 |
AboutOpen | 2023; 10: 64-68 ISSN 2465-2628 | DOI: 10.33393/ao.2023.2566 ORIGINAL RESEARCH ARTICLE |
 |
Clinical research: the great absentee of the Italian University training programs?
ABSTRACT
Background: In an era where clinical trials have become more and more complex and regulatory authorities impose very high quality standards, the education of clinical research professionals becomes crucial. As one of ICH-GCP guiding principles, adequate training should be ensured and included in educational programs.
Methods: In 2021, the Italian Group of Data Managers and Clinical Research Coordinators shared among professionals involved in clinical research an online survey aimed at investigating quality and characteristics of clinical research training provided during undergraduate and postgraduate Italian programs.
Results: The survey was completed by 280 professionals: 178 study coordinators, 29 clinical research associates, 20 project managers, 7 study nurses, and 44 others. The majority were 25-45 years old (n = 242, 86.4%), worked at experimental sites (n = 211, 75.4%), and almost all (n = 252, 90.0%) had at least a master’s degree, mainly in biology/biotechnology (n = 162, 57.9%) and pharmacy (n = 64, 22.9%). Clinical research education during the degree courses was considered poor by 73.6% (n = 206). The knowledge on clinical research professional world at the time of graduation was considered poor by 71.1% of participants (n = 199), like the knowledge of related career opportunities (71.1%, n = 199, poor). According to 85.0% of professionals (n = 238) additional postgraduate trainings were needed, mainly university master courses (47.50%, n = 133) and private institution courses (47.86%, n = 134). Postgraduate trainings were considered very useful by 71.4% (n = 200) of responders.
Conclusion: Our data suggest undergraduate programs on clinical research education failing at providing even the basic information on clinical research. Therefore, most professionals resort to specific additional postgraduate courses.
Received: January 28, 2023
Accepted: April 3, 2023
Published online: April 28, 2023
AboutOpen - ISSN 2465-2628 - www.aboutscience.eu/aboutopen
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Training of professionals and operators represents one of the critical and crucial points of clinical and translational research (1,2). In particular, training on Good Clinical Practice (GCP) and methodology of clinical research is fundamental to guarantee the quality of clinical trials and related processes (2,3,4), both for-profit and nonprofit spontaneous studies (5). Training is an important requirement as stated in ICH-GCPs, which require adequate and specific training both for personnel operating in clinical centers and professionals working on behalf of sponsors or ethics committees (3). These training requirements have evolved over time and have been implemented by drug regulatory agencies, including the Italian Agenzia Italiana del Farmaco (AIFA), with specific regulations such as the Ministerial Decree 15.11.2011, which establishes the minimum training requirements for the staff of clinical research organizations (CRO) operating in Italy (6), and AIFA Resolution 809/2015, which establishes the minimum training requirements for professionals involved in the conduct of Phase I clinical trials (7).
Moreover, among the deviations detected by Regulatory Authorities during site inspections, training is certainly a particularly affected area (8,9,10). Specific clinical research training should be therefore considered as an integral part of the basic training to be guaranteed to medical and scientific health personnel (11). Furthermore, it is extremely important that this training is not meant as a short online training but as a robust, well-planned, traceable, and documented course (4). In Italy, the growing need for adequate training in clinical research has sparked the urge, acknowledged among other things by the recently implemented legislation (12), for a complete review of university training programs, both for medical personnel and other healthcare professionals. In this context, the Gruppo Italiano Data Manager e Coordinatori di Ricerca Clinica (GIDMcrc) has decided to promote a survey among Italian clinical research professionals, to collect information regarding the training received during their academic career, their perceptions on quality and completeness of this training, and on areas needing implementation in the field of clinical research and related topics.
Materials and methods
During 2021, the GIDMcrc promoted and distributed an online questionnaire intended for clinical research operators in order to investigate their pre- and postgraduate training experience related to their professional field.
This questionnaire, set up on the Google Forms app, presented 12 multiple choice questions (multiple checkbox) or single choice (radio button), depending on the type of question.
After an initial design phase, the questionnaire was validated on a sample of 10 respondents, in order to test the correct functioning and the correct capture and extraction of data by the system. Once the validation phase was complete, the questionnaire was made available online from February 25 to March 27, 2021, and finally shared through the GIDMcrc web channels.
All data were collected anonymously and processed in aggregate form for the purposes stated in the questionnaire itself.
The answers were considered valid only if they came from professionals operating in the field of clinical research.
Given the type of survey and the characteristics of the channels used for the dissemination of the questionnaire, an a priori sample was not defined. However, considering possible future uses of the collected results, it was decided to proceed with the analysis only if the following criteria were met:
– at least 100 responses received;
– at least 3 different professional categories represented;
– fewer responses from data managers/clinical research coordinators compared to 80% of the total.
A copy of the questionnaire is available in Appendix 1.
Results
At the end of the survey, 280 responses were collected from professionals working in clinical research.
A total of 51.7% (n = 143) of the respondents were aged between 25 and 35 years, while 35.36% (n = 99) were in the 36–45 years range and 11.79% (n = 33) in the over-45 years range.
Clinical research coordinators are the most represented professional figure, with 63.57% of responses (n = 178), followed by clinical research associates (CRAs) or monitors with 10.36% (n = 29) and by project managers with 7.86% (n = 22). However, professional categories such as research nurses (2.50%, n = 7), clinical trial assistants (2.14%, n = 6), and data entry personnel (2.14%) are also represented, albeit in a smaller percentage (2.14%, n = 6).
Precisely 75.36% of respondents are employed in hospitals, university hospitals, or research institutes (IRCCS), 19.28% in pharmaceutical companies or contract research organizations (CROs), while the remaining 5.36% is represented by freelancers and staff belonging to foundations or scientific companies.
The most represented degrees were biology and biotechnology (57.86%, n = 162), followed by degrees in pharmacy/pharmaceutical chemistry and technologies (22.86%, n = 64), a small group of degrees in nonscientific stream (9.29%, n = 26), and degrees in health subjects other than those previously mentioned, such as nursing and medicine (8.93%, n = 25). Respondents with a qualification no lower than a master’s or single-cycle degree are 90% of the total (n = 252), while non-graduates represent a truly minority share (1.7% of the total, n = 3).
When asked “how do you rate the training provided in clinical research and related areas received during university studies,” 29.3% (n = 82) of respondents assigned a score of 0 on a scale of 0 to 10, and the cumulative percentage of those who assigned a score between 0 and 3 is equal to 73.57% (n = 206) (Fig. 1).
In terms of quantification of the level of knowledge on the world of clinical research at the end of the academic career, 71.07% of the answers (n = 199) recorded a score between 0 and 3 on a scale of 0 to 10, 27. 86% (n = 78) assigned a score between 4 and 7, while only 1.07% (n = 3) assigned a score greater than or equal to 8 (Fig. 2).
Similarly, 71.07% of respondents (n = 199) quantified their level of knowledge with a score between 0 and 3, again on a 0-10 scale, with respect to possible postgraduate career opportunities in clinical research and related professional figures (Fig. 3).
Eighty-five percent of respondents (n = 238) reported having completed their training in clinical research with supplementary training after graduation. Of these, 25.2% (n = 61) obtained a first-level university master’s and 29.8% (n = 72) a second-level university master’s. Training provided by private entities of various kinds was participated by 55.4% of the participants (n = 134), while 31.4% of professionals (n = 76) were able to take advantage of a training period alongside expert staff. About 30.3% of professionals (n = 73) also declared that they had benefited from self-training in clinical research or related topics (Fig. 4).
On a scale of 0 to 10, the training performed in the postgraduate period was rated both useful and enriching with a score between 8 and 10 by 71.3% of the respondents who resorted to it (n = 200), moderately useful by 12.86% (n = 36), assigning a score between 4 and 7, while only 2 people (0.71%) assigned a score between 0 and 3 (Fig. 5).
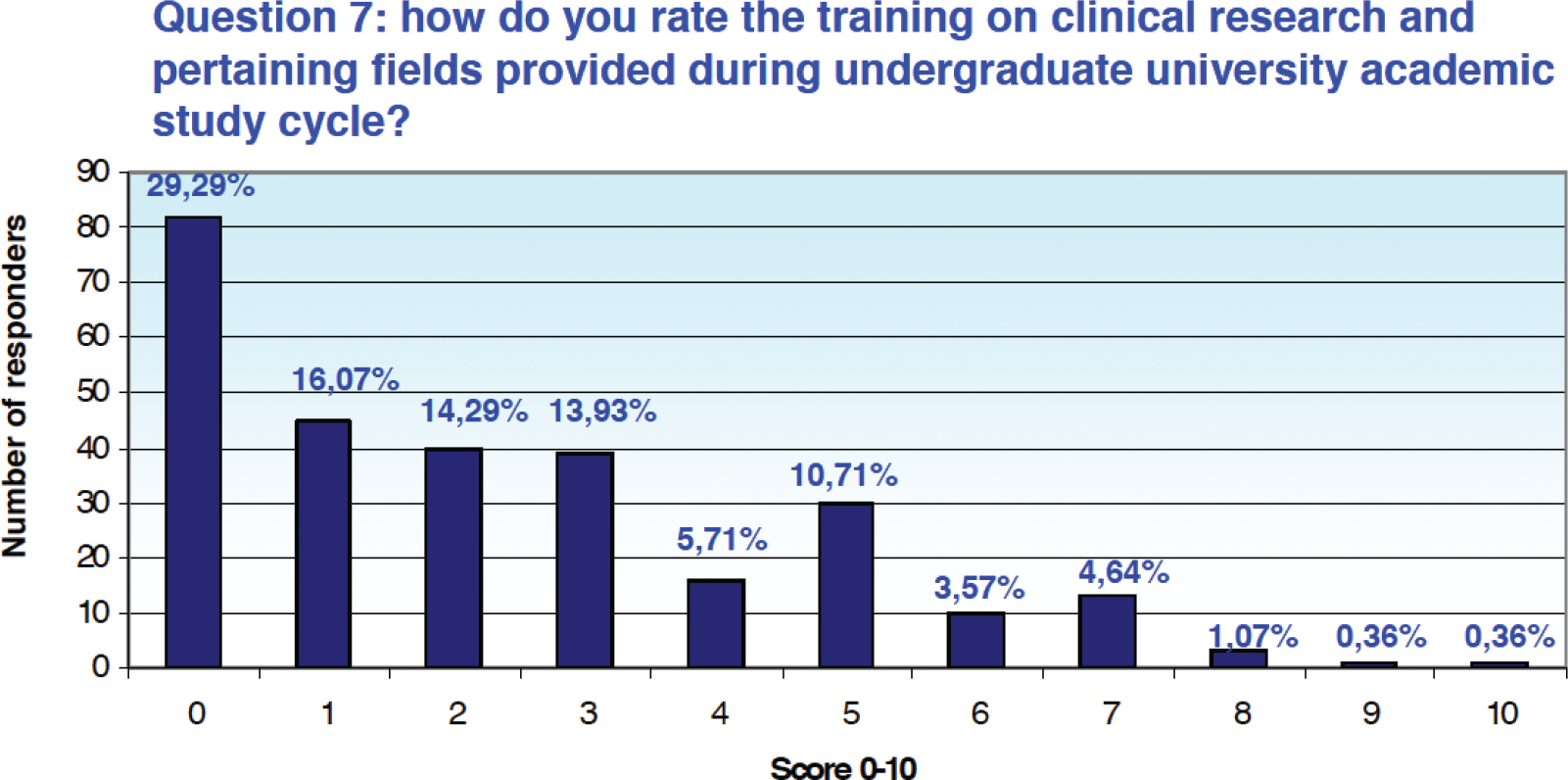
Fig. 1 - Results from Question 7 of the survey.
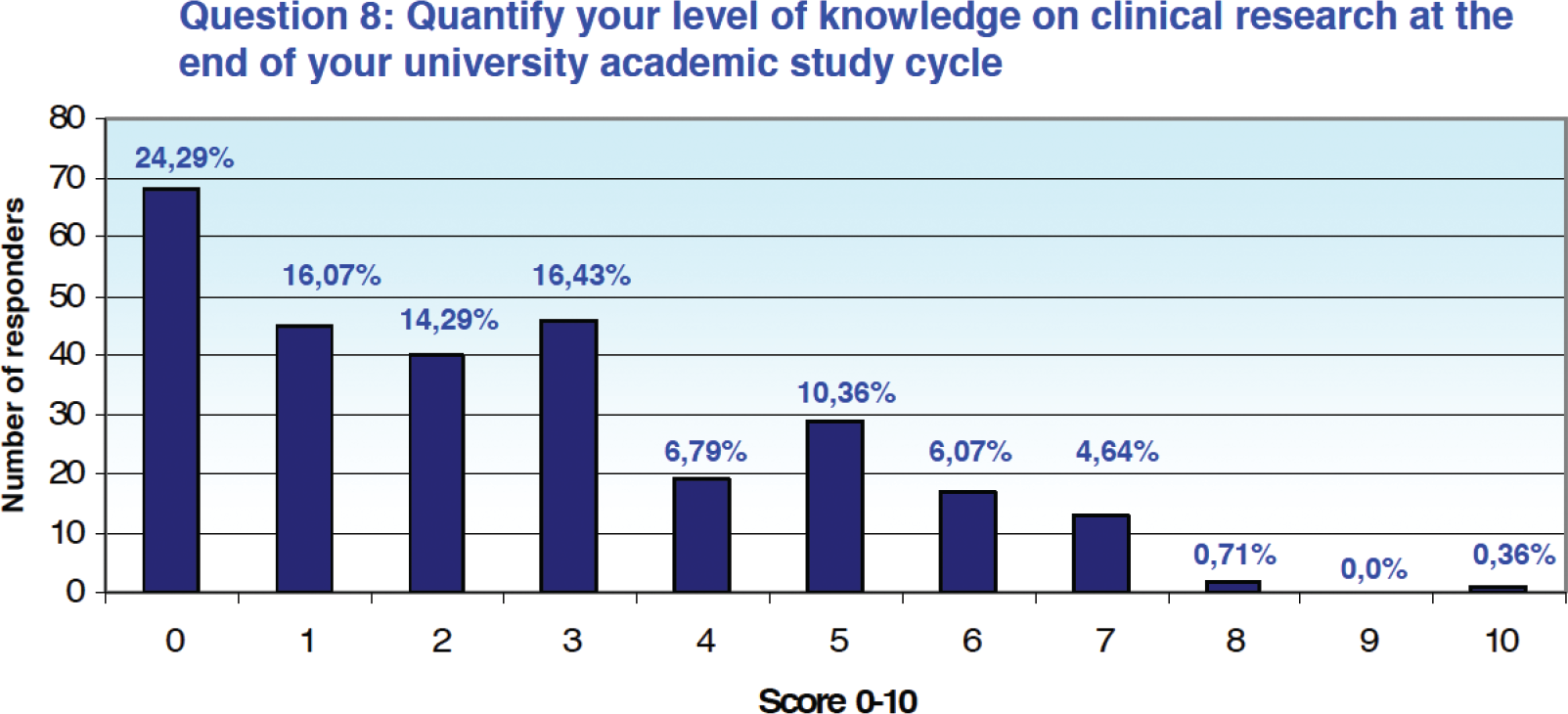
Fig. 2 - Results from Question 8 of the survey.
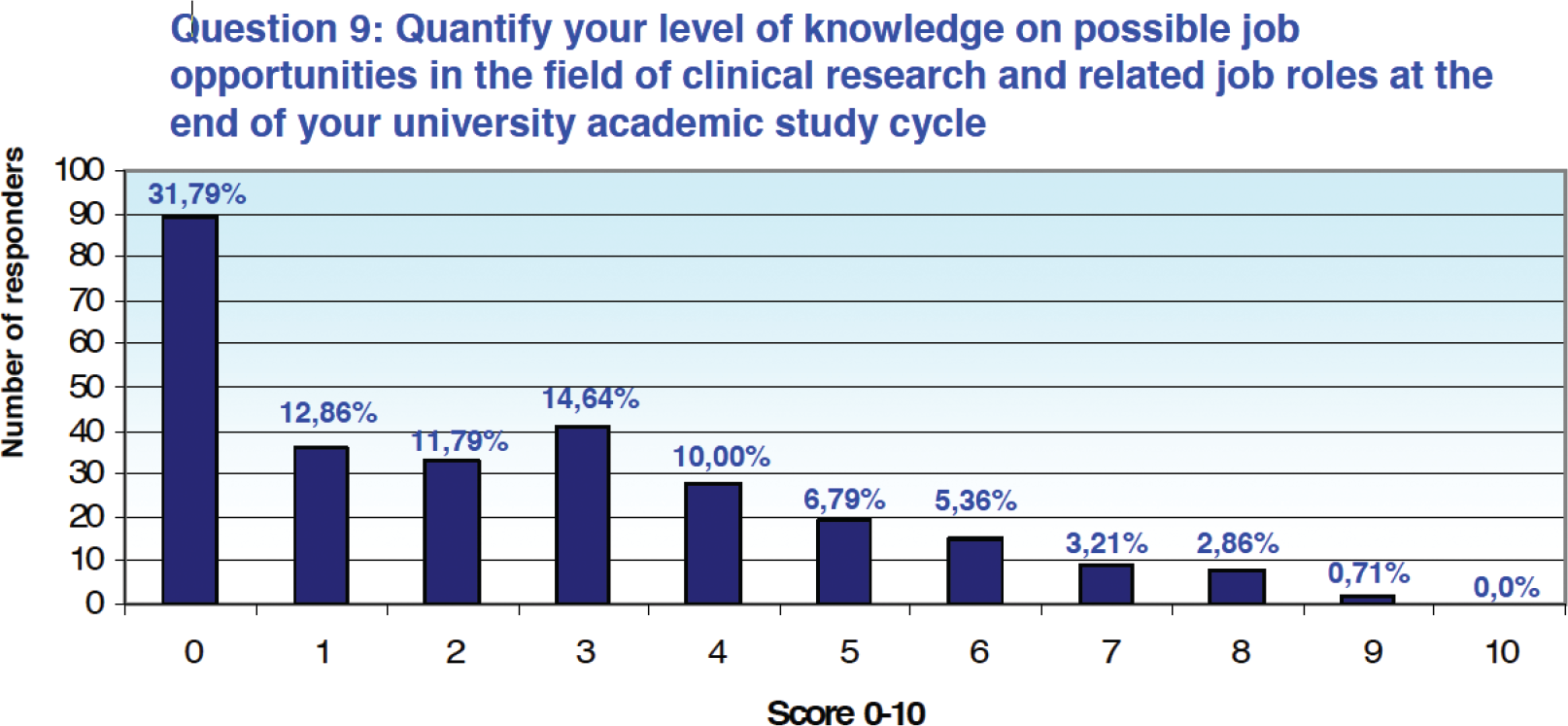
Fig. 3 - Results from Question 9 of the survey.
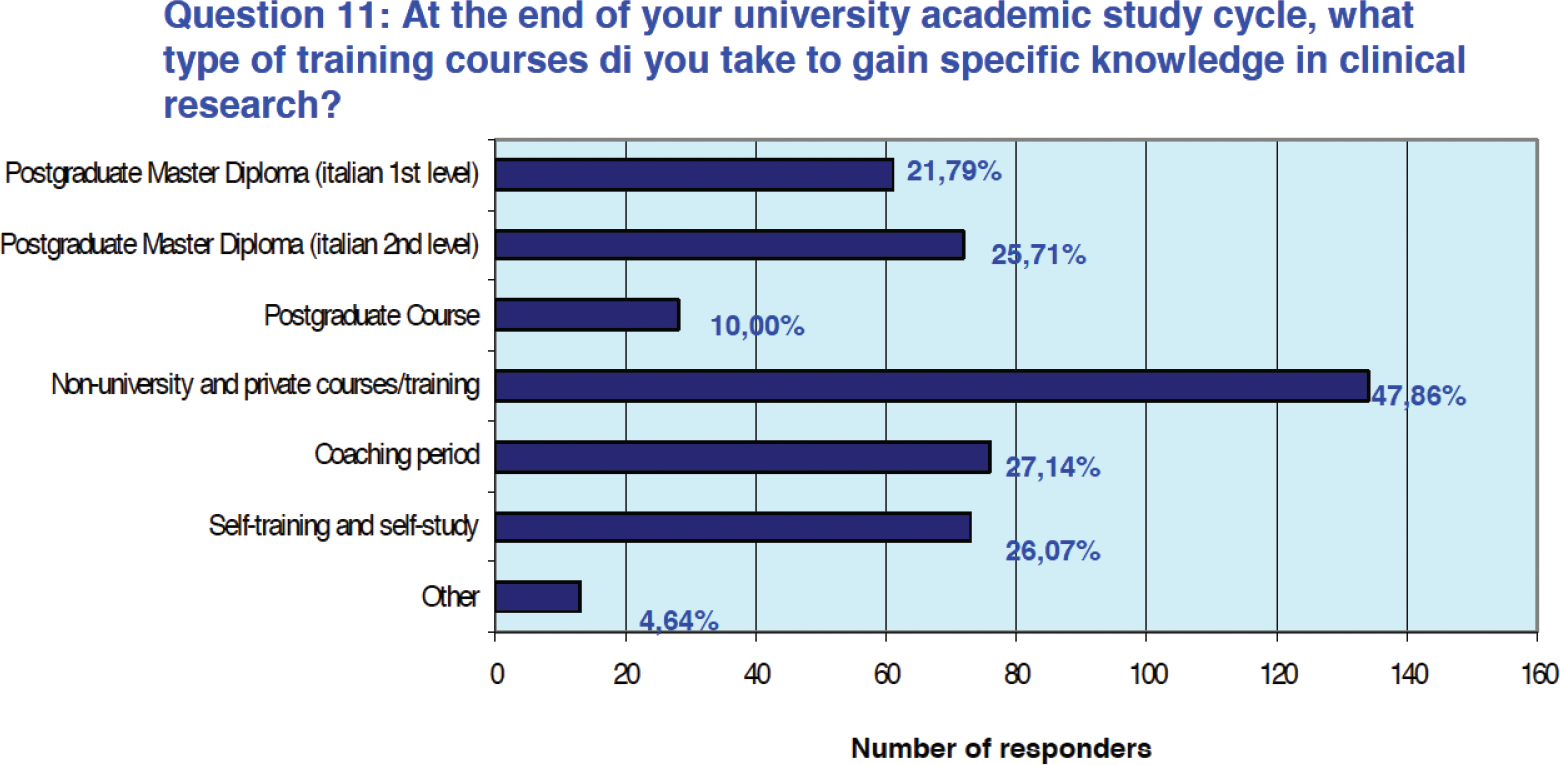
Fig. 4 - Results from Question 11 of the survey.
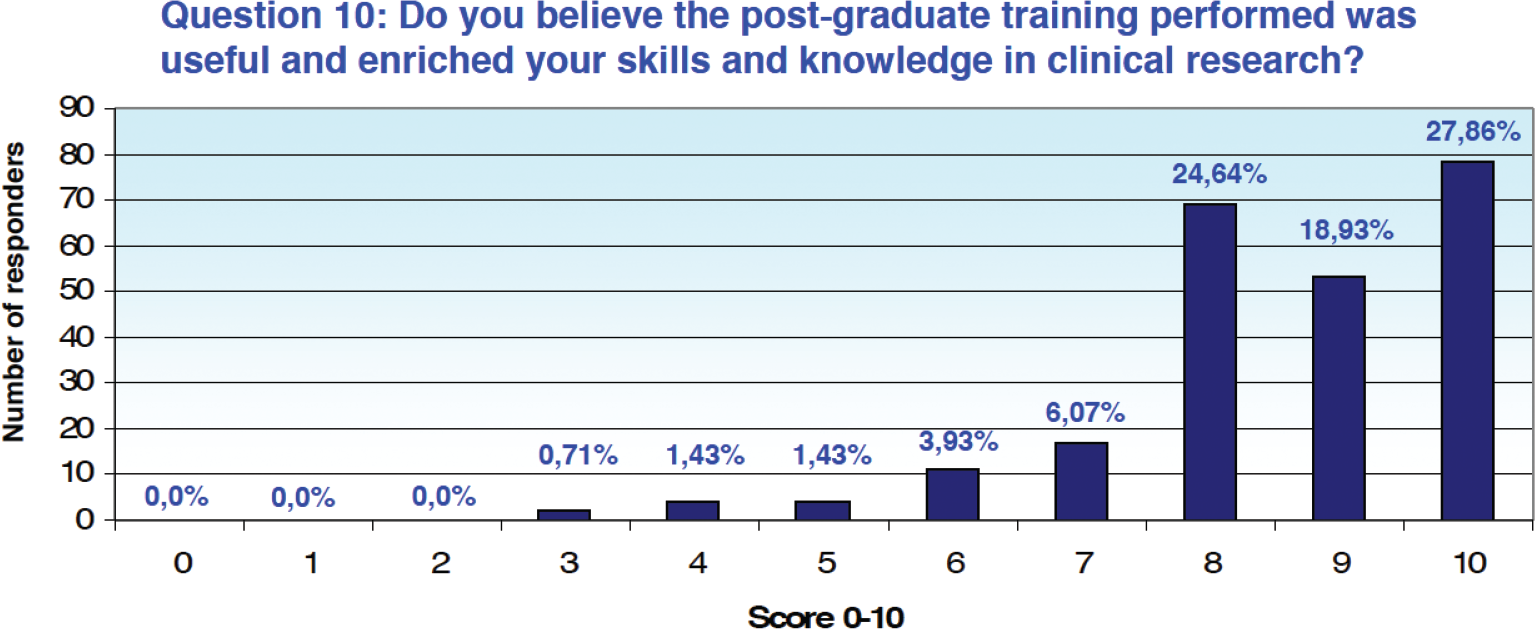
Fig. 5 - Results from Question 10 of the survey.
Conclusions
The data collected provide a snapshot of reality and experiences lived by Italian clinical research professionals.
The professional figures working in research centers are the most represented in terms of responses, with a clear majority of clinical research coordinators. Although operators of clinical centers are the most represented, the degree classes represented are almost the same as those of professionals working in CROs or pharmaceutical companies, with the same university training background. The only three degree classes of biology, biotechnology, and pharmacy/CTF (or any equivalent/equivalent degrees) in fact gather over 80% of the responding professionals. This seems to be in line with the requirements of most of the public tenders in the last 5 years, which are clearly restricting the field to health science degrees only.
In addition, more than 90% of respondents declared that they have a master’s or single-cycle degree, professionals who have therefore undergone the complete university training process in the context of their specific degree class.
As regards training in clinical research received during the normal academic career, almost three-fourths of the respondents declared that it was absent and/or inadequate, which suggests a possible gap in the university degree training courses in providing any knowledge specific to the field of clinical research and related subjects. A certain consistency and coherence can be seen between the various answers collected, a similar and constant share of respondents, around three-fourths of the professionals involved, claim to have completed their university career acquiring little knowledge on clinical research. Similarly, it can be seen that there is little, if any, awareness on possible professional opportunities in the context of clinical research at the end of the degree program (for professional figures operating in clinical research centers, sponsors and pharmaceutical companies, and CROs).
The responses recorded suggest the presence of a detachment between the university and the employment reality, as well as detecting gaps that need to be filled by the various professions through additional postgraduate training, both provided by private entities and by ad hoc master programs.
Overall, post-university training appears to be effective as three-fourths of the respondents claim to have benefited from it and rate it positively.
The data collected also suggest the need for a profound reflection on the orientation to work and professions, and to the related post-university training, which as suggested by the data in the literature represents a pinnacle step toward building a solid and expendable professional profile (13,14). In fact, doubts arise as to how a newly graduate student should tackle the employment real world or even that of postgraduate training if, as highlighted from the survey, during his/her degree program no tools have been provided to acquire the basic knowledge necessary to navigate the extensive clinical research field.
It is therefore desirable that topics such as clinical research be given the necessary space within the training programs of scientific degree courses promoted by Italian universities, especially for those degrees that are more relevant based on curricula and characteristics, such as biology, biotechnology, and pharmacy or chemistry and pharmaceutical technologies, to any professional opportunities in this field.
This discussion is certainly extendable also to the Faculty of Medicine and Surgery, given the increasing tendency to consider treatment and research as complementary activities, as demonstrated by the national competent authority (AIFA) data, which confirm how much clinical research is conducted also outside of those centers, namely the IRCCS, that until a few years ago were considered the only ones authorized to carry out research (15).
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: Data are available from the authors.
References
- 1. Samuels E, Ianni PA, Chung H, et al. Practical tips and/or guidelines Open Access Guidelines for Evaluating Clinical Research Training using Competency Assessments [Version 2]. MedEdPublish. 2019;8(3). CrossRef
- 2. Bechtel J, Chuck T, Forrest A, et al. Improving the quality conduct and efficiency of clinical trials with training: recommendations for preparedness and qualification of investigators and delegates. Contemp Clin Trials. 2020;89:105918. CrossRef PubMed
- 3. Guideline for good clinical practice E6(R2) Step 5 – Committee for Human Medicinal Products, 1 December 2016 EMA/CHMP/ICH/135/1995. Online Accessed January 2023.
- 4. Swezey T, McGuire FH, Hurley P, et al. More than a box to check: research sponsor and clinical investigator perspectives on making GCP training relevant. Contemp Clin Trials Commun. 2020;19:100606. CrossRef PubMed
- 5. Magnin A, Iversen VC, Calvo G, et al. European survey on national training activities in clinical research. Trials. 2019;20(1):616. CrossRef PubMed
- 6. Decreto Ministeriale del 15 novembre 2011. Definizione dei requisiti minimi per le organizzazioni di ricerca a contratto (CRO) nell’ambito delle sperimentazioni cliniche di medicinali. Gazzetta Ufficiale n. 11 del 14 gennaio 2012. Online. Accessed January 2023.
- 7. Determina AIFA 809 del 19 giugno 2015 – Determina inerente i requisiti minimi necessari per le strutture sanitarie che eseguono sperimentazioni di fase I (GU Serie Generale n. 15 8 del 10.07.2015). Online. Accessed January 2023.
- 8. Rapporto Ispezioni GCP 2015-2017: Classificazione e analisi delle deviazioni alla Good Clinical Practice. Agenzia Italiana del Farmaco (AIFA). Online. Accessed January 2023.
- 9. Annual Report of the Good Clinical Practice Inspectors’ Working Group 2019 – 5 February 2021 EMA/INS/GCP/588463/2020. Inspections Office Quality and Safety of Medicines Department – European Medicines Agency; 2020. Online. Accessed January 2023.
- 10. FDA Data Dashboard. Inspections. Online. Accessed January 2023.
- 11. Alfaar AS, Hassan WM, Bakry MS, Ezzat S. Clinical Research Recession: Training Needs Perception Among Medical Students. J Cancer Educ. 2017;32(4):728-733. CrossRef PubMed
- 12. LEGGE 11 gennaio 2018, n. 3 : Delega al Governo in materia di sperimentazione clinica di medicinali nonche’ disposizioni per il riordino delle professioni sanitarie e per la dirigenza sanitaria del Ministero della salute. (GU Serie Generale n.25 del 31-01-2018). Online. Accessed January 2023.
- 13. Loiodice I, Dato D. I servizi di orientamento universitario (in entrata, in itinere, in uscita) per il successo formativo, l’inclusione sociale e l’occupabilità in Successo formativo, inclusione e coesione sociale: strategie innovative. 2017. Armando Editore, Roma. Online. Accessed January 2023.
- 14. Cunti A, Priore A. Prefigurarsi il lavoro. L’orientamento universitario tra ricerca, didattica e formazione. Educational Reflective Practices. 2020;2:178-195. Online. Accessed January 2023.
- 15. La Sperimentazione Clinica dei Medicinali in Italia – 19° Rapporto Nazionale AIFA. Anno; 2020. AIFA. Online. Accessed January 2023.