 |
AboutOpen | 2024; 11: 37-41 ISSN 2465-2628 | DOI: 10.33393/ao.2024.3066 ORIGINAL RESEARCH ARTICLE |
 |
Using the Nephrology Referral Form in Italian primary care to improve the care pathway of patients with anemia or other complications related to chronic kidney disease: survey findings
ABSTRACT
Introduction: Anemia is a potentially reversible condition in early chronic kidney disease (CKD) that requires timely intervention. General practitioners (GPs) play a crucial role in recognizing CKD. A new Nephrology Referral Form (NRF) was developed and tested in the Italian setting.
Methods: This mixed-methods survey, conducted between 2021 and 2022, introduced the NRF through focus group discussions involving a scientific committee. The NRF was tested in a 6-month trial involving 24 GPs each from Lazio and Puglia regions. GPs provided feedback on the use of the NRF in clinical practice through a questionnaire sent via Microsoft Form. The data were analyzed descriptively.
Results: After 6 months, 41.67% of the GPs were using the NRF at least once a week. Diabetes mellitus and hypertension were common triggers for NRF assessments. GPs overwhelmingly agreed (96%) on the NRF’s utility in identifying CKD cases, with 92% citing its effectiveness in diagnosing well-defined cases. The NRF facilitated specialist referrals, with 83% of GPs reporting increased referrals compared to the prior 6 months. Feedback underscored the NRF’s positive impact, suggesting improvements such as additional referral centers, regional/national networks, enhanced GP training, and increased collaboration.
Conclusion: GPs regularly used the NRF to identify and diagnose cases of CKD, streamlining the referral process and increasing referrals to specialists. Feedback emphasized the NRF’s positive impact and highlighted its potential as a valuable tool for enhancing early CKD detection, interventions, and fostering multidisciplinary management in primary care for better patient outcomes.
Keywords: Anemia, Chronic kidney disease, General practitioners, Nephrology Referral Form, Questionnaire
Received: March 14, 2024
Accepted: April 24, 2024
Published online: May 15, 2024
This article includes supplementary materials.
AboutOpen - ISSN 2465-2628 - www.aboutscience.eu/aboutopen
© 2024 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Chronic kidney disease (CKD) is characterized by reduced glomerular filtration rate or high albuminuria, affecting 15%-20% of adults globally, often overlooked by both patients and physicians (1). CKD significantly increases the risk of adverse outcomes, with cardiovascular disease (CVD) being a primary concern, including coronary heart disease, stroke, peripheral artery disease, arrhythmias, and heart failure, accounting for a substantial portion of mortality (2). Over the past 30 years, there has been a 43% increase in cases requiring dialysis and a 41% rise in mortality related to CKD; it is estimated that CKD will be the fifth cause of death in the world in the next years (3) The epidemiological data on CKD prevalence are particularly concerning in European Union countries, with Italy showing a prevalence of approximately 6%-7% among adults (4). Early-stage CKD is asymptomatic, with symptoms emerging in later stages alongside complications such as declining kidney function and the presence of other disease-associated comorbidities such as anemia, hyperlipidemia, nutrition, osteodystrophy, and cardiovascular risk (5). In the advanced CKD stages when kidney function is severely impaired, patients typically require dialysis or transplantation, which poses a substantial cost burden on the National Health System (NHS). A critical issue in CKD treatment is the limited therapy options available, particularly in the early stages of the disease, as the prevalence of associated comorbidities continues to increase. An estimated 77% of individuals with mild to moderate renal function decline (stage 3) remain undiagnosed, with a median time to diagnosis of 3.64 years (6). Anemia is a common comorbidity of CKD, significantly impacting a patient’s quality of life and increasing healthcare resource utilization (7); it is associated with an increased risk of CKD progression, cardiovascular events, and overall mortality. The underlying mechanisms of anemia in CKD are multifactorial, involving a progressive decrease in endogenous erythropoietin (EPO) levels and other contributing factors, such as absolute iron deficiency, impaired iron absorption, high hepcidin levels, systemic inflammation, reduced bone marrow response to EPO due to uremic toxins, shortened red cell life span, and deficiencies in vitamin B12 and folic acid (8). Anemia serves as an early warning sign of potential CKD and can be easily detected through simple blood tests. Early identification of CKD is crucial for initiating pharmacological interventions to preserve renal function (9). The primary goals of CKD treatment are to slow disease progression, manage symptoms and complications, delay or prevent end-stage renal disease, and enhance patients’ quality of life by avoiding the need for dialysis or kidney transplantation. Treatment strategies typically involve controlling blood pressure and glucose levels, making lifestyle modifications, and prescribing appropriate medications (10). General practitioners (GPs) play a vital role in recognizing CKD and are often the first to encounter patients with declining kidney function (11). Therefore, prompt training is essential to enable GPs to identify and refer patients to the nephrologist for comprehensive care, facilitating multidisciplinary management (12). In Italy, GPs often fail to detect early-stage CKD due to inadequate evaluation of key parameters, leading to delayed referrals to nephrologist for further diagnosis (13). To address this issue, a scientific committee developed a new tool known as the Nephrology Referral Form (NRF), which was subsequently tested in the clinical practice of a small sample of GPs. A questionnaire was administered to assess the NRF’s effectiveness in improving the care pathway of patients with anemia or other complications related to CKD in the Italian primary care setting.
Methods
Mixed methods were used in this study (14). Qualitative and quantitative methods were performed throughout the focus group and survey, respectively, in two different steps (15). In the first step, in 2021, the Integrated Strategies for Health Enhancing Outcomes (ISHEO)’s research team promoted the creation of a scientific committee (SC) composed of two internists, two nephrologists and conducted a focus group afterward in order to develop the NRF tool. The NRF, a paper form, requires the GP to complete three short sequential steps that can lead to the identification of the patient to be referred to the nephrology specialist: (1) patient eligibility according to prespecified criteria that must be met to proceed to step 2; (2) prespecified renal function parameters that the patient must meet to be referred to the nephrologist; (3) referral of the patient to the nephrologist with a prescription for ultrasound of the complete urinary system and laboratory tests (Supplementary appendix 1). In the second focus group conducted by ISHEO, two pilot Italian regions, Lazio and Puglia, were identified by SC, which decided to involve 24 primary care physicians (12 per region) to pilot the NRF in clinical practice for 6 months. It was estimated by SC that the number of 24 physicians was sufficient for a pilot study as they covered a population of about 30,000 patients. Further, a questionnaire (nine multiple choices and one open-ended question) was created with the aim to evaluate the effectiveness of the tool in the clinical practice of the 24 GPs (Supplementary appendix 2). In the second step, after 6 months of NRF use, the questionnaire was administrated to 24 GPs via Microsoft Form. The collected responses were anonymized, and descriptive statistical analysis was performed (16).
Results
Six months after the introduction of NRF into clinical practice, all 24 GPs responded to the questionnaire (100% response rate). It was used for more than half of respondents every 2 to 4 weeks (54.16%), at least once a week by 41.67%, and every 2 or more months by 4.17% (Fig. 1).
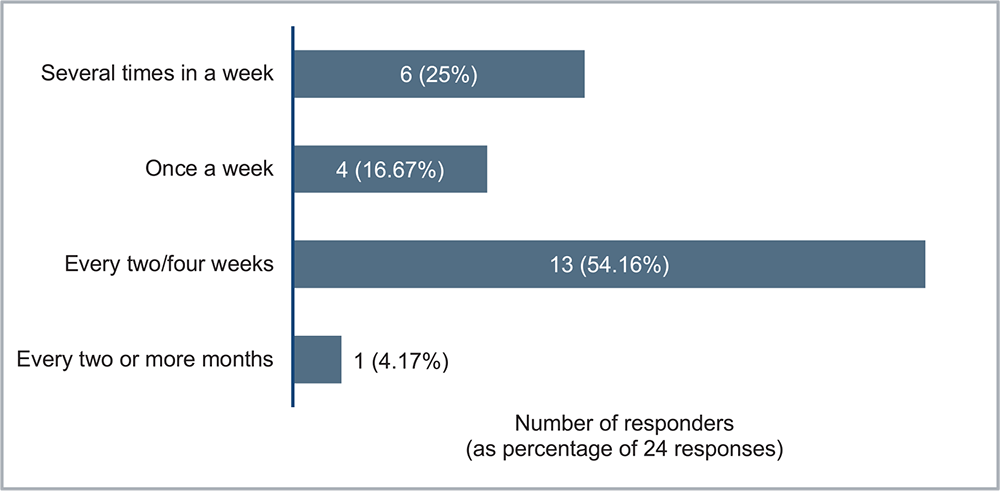
FIGURE 1 - How often did you use the Nephrology Referral Form for the diagnosis of chronic kidney disease?
The most frequently observed clinical conditions leading to patient assessment by NRF were diabetes mellitus (33.33%), high blood pressure (23.19%) at 6 months, followed by other health issues (Fig. 2).
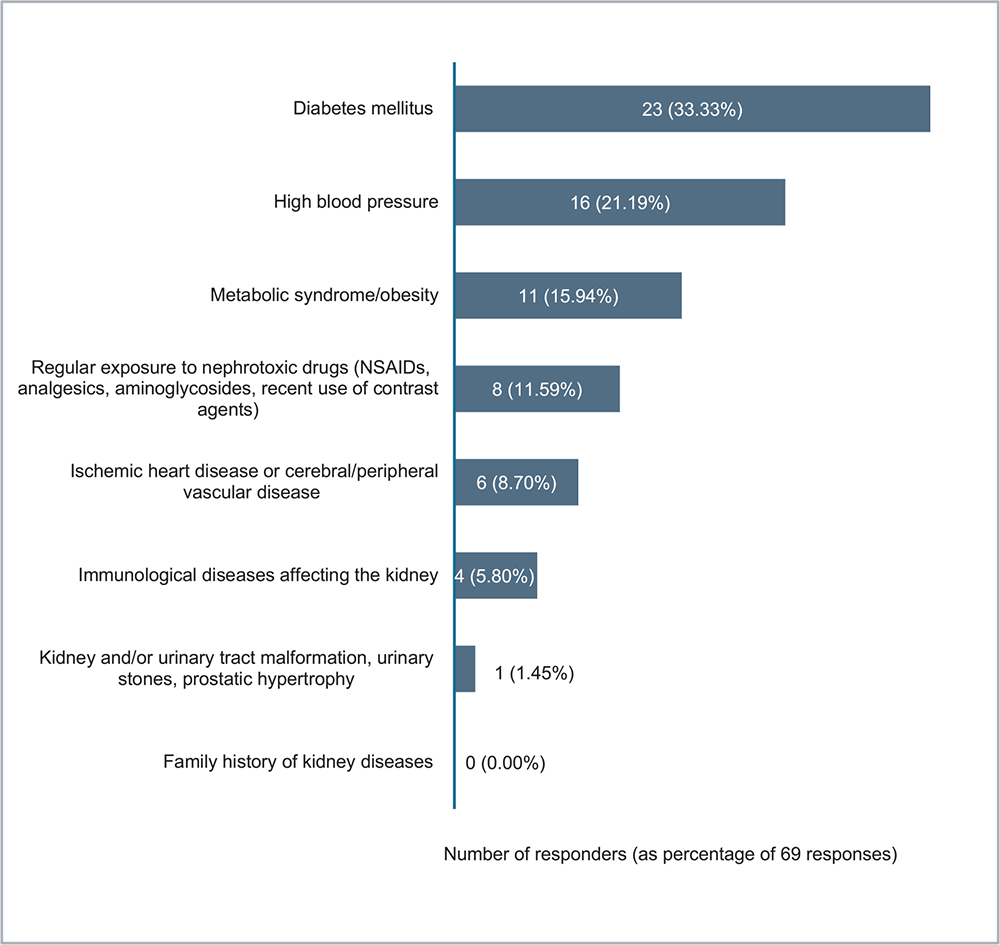
FIGURE 2 - What are the most frequently observed clinical conditions that led to the evaluation of the patient via the Nephrology Referral Form? Indicate one or more options. NSAID = nonsteroidal anti-inflammatory drug.
About 96% of GPs agreed that NRF was a useful tool for identifying cases of CKD, while for 92% of GPs agreed the use of NRF allowed them to diagnose cases where CKD was well defined (Tabs 1 and 2).
| No of respondents at 6 months (%) | |
|---|---|
| Totally agree | 23 (96%) |
| Disagree | 1 (4%) |
| I don’t know | 0 |
| No of respondents at 6 months (%) | |
|---|---|
| Yes, in several situations | 4 (17%) |
| Yes, sometimes | 18 (75%) |
| No | 2 (8%) |
| Don’t know | 0 |
The GP was also asked whether the use of the NRF facilitated the link with a referring nephrologist/internist: 62.5% of GPs did not have any referral nephrologist, a network was established with several nephrologists for 25%, and 12.5% patients who identified with CKD were always sent to the same nephrologist (Fig. 3).
Eighty-three percent of the GPs noted a rise in patient referrals to specialists compared to the preceding 6 months before using the NRF. Among these, 62% reported a modest increase of less than 50%, while 21% reported a more substantial increase exceeding 50% (Fig. 4).
A total of 33% of GPs report having received information questions from colleagues and specialists regarding the use of the NRF, while 67% did not receive any information requests. The establishment of new nephrology referral centers in Italy, a regional/national nephrology network, more training and involvement of GPs were the main suggestions that emerged from the open-ended questions to improve the use of the NRF.
Discussion
CKD presents a significant global health challenge, affecting 15%-20% of adults worldwide, yet its impact is often underestimated by both patients and healthcare providers (1). The increasing prevalence of CKD, coupled with its link to adverse outcomes like CVD (2), emphasizes the need for effective management strategies. Despite CKD’s rising incidence, early stages of the disease are asymptomatic, highlighting the need for innovative approaches to timely identification and intervention (17).
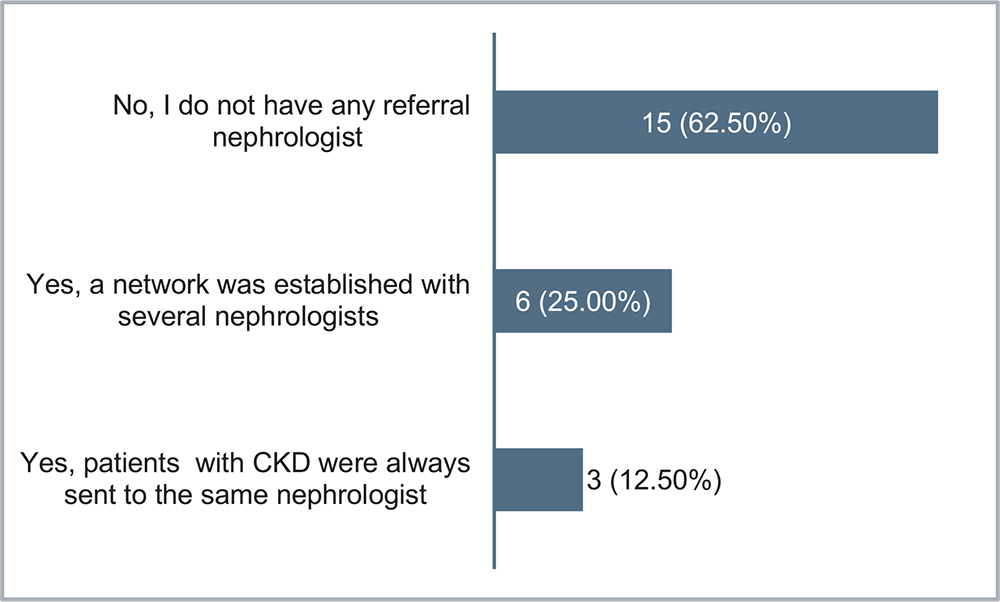
FIGURE 3 - Considering your experience, has the use of the Nephrology Referral Form facilitated the connection with a referring nephrologist/internist?
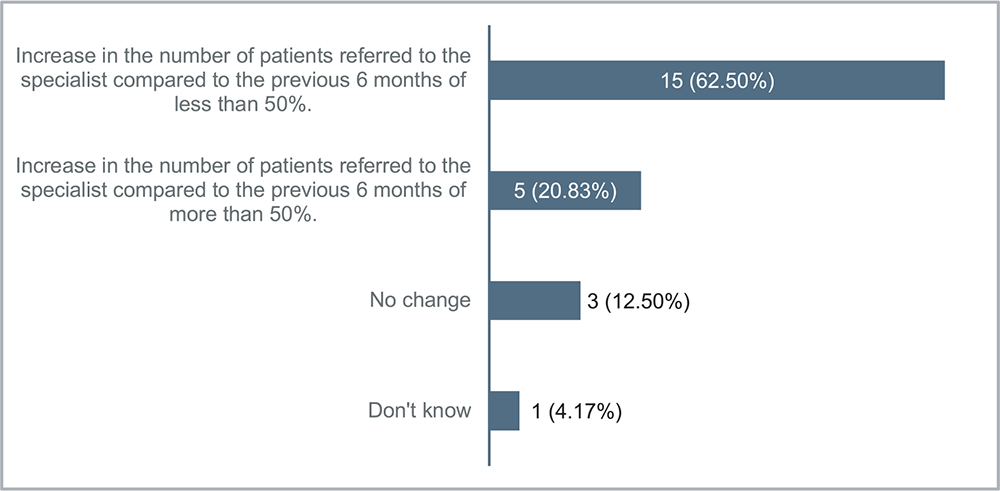
FIGURE 4 - Considering your experience, has the number of patients referred to the specialist increased since the use of the Nephrology Referral Form compared to 6 months previously?
Current clinical guidelines for CKD stress prompt identification and intervention to slow disease progression. This involves regular monitoring of renal function, maintenance of optimal blood pressure, adopting a healthy diet, engaging in regular physical activity, and quitting smoking. The guideline also recommends medications to manage CKD-associated comorbidities such as anemia, bone disorders, and CVD (10).
Anemia often serves as a “sentinel” of CKD, providing an opportunity for early intervention to preserve renal function (18) and GPs play a pivotal role in the early CKD detection and referral due to their role in primary healthcare. However, in Italy, the current system often fails to identify CKD in its initial stages, resulting in delayed referrals to nephrologists for comprehensive diagnosis (17). To address this gap, a novel tool called the NRF was developed and pilot-tested among primary care physicians. Results showed a promising rate of NRF utilization, with 41.67% of GPs using it in their practice at least once a week. Diabetes mellitus and hypertension emerged as the most common clinical conditions prompting NRF assessments, aligning with established risk factors for CKD. The high agreement among GPs (96%) on the utility of the NRF for identifying CKD cases highlights its effectiveness as a diagnostic aid. The NRF also streamlined the referral process, improving connectivity between GPs and nephrologists/internists. This was evidenced by increased patient referrals, with 83% of GPs reporting an increase compared to the prior 6 months. Feedback from GPs emphasized the need for more nephrology referral centers, regional/national nephrology networks, enhanced GP training, and increased healthcare collaboration. These suggestions support the goal of establishing a comprehensive, multidisciplinary approach to CKD management (19,20).
Conclusion
The results of the questionnaire on the use of the NRF in the primary care setting to improve the care pathway of the patient with anemia or other complications related to CKD showed promising results. The NRF, developed by a SC, aimed to empower GPs as frontline healthcare providers in the early identification and management of CKD. The NRF trial was conducted in two pilot Italian regions, involving 24 GPs who used it for 6 months. The results demonstrated a noteworthy frequency of NRF utilization. Importantly, a significant percentage of GPs expressed agreement on the utility of the NRF in identifying and diagnosing cases of CKD, demonstrating its effectiveness as a diagnostic aid. The NRF facilitated a streamlined referral process, connecting GPs with nephrologists and internists more efficiently. This was reflected in the observed increase in patient referrals to specialists, with 83% of GPs reporting a rise compared to the 6 months prior to NRF implementation. In light of these positive outcomes, the NRF emerges as a promising tool in the primary care setting for managing anemia or other complications related to CKD. The encouraging findings from the survey highlight the NRF’s potential to address gaps in early CKD diagnosis and facilitate timely interventions and enhanced multidisciplinary care. Future research and broader adoption of the NRF are necessary to validate its effectiveness on a larger scale and ultimately improve patient outcomes in CKD management.
Acknowledgments
All authors contributed equally to the study. Answers to questionnaire questions not included in the article can be requested from the corresponding author.
Disclosures
Conflict of interest: All authors have no conflicts of interest to declare.
Financial support: This study was conducted with ISHEO’s own resources.
References
- 1. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825-830. CrossRef PubMed
- 2. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013 Jul 20;382(9888):260-272. CrossRef PubMed
- 3. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020 Feb 29;395(10225):709-733. CrossRef PubMed
- 4. Ministero della Salute. Malattia renale cronica. Data di pubblicazione: 23 ottobre 2020, ultimo aggiornamento 9 novembre 2020. Malattia renale cronica (salute.gov.it) Accessed March 2024.
- 5. Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35(2):329-344, vii. CrossRef PubMed
- 6. Tangri N, Moriyama T, Schneider MP, et al. Prevalence of undiagnosed stage 3 chronic kidney disease in France, Germany, Italy, Japan and the USA: results from the multinational observational REVEAL-CKD study. BMJ Open. 2023;13(5):e067386. CrossRef PubMed
- 7. Locatelli F, Del Vecchio L. Quality of life: a crucial aspect for the patients, a neglected goal in the treatment of anemia in patients with CKD. Kidney Int. 2023 Jun;103(6):1025-1027. CrossRef PubMed
- 8. KDIGO Anemia Working Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012;2:279-335.
- 9. Levin A, Stevens P, Bilous RW, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150.
- 10. Elendu C, Elendu RC, Enyong JM, et al. Comprehensive review of current management guidelines of chronic kidney disease. Medicine (Baltimore). 2023;102(23):e33984. CrossRef PubMed
- 11. Haley WE, Beckrich AL, Sayre J, et al. Improving care coordination between nephrology and primary care: a quality improvement initiative using the renal physicians association toolkit. Am J Kidney Dis. 2015;65(1):67-79. https://doi.org/10.1053/j.ajkd.2014.06.031
- 12. Sperati CJ, Soman S, Agrawal V, et al; National Kidney Foundation Education Committee. Primary care physicians’ perceptions of barriers and facilitators to management of chronic kidney disease: A mixed methods study. PLoS One. 2019;14(8):e0221325. CrossRef PubMed
- 13. Greer RC, Liu Y, Cavanaugh K, et al. Primary care physicians’ perceived barriers to nephrology referral and co-management of patients with CKD: a qualitative study. J Gen Intern Med. 2019;34(7):1228-1235.
- 14. Bailey PK, Hole BD, Plumb LA, Caskey FJ. Mixed-methods research in nephrology. Kidney Int. 2022;101(5):895-905. CrossRef
- 15. Palinkas LA, Mendon SJ, Hamilton AB. Innovations in mixed methods evaluations. Annu Rev Public Health. 2019;40:423-442. CrossRef
- 16. Nassar-McMillan S, Borders L. Use of focus groups in survey item development. Qual Rep. 2015;7(1):1-12. CrossRef
- 17. Campbell GA, Bolton WK. Referral and comanagement of the patient with CKD. Adv Chronic Kidney Dis. 2011;18(6):420-427. CrossRef PubMed
- 18. Levin A, Okpechi IG, Caskey FJ, et al. Perspectives on early detection of chronic kidney disease: the facts, the questions, and a proposed framework for 2023 and beyond. Kidney Int. 2023;103(6):1004-1008. CrossRef
- 19. Ravani P, Marinangeli G, Tancredi M, Malberti F. Multidisciplinary chronic kidney disease management improves survival on dialysis. J Nephrol. 2003;16(6):870-877. PubMed
- 20. Terlizzi V, Sandrini M, Vizzardi V, et al. Ten-year experience of an outpatient clinic for CKD-5 patients with multidisciplinary team and educational support. Int Urol Nephrol. 2022;54(4):949-957. CrossRef PubMed