 |
AboutOpen | 2023; 10: 110-118 ISSN 2465-2628 | DOI: 10.33393/ao.2023.2630 REVIEW |
 |
How to manage symptoms in pediatric cancer patients enrolled in clinical trials? A review of principal patient-reported outcome screening tools
ABSTRACT
Background: In adult oncology, the practice of tracking symptoms and toxicities using patient-reported outcomes (PROs) has increased and correlates with increased survival. In contrast, symptom monitoring using PROs is not common in pediatric oncology. Only in the last couple of years attention has also been paid to the patient’s perception in pediatrics and listening to the voice of children and to making them participate in the treatment.
Methods: A comprehensive literature search was conducted in MEDLINE/PubMed and PsycINFO to identify relevant articles published through December 2022.
Results: From 58 non-duplicate articles, 33 met our eligibility criteria. Of these, 17 were used in clinical trials.
Conclusions: The dissemination and use of these tools will therefore have surprising repercussions on the control of pain and physical symptoms of small patients as well as on physical and psychological aspects. The administration and use of the PROs ensures optimal use of the drugs currently present in clinical trials by researcher and nurse and aims at a safer and more controlled approval of new drugs.
Keywords: Cancer symptoms, Childhood cancers, Outcome screening tools, Patient-reported outcomes, Pediatrics
Received: July 5, 2023
Accepted: September 19, 2023
Published online: October 6, 2023
AboutOpen - ISSN 2465-2628 - www.aboutscience.eu/aboutopen
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Although childhood cancers are considered rare, the latest epidemiological data indicate an increase in the frequency of childhood tumors by 2% per year (1). In Italy, the incidence of tumor is 164 cases per million/year in the 0–14 age group and 269 cases per million/year in the 15–19 age group (2).
To date, despite the increase in frequency, more than 80% of children with cancer survive for at least 5 years after diagnosis, the majority of which can therefore be considered cured (3). For children and adolescents, both the cancer and the treatment generate early and long-term symptoms and adverse events (4). Treatments often put patients at risk of developing toxicity and side effects, reasons why attention must be paid to their physical and psychological state during cancer treatment. For example, most of them suffer from symptoms that are not always easily ascertained such as treatment-related fatigue, pain, nausea, cough, lack of appetite, and psychological deterioration (5). Very often children underreport their symptom severity to avoid complaining or “bothering” the physician with a symptom perceived as inherent in the treatment, or in an effort to protect their family from worries (6). Children appear very fearful and frightened during treatment and for this reason they often do not express what they feel to their parents or to the medical and nursing staff. It can happen that the voice and the needs of children are mediated by those of the parent, solicited by the doctor’s perception or ignored. In fact, some research shows that reports from doctors, nurse, or parents may not exactly reflect opinions from the child’s perspective (7).
This risk is to create barriers that do not allow children to be given a voice and these barriers can, in part, be surmounted by the use of patient-reported outcomes (PROs) that allow patients and their caregivers to feel more in control in managing their health and support clinicians in improving outcomes of care (8,9).
In adult oncology, the practice of tracking symptoms and toxicities using PROs has increased and correlates with increased survival (8). A PRO is a direct report of a patient’s condition, not interpreted or modified from a clinician. PROs are now considered the gold standard for the assessment of subjective symptoms, both in clinical practice and clinical trials so that in recent years the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have underlined the importance of PROs, also in the process of producing the evidence that leads to the approval of a treatment for use in clinical practice (10), whereas symptom monitoring using PROs is not common in pediatric oncology (11).
Only in the last couple of years has attention also been paid to the patient’s perception in pediatrics and listening to the voice of children and to making them participate in the treatment.
There is evidence that good symptom management decreases treatment-related complications and increases quality of life (4). Some study groups are committed to creating ad hoc tools for the child that are easy to understand and provide feedback to the clinicians to improve care (12,13). Greater attention must be paid to the group of patients who, due to the complexity of the disease, are enrolled in clinical trials.
Over the past 40 years pediatric oncology in Europe has made considerable progresses in increasing patient survival rates up from 10% to 80%. This was only achievable through close collaboration in multinational clinical trials (14). To date the number of children enrolled in experimental studies and clinical trials is increasing: each year more than 60% are enrolled in clinical trials (4).
Today, drug development in pediatric oncology focuses on improving survival for those patients with largely incurable tumors and attempting to use novel agents as an alternative to standard of care, thus improving the quality of survivorship (15). Patients enrolled in clinical trials are therefore often exposed to severe and demanding treatments from a physical and psychological point of view and for this reason it is desirable to offer them easy ways to express their feelings about the therapy. It is necessary to propose easily understandable detection tools that investigate not only the presence but also the frequency and severity of symptoms, in particular those not directly observable.
In this review we want to investigate the PROs used in pediatric oncology to measure self-reported symptoms in children and adolescents undergoing cancer treatment. The outcomes of interest for this narrative review include the PROs most used by clinicians and researchers and the PROs usually proposed in pediatric oncology clinical trials.
Methods
Literature search strategy
A comprehensive literature search was conducted in MEDLINE/PubMed and PsycINFO to identify relevant articles published through December 2022. Keyword searches focused on terms used to describe self-reported symptom measurements in children and adolescents with cancer, such as “child,” “adolescents,” “pediatric oncology,” “cancer children,” “patient-reported outcome,” and “measurement.” We found 58 articles.
A first screening was done based on the title, then on the abstract, and finally on the text of each eligible article. We used prespecified inclusion and exclusion criteria and two reviewers carefully screened and selected articles on the basis of eligibility criteria.
Inclusion and exclusion criteria
Criteria for inclusion of studies regarding PROs were: (1) English language; (2) only empirical studies with children and adolescent self-report validated instruments; (3) focused on measuring physical or psychological symptoms during treatment; (4) recruiting in clinical trial in pediatric oncology.
Exclusion criteria: (1) all survey instruments or interviews; (2) tool used for pediatric cancer survivors; (3) caregivers’ self-reported tool; (4) instrument used in adult oncology.
Two members of the study team evaluated each eligible article, inclusion/exclusion criteria, participant characteristics (sample, mean age), and reliability of each measure. Data collected included instrument names in alphabetical order, age range, symptoms investigated, description of tool, and reference period.
Data abstraction: use of instrument in clinical trial
We were interested in which PROs were currently being used in oncology clinical trials. We checked in Clinicaltrial.gov database using the following criteria: (1) instrument name (or abbreviated name); (2) field of study “pediatric cancer.” We have identified the tools that have been used in clinical trials. Findings are presented in Table I.
| Instrument name (alphabetical order) | Age range (years) | Symptoms | Description | Reference period | Trial |
|---|---|---|---|---|---|
| Adolescent Pediatric Pain Tool (APPT) | 8–17 | Pain intensity, location, and quality | A multidimensional self-administered pain assessment tool for children and adolescents experiencing pain for various reasons, such as sickle cell disease (SCD), postoperative pain, allergy testing, orthopedic, traumatic injury, and cancer | Present | |
| Behavioral, Affective, and Somatic Experiences Scale (BASES) | 5–18 | Somatic distress | A 38-item nurse/children/parents-reported instrument, with five subscales labeled Somatic Distress, Compliance, Mood/Behavior, Interactions, and Activity | At baseline (before/during conditioning); 7 days after the stem cell infusion (day 17); 21 days after the stem cell infusion (day 121) |
|
| Cancer Need Questionnaire Young People (CNQ-YP) | 12–24 | Need of young people | A 70-item and 6-factor tool: Treatment Environment and Care (33 items); Feelings and Relationships (14 items); Daily Life (12 items); Information and Activities (5 items); Education (3 items); and Work (3 items), to assess the unmet needs of Adolescent and Young Adult (AYAs) | ||
| Cancer and Treatment Distress (CTXD) | Distress | Measures cancer-related distress over the past week | “Past week” | ||
| Distress Thermometer (DT) | 9–18 | Distress | Measures the level of distress | “Past week including today” | Find in clinicaltrial.gov |
| European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORT QLQ-C30) | 12–17 | Health status | Thirty self-report questions assessing the health-related quality of life (HRQoL) of cancer patients participating in international clinical trials | Present | Find in clinicaltrial.gov |
| Edmonton Symptom Assessment System (ESAS) | 8–18 | Pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath | Nine‐item patient‐rated symptom Visual Analog Scale developed for use in assessing the symptoms of patients receiving palliative care | “Over the past 24 hours” | |
| Functional Assessment of Cancer Therapy—General (FACT-G) | 18+ | Symptoms of cancer | A 27-item questionnaire designed to measure four domains of HRQoL in cancer patients: physical, social, emotional, and functional well-being | “Past 7 days” | |
| Global Impressions of Change (GIC) | 7–18 | Pain, fatigue, anxiety, depression, stress, and mobility | Questions asked about overall health and changes in their health compared to the last time you completed this survey | “Last time” | Find in clinicaltrial.gov |
| KLIK PROfile | 8–18 | Mobility, scared, worry, angry, sleep, attention, concentration | The KLIK (Dutch acronym for Quality of Life in Clinical Practice) method is an online system (www.hetklikt.nu) to enable routine monitoring and discussion of electronic patient-reported outcomes (ePROs) for children with a chronic disease. Physical, emotional, social, and school areas are investigated. | Present | |
| Memorial Symptom Assessment Scale (MSAS) |
7–12 10–18 |
Fatigue, sadness, itch, pain, worry, anorexia, nausea, insomnia Concentration, cough, dry mouth, nausea, worry, neuropathy, insomnia, diarrhea, sadness, vomiting, problems with urination, nervousness, difficulty swallowing, anorexia, hair loss, weight change, dizziness, headache, sweating, irritability, dyspnea, mucositis, constipation, lymphedema |
The MSAS 7–12 assesses eight symptoms with eight questions plus conditional questions for reported symptoms. The MSAS 10–18 assesses 31 symptoms with 31 questions plus additional conditional questions for reported symptoms. | “Past week” | Find in clinicaltrial.gov |
| Miami Pediatric Quality of Life Questionnaire (MPQOLQ) | 7+ | Mobility, angry, worry, nausea, nightmares, attention, concentration | Evaluating HRQoL issues in children treated for cancer. Social, emotional, and self-competence | Present and across 1-month intervals | |
| Oral Mucositis Daily (OMDQ) | 12–18 | Mouth and throat soreness (MTS), diarrhea, overall health | 10 items that assess the severity and impact of oral mucositis by evaluating mouth and throat soreness and the degree to which MTS interferes with activities of daily life such as eating, swallowing, drinking, talking, and sleeping | Past 24 hours | |
| Pediatric Quality of Life and Evaluation of Symptoms Technology (PEDIQUEST) | 2+ | Child distress | Longitudinal self-report study that relayed information to the treatment team for clinical decision-making | Present Once a week and the least once a month |
Find in clinicaltrial.gov |
| Pediatric Quality of Life Inventory (PEDSQL) | 5–7 8–12 13–18 18–25 |
Physical, emotional, and social functioning School functioning (e.g., concentration problems) |
Modular approach to measuring HRQoL in healthy children and adolescents and those with acute and chronic health conditions | “Past 7 days” “Past 1 month” |
Find in clinicaltrial.gov |
| Pediatric Fatigue Short Form measures (PROMIS Fatigue) | 8–17 | Fatigue | To capture cancer-related fatigue change in pediatric patients with cancer | At three time points during chemotherapy | Find in clinicaltrial.gov |
| Pediatric Nausea Assessment Tool (PeNAT) | 4–18 | Nausea | Assess symptoms of nausea | “Right now” | Find in clinicaltrial.gov |
| Pediatric Quality of Life (PED QOL) | 5–7 8–12 13–18 |
Physical, social, and emotional functioning | A brief measure of HRQoL in children and young people | Present | Find in clinicaltrial.gov |
| Play-Performance Scale (PPS) | <16 | Disease progression | Caregivers completed 10 questions about medications their child had taken in “the past 7 days” for nausea, insomnia, constipation, diarrhea, mucositis, neuropathy, headache, depression, anxiety, and pain | Present | |
| Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE) | 7–15 | Adverse event: abdominal pain, anorexia, anxiety, constipation, cough, depression, diarrhea, fatigue, headache, insomnia, mucositis oral, nausea, pain, neuropathy, vomiting | Assess symptomatic adverse events via child self- or proxy-report | Past 7 days | Find in clinicaltrial.gov |
| Patient-Reported Outcomes Measurement Information System (PROMIS) | 8–17 | Depression, anxiety, anger, pain, interference, fatigue, mobility, upper extremity functioning | Testing measures of physical function mobility, pain interference, fatigue, depressive symptoms, and anxiety | “Past 7 days” | Find in clinicaltrial.gov |
| Quality of Life in Childhood Oncology (QLIC-ON) | 8–12 13–18 |
Quality of life, physical, emotional problems, social and school functioning | Tracked four HRQoL domains in children who had completed treatment for their cancer | “In the past one week…” | |
| Quality of Life for Children with Cancer (QOLCC) | 7–12 13–18 |
Physical, psychological, social, disease, cognitive, communication, understanding | To assess the quality of life for children and adolescents who suffer from cancer | Present | Find in clinicaltrial.gov |
| Royal Marsden Hospital Pediatric Oncology Quality of Life Questionnaire (RMH-PQLQ) | 7–16 | Physical symptoms | A parent-reported measure that assesses HRQoL in children undergoing treatment for cancer as well as children who have completed treatment | “Baseline” and “Follow-up” | |
| Symptom Distress Scale (SDS) | 10–17 | Sadness, worry, anger, fatigue, mouth sores, pain, fatigue, bowel discomfort, concentration, dyspnea, cough | SDS is a 10-item instrument (appearance, mobility, tiredness, sleep, mood, pain, appetite, nausea, bowel pattern, and ability to concentrate) based on their level of distress today. | “Lately” | Find in clinicaltrial.gov |
| Symptom Screening in Pediatrics (SSPedi) | 8–18 | Sadness, worry, anger, fatigue, mouth sores, pain, headache, neuropathy, anorexia, vomiting, nausea, diarrhea, constipation, concentration, memory, dysgeusia | Symptom screening in pediatric oncology patients. Includes 15 items. Captures symptom interference (“bother”) without symptom prevalence or severity | “Yesterday” or “today” | Find in clinicaltrial.gov |
| State-Trait Anxiety Inventory (STAI) | 9–12 | Anxiety, apprehension, tension, worry | To measure anxiety | “Feelings now, at this moment” | Find in clinicaltrial.gov |
| Visual Analog Scale (VAS) | 9–18 | Anxiety, nausea | To measure anxiety and nausea | “Right now” | Find in clinicaltrial.gov |
| Wong Baker Face Pain Rating Scale | 3+ | Pain | A self-assessment tool pain scale for children | “Right now” | Find in clinicaltrial.gov |
Results
Literature search results
A PRISMA flow diagram was generated (Fig. 1) outlining the number of records identified, included, and excluded and the selection of articles through the different phases of this narrative review. A total of 63 articles were identified through database searching, of which 58 were non-duplicates.
A total of 58 abstracts were screened and 20 articles were excluded during the initial abstract screening phase, leaving us with 38 articles eligible for full-text review. An additional 5 articles were excluded during the full-text review phase, leaving 33 articles that met all eligibility criteria for inclusion in this study. In these 33 articles on PROs, we found 29 tools that met our eligibility criteria and after research we found 17 of them in clinicaltrial.gov.
Study selection
Two members of the research team independently screened all titles. After a first screening a comparison was made between the two members of the team. At a later time, the two team members read the abstracts separately and found an agreement using inclusion and exclusion criteria described earlier. The members of the team screened the initial 63 abstracts. Studies with titles and abstracts that met the inclusion criteria or lacked adequate information to determine inclusion or exclusion underwent full-text review. The two members of the research team reached an agreement.
Then, the two trained members of the research team independently reviewed each full-text article for inclusion or exclusion based on eligibility criteria described earlier. If both reviewers agreed that a study did not meet eligibility criteria, the study was excluded. If they disagreed, a third member of the team, expert in pediatric oncology and clinical trial, helped them seek an agreement.
Table I presents characteristics of the 29 self-reported English symptom instruments used in children and adolescents undergoing cancer treatment that met our eligibility criteria. Table I also includes a summary of instruments’ age range, types of symptoms assessed, recall period, and their use in clinicaltrial.gov.
Pediatric self-report instrument characteristics
Instruments’ age range are included between 2 and 25 years. Some tools are symptom specific, while others include a range of symptoms experienced by children and adolescents undergoing cancer treatment. The most commonly assessed symptoms were pain, distress, anxiety, depression, fatigue, mobility, concentration, and nausea.
Most instruments used a version of the Likert scale, a psychometric scale named after its inventor, American social psychologist Rensis Likert, which is commonly used in research questionnaires. From 29 pediatric report outcome screening tools, 17 have been used in studies that are registered in clinicaltrial.gov.
Instruments used in clinical trials
Table I includes instruments that have been used in studies that are registered in clinicaltrial.gov. A total of 17 instruments were identified in this search. Distress Thermometer Scale (16), Memorial Symptom Assessment Scale (MSAS) (17–20), Pediatric Quality of Life Inventory (PEDSQL) (18), Pediatric Quality of Life (PED QOL) (21), Patient-Reported Outcomes Measurement Information System (PROMIS) (16,19,22–26), Quality of Life for Children with Cancer (QOLCC) (27), Symptom Distress Scale (SDS) (24,26), Symptom Screening in Pediatrics (SSPedi) (9), and Visual Analog Scale (VAS) (27) were the instruments most often cited in clinicaltrial.gov.
Discussion
Our narrative review highlighted the most used PROs in pediatric oncology setting, by identifying 29 self-report instruments. The tools identified are aimed at giving voice to both the psychological and physical implications experienced by children and adolescents during cancer treatment.
Most of the tools are used for both aspects: Behavioral, Affective and Somatic Experiences Scale (BASES); Cancer Need Questionnaire Young People (CNQ-YP); Edmonton Symptoms Assessment System (ESAS); Functional Assessment of Cancer Therapy-General (FACT-G); Global Impression of Change (GIC); KLIK Profile; Memorial Symptom Assessment Scale (MSAS); Miami Pediatric Quality of Life Questionnaire (MPQOLQ); Play-Performance Scale (PPS); Quality of Life in Childhood Oncology (QLIC-ON); Pediatric Quality of Life Inventory (PEDSQL); Pediatric Quality of Life (PED QOL); Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE); Patient-Reported Outcomes Measurement Information System (PROMIS); Quality of Life for Children with Cancer (QOLCC); Symptom Distress Scale (SDS); Symptom Screening in Pediatrics (SSPedi); Visual Analog Scale (VAS).
Only six tools assess exclusively physical symptoms: Adolescent Pediatric Pain Tool (APPT); European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QOL-C30); Oral Mucositis Daily (OMDQ); Pediatric Nausea Assessment Tool (PeNAT); Royal Marsden hospital Pediatric Oncology Quality of Life Questionnaire (RMH-PQLQ); Wong Baker Face Pain Rating Scale.
Five of them assess only psychological implications: Cancer and Treatment Distress (CTXD); Distress Thermometer (DT); Pediatric Quality of Life and Evaluation of Symptoms Technology (PEDIQUEST); Pediatric Fatigue Short Form measures (PROMIS); State-Trait Anxiety Inventory (STAI).
Of these 29 instruments, we have identified 17 which are used in clinical trials in pediatric oncology and which therefore investigate the symptoms of children or adolescents who are following a clinical trial treatment protocol. Of these 17, most of the tools investigate both psychological and physical aspects. This is an important feature that completed the evaluation of all the aspects involved in pediatric oncology field according to a biopsychosocial way.
Increasing attention to drug development for children with cancer by regulators and pharmaceutical companies holds the promise of accelerating the availability of new therapies especially for children with resistant or relapsing cancer, potentially improving survival and decreasing the acute and chronic toxicities of therapy (15).
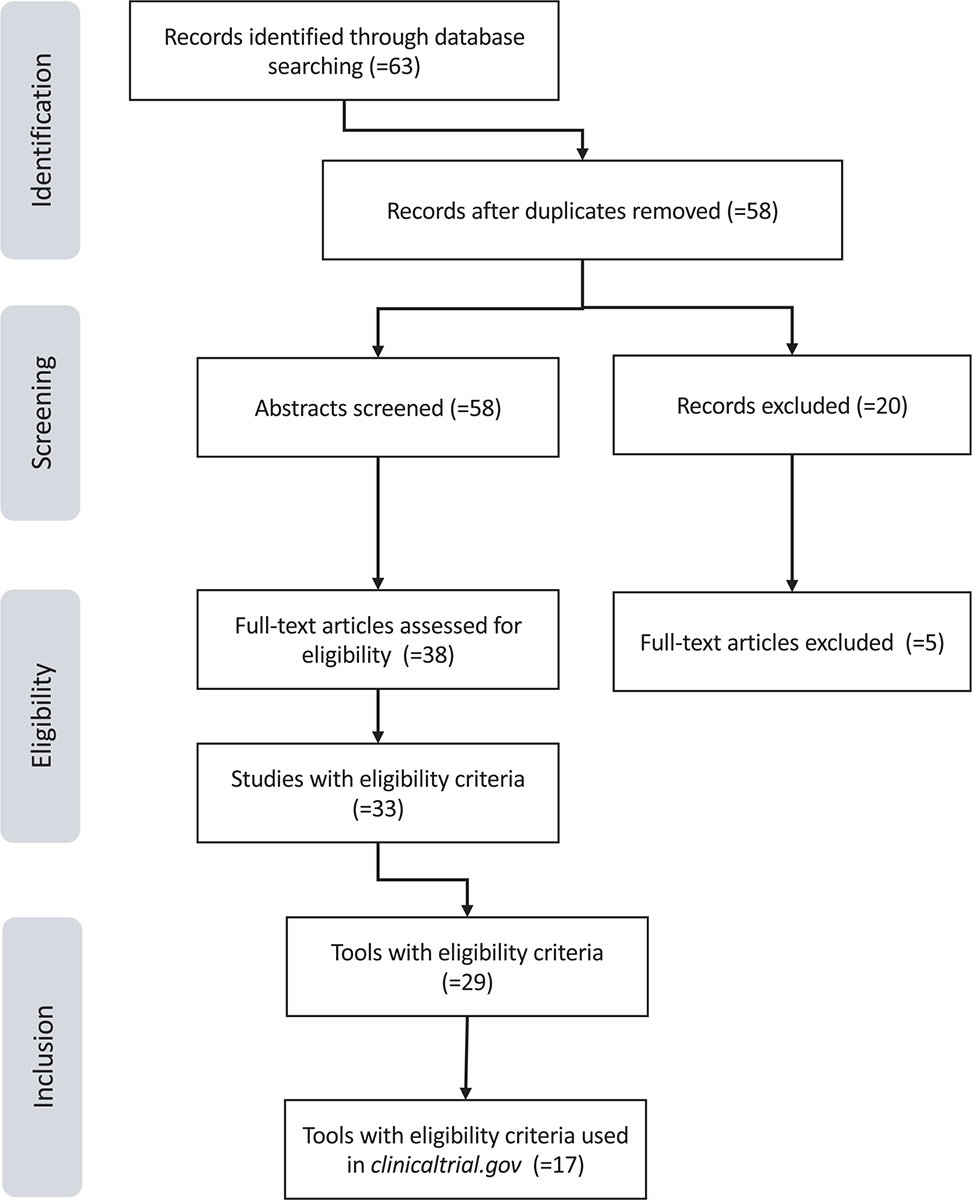
Fig. 1 - PRISMA flow diagram of literature search results.
As novel therapies move to the front-line setting, it will be important to develop mechanisms to evaluate the potential adverse effects of these agents.
The US FDA has encouraged the use of PROs in cancer registration trials since symptomatic adverse events in pediatric cancer registration trials have traditionally been collected using clinician-reported Common Terminology Criteria for Adverse Events (CTCAE) and have not been complemented with patients’ self-report (28).
This narrative review aids pediatric oncology researchers and nursing in choosing the correct tool to measure the prevalence and the severity of symptomatology self-reported by patients during a cancer clinical treatment or trials in order to offer a better symptom management. What is also confirmed by our study is that in order to have complete information the best approach consists in the use of tools that investigate both physical and psychological aspects or that foresee the integration of the two.
Our review investigated many different tools, and thanks to the research we were able to demonstrate over time which aspects may be the most relevant to investigate. It would be useful to be able to standardize them to obtain a single clinical trial tool in pediatric oncology. Considering clinical trials, to date, it is known that the spread of the Ped-PRO-CTCAE recently developed and validated by the National Cancer Institute in several European languages offers a novel opportunity to elevate the child’s voice in drug development to inform the incidence and impact of symptomatic side effects in pediatric oncology patients.
Nonetheless, the literature on PROs goes very fast; therefore, a limit of this study is that it could be liable to bias or oversights. Those presented are certainly the main ones to our knowledge and research. Moreover, more attention should be paid to children in the 2–6 age range that is less represented. That is probably due to the fact that the competences are less developed to give an effective report about symptoms. Futures studies should create new tools appropriated to this age range.
Future objectives concern the possibility of integrating these tools in clinical practice in pediatric oncology and perhaps making them become an integral part of clinical research protocols over time with their data recorded in the medical records.
On some of these aspects there is still no uniformity, therefore it will be necessary to continue to raise awareness about the use of PROs in the pediatric oncology community.
Conclusion
We now have the knowledge to make the child’s voice an integral and fundamental part of clinical work. The dissemination and use of these tools will therefore have surprising repercussions on the control of pain and physical symptoms of small patients as well as on physical and psychological aspects.
The administration and use of the PROs ensures optimal use of the drugs currently present in clinical trials by researcher and nurse and aims at a safer and more controlled approval of new drugs.
Acknowledgments
We are grateful to UNIONE GENITORI ITALIANI – UGI and ADISCO Sezione Regionale Piemonte to sustain our project.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors’ contribution: All authors contributed equally to this manuscript.
Data availability statement: The data presented in this study are available on request from the corresponding author.
References
- 1. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. CrossRef PubMed
- 2. AIRTUM Working Group. I numeri del cancro in Italia 2013. Intermedia Editore; 2014. Online Accessed June 2023.
- 3. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Online. Accessed June 2023.
- 4. Ullrich CK, Billett AL, Berde CB. Symptom Management in Children with Cancer. In: Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE, editors. Nathan and Oski’s Hematology of Infancy and Childhood. Elsevier Health Sciences; 2009. pp. 2349–96. CrossRef
- 5. Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage. 2000;19(5):363–377. CrossRef PubMed
- 6. Leahy AB, Feudtner C, Basch E. Symptom monitoring in pediatric oncology using patient-reported outcomes: why, how, and where next. Patient. 2018;11(2):147–153. CrossRef PubMed
- 7. Schepers SA, Haverman L, Zadeh S, Grootenhuis MA, Wiener L. Healthcare professionals’ preferences and perceived barriers for routine assessment of patient-reported outcomes in pediatric oncology practice: moving toward international processes of change. Pediatr Blood Cancer. 2016;63(12):2181–2188. CrossRef PubMed
- 8. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. CrossRef PubMed
- 9. Leahy AB, Steineck A. Patient-reported outcomes in pediatric oncology: the patient voice as a gold standard. JAMA Pediatr. 2020;174(11):e202868. CrossRef PubMed
- 10. 10. Di Maio M. [Outcomes reported by the patient: a tool for respecting the patient’s rights and for involvement in research]. Recenti Prog Med. 2020 Nov;111(11):685–689. [Article in Italian]. CrossRef. Accessed June 2023.
- 11. Pinheiro LC, McFatrich M, Lucas N, et al. Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review. Qual Life Res. 2018;27(2):291–319. CrossRef PubMed
- 12. OʼSullivan C, Dupuis LL, Sung L. A review of symptom screening tools in pediatric cancer patients. Curr Opin Oncol. 2015;27(4):285–290. CrossRef PubMed
- 13. Cheng L, Wang L, He M, Feng S, Zhu Y, Rodgers C. Perspectives of children, family caregivers, and health professionals about pediatric oncology symptoms: a systematic review. Support Care Cancer. 2018;26(9):2957–2971. CrossRef PubMed
- 14. The European Society for Paediatric Oncology. SIOP Europe. Online. Accessed June 2023.
- 15. Laetsch TW, DuBois SG, Bender JG, Macy ME, Moreno L. Opportunities and challenges in drug development for pediatric cancers. Cancer Discov. 2021;11(3):545–559. CrossRef PubMed
- 16. Wiener L, Devine KA, Thompson AL. Advances in pediatric psychooncology. Curr Opin Pediatr. 2020;32(1):41–47. CrossRef PubMed
- 17. Reeve BB, McFatrich M, Mack JW, et al. Validity and reliability of the pediatric patient-reported outcomes version of the common terminology criteria for adverse events. J Natl Cancer Inst. 2020;112(11):1143–1152. CrossRef PubMed
- 18. Ilowite MF, Al-Sayegh H, Ma C, et al. The relationship between household income and patient-reported symptom distress and quality of life in children with advanced cancer: a report from the PediQUEST study. Cancer. 2018;124(19):3934–3941. CrossRef PubMed
- 19. Reeve BB, McFatrich M, Mack JW, et al. Expanding construct validity of established and new PROMIS pediatric measures for children and adolescents receiving cancer treatment. Pediatr Blood Cancer. 2020;67(4):e28160. CrossRef PubMed
- 20. Madden K, Magno Charone M, Mills S, et al. Systematic symptom reporting by pediatric palliative care patients with cancer: a preliminary report. J Palliat Med. 2019;22(8):894–901. CrossRef PubMed
- 21. Dussel V, Orellana L, Soto N, et al. Feasibility of conducting a palliative care randomized controlled trial in children with advanced cancer: assessment of the PediQUEST study. J Pain Symptom Manage. 2015;49(6):1059–1069. CrossRef PubMed
- 22. Macpherson CF, Wang J, DeWalt DA, Stern ED, Jacobs S, Hinds PS. Comparison of legacy fatigue measures with the PROMIS pediatric fatigue short form. Oncol Nurs Forum. 2018;45(1):106–114. CrossRef PubMed
- 23. Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. 2014;61(7):1282–1288. CrossRef PubMed
- 24. Wang J, Jacobs S, Dewalt DA, Stern E, Gross H, Hinds PS. A longitudinal study of PROMIS pediatric symptom clusters in children undergoing chemotherapy. J Pain Symptom Manage. 2018;55(2):359–367. CrossRef PubMed
- 25. Hinds PS, Wang J, Stern ED, et al. Voices of children and adolescents on phase 1 or phase 2 cancer trials: A new trial endpoint? Cancer. 2017;123(19):3799–3806. CrossRef PubMed
- 26. Hinds PS, Wang J, Cheng YI, et al. PROMIS pediatric measures validated in a longitudinal study design in pediatric oncology. Pediatr Blood Cancer. 2019;66(5):e27606. CrossRef PubMed
- 27. Janic A, Bowden S, Levy S, Stinson J, Dimaras H. Patient-reported outcome measures for retinoblastoma: a scoping review. J Patient Rep Outcomes. 2020;4(1):66. CrossRef PubMed
- 28. Murugappan MN, King-Kallimanis BL, Reaman GH, et al. Patient-reported outcomes in pediatric cancer registration trials: a US Food and Drug Administration perspective. J Natl Cancer Inst. 2022 Jan 11;114(1):12–19. CrossRef PubMed