. Physiother Res Int. 2020;25(1):e1812. CrossRef PubMed
- 37. Courtney CA, Steffen AD, Fernández-de-Las-Peñas C, Kim J, Chmell SJ. Joint mobilization enhances mechanisms of conditioned pain modulation in individuals with osteoarthritis of the knee. J Orthop Sports Phys Ther. 2016;46(3):168-176. CrossRef PubMed
- 38. Crossley KM, Vicenzino B, Lentzos J, et al. Exercise, education, manual-therapy and taping compared to education for patellofemoral osteoarthritis: a blinded, randomised clinical trial. Osteoarthritis Cartilage. 2015;23(9):1457-1464. CrossRef PubMed
- 39. Cruz-Montecinos C, Flores-Cartes R, Montt-Rodriguez A, Pozo E, Besoaín-Saldaña A, Horment-Lara G. Changes in co-contraction during stair descent after manual therapy protocol in knee osteoarthritis: a pilot, single-blind, randomized study. J Bodyw Mov Ther. 2016;20(4):740-747. CrossRef PubMed
- 40. Dwyer L, Parkin-Smith GF, Brantingham JW, et al. Manual and manipulative therapy in addition to rehabilitation for osteoarthritis of the knee: assessor-blind randomized pilot trial. J Manipulative Physiol Ther. 2015;38(1):1-21.e2. CrossRef PubMed
- 41. Fitzgerald GK, Fritz JM, Childs JD, et al. Exercise, manual therapy, and use of booster sessions in physical therapy for knee osteoarthritis: a multi-center, factorial randomized clinical trial. Osteoarthritis Cartilage. 2016;24(8):1340-1349. CrossRef PubMed
- 42. Forestier R, Genty C, Waller B, et al. Crenobalneotherapy (spa therapy) in patients with knee and generalized osteoarthritis: a post-hoc subgroup analysis of a large multicentre randomized trial. Ann Phys Rehabil Med. 2014;57(4):213-227. CrossRef PubMed
- 43. Jeyakumar S, Alagesan J, Ramachandran A. A comparative study on the efficacy of Maitland’s mobilisation and Mulligan’s mobilisation in sub-acute osteoarthritis knee. Biomedicine (Taipei). 2017;37(4):518-520.
- 44. Jin L, Ma B, Liu X, Teng W. A randomized clinical trial assessment of nonsteroidal anti-inflammatory drugs and Chinese bone setting manipulation therapy in knee osteoarthritis. Int J Clin Exp Med. 2017;10(3):5106-5115.
- 45. Kaya Mutlu E, Ercin E, Razak Ozdıncler A, Ones N. A comparison of two manual physical therapy approaches and electrotherapy modalities for patients with knee osteoarthritis: a randomized three arm clinical trial. Physiother Theory Pract. 2018;34(8):600-612. CrossRef PubMed
- 46. Kornkamon C, Wanitcha K. Immediate effects of self-manual therapy and supervised manual therapy in individuals with knee osteoarthritis. Indian J Public Health Res Dev. 2019;10(11):2992-2998. CrossRef
- 47. Lalit SY, Suhas MB, Amita M. Effect of manual therapy techniques on knee proprioception in patients with osteo-arthritis of knee. Indian J Physiother Occup Ther. 2012;6(3):285. Online
- 48. Lizis P, Manko G, Kobza W, Para B. Manual therapy with cryotherapy versus kinesiotherapy with cryotherapy for knee osteoarthritis: a randomized controlled trial. Altern Ther Health Med. 2019;25(4):40-45. PubMed
- 49. Mahmooda S, Ishaq I, Safdar M, Sabir M, Tahir A, Irshad S. Effects of mulligan’s mobilization with movements versus myofascial release in addition to usual care on pain and range in knee osteoarthritis. Rawal Med J. 2020;45(2):353-357.
- 50. Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther. 2007;12(2):109-118. CrossRef PubMed
- 51. Narang S, Ganvir S. Efficacy of Kaltenbohn mobilization on patients with osteoarthritis of knee joint. Indian J Physiother Occup Ther. 2014;8(3):162. Online
- 52. Nigam A, Satpute KH, Hall TM. Long term efficacy of mobilisation with movement on pain and functional status in patients with knee osteoarthritis: a randomised clinical trial. Clin Rehabil. 2021;35(1):80-89. CrossRef PubMed
- 53. Pollard H, Ward G, Hoskins W, Hardy K. The effect of a manual therapy knee protocol on osteoarthritic knee pain: a randomised controlled trial. J Can Chiropr Assoc. 2008;52(4):229-242. PubMed
- 54. Pozsgai M, Péter IA, Farkas N, Than P, Nusser N. End-range Maitland mobilization decreasing pain sensitivity in knee osteoarthritis: randomised, controlled clinical trial. Eur J Phys Rehabil Med. 2022;58(3):442-541. CrossRef
- 55. Rao RV, Balthillaya G, Prabhu A, Kamath A. Immediate effects of Maitland mobilization versus Mulligan mobilization with movement in osteoarthritis knee: a randomized crossover trial. J Bodyw Mov Ther. 2018;22(3):572-579. CrossRef PubMed
- 56. Razek RA, Shenouda MM. Efficacy of Mulligan’s mobilization with movement on pain, disability, and range of motion in patients with knee osteoarthritis: a randomized controlled pilot study. Indian J Physiother Occup Ther. 2014;7(1):242-247. Online
- 57. Reza MK, Shaphe MA, Qasheesh M, Shah MN, Alghadir AH, Iqbal A. Efficacy of specified manual therapies in combination with a supervised exercise protocol for managing pain intensity and functional disability in patients with knee osteoarthritis. J Pain Res. 2021;14:127-138. CrossRef PubMed
- 58. Sharma SS. A randomized comparison of effectiveness of clinical exercises and manual therapy procedures versus clinical exercises alone in the treatment of osteoarthritis of knee. Indian J Physiother Occup Ther. 2013;7(3):198. Online
- 59. Sit RWS, Chan KKW, Zou D, et al. Clinic-based patellar mobilization therapy for knee osteoarthritis: a randomized clinical trial [Internet]. Vol. 16. Ann Fam Med. 2018;16(6):521-529. CrossRef PubMed
- 60. Syed S, Wani S. Effect of two different manual therapy protocols on osteoarthritic knee pain & functional disability: a comparison study. Rom J Phys Ther/Revista Romana de Kinetoterapie. 2014;20(34). Online. Accessed June 2023.
- 61. Taj S, Anwar K, Arshad H, Khalid M, Ali MQ, Hussain E. Effectiveness of Maitland mobilization versus pain relief phenomena for pain, range of motion and disability in early knee osteoarthritis. Pak J Med Health Sci. 2023;17(1). CrossRef
- 62. Tucker M, Brantingham JW, Myburg C. Relative effectiveness of a non-steroidal anti-inflammatory medication (Meloxicam) versus manipulation in the treatment of osteo-arthritis of the knee. Eur J Chiropractic. 2003;50:163-183.
- 63. Witwit RT, Shadmehr A, Mir SM, Fereydounnia S, Jalaei S. Comparison of non-thrust manipulation vs muscle energy techniques in management of patients with knee osteoarthritis: a randomized clinical trial. Neuroquantology. 2022; 20(67):6843-6859.
- 64. Maddox CD, Subialka JA, Young JL, Rhon DI. Over half of clinical trials of mobilization and manipulation for patients with low back pain may have limited real-world applicability: a systematic review of 132 clinical trials. J Orthop Sports Phys Ther. 2022;52(8):532-545. CrossRef PubMed