 |
AboutOpen | 2023; 10: 50-54 ISSN 2465-2628 | DOI: 10.33393/ao.2023.2547 ORIGINAL RESEARCH ARTICLE |
 |
New technologies in the surgical management of endometriosis
ABSTRACT
Introduction: Endometriosis is a very common disease that affects up to 10% of the female population. Although medical therapy represents the first-line treatment for endometriosis, it does not always manage to control symptoms. Laparoscopy represents the standard surgical treatment in endometriosis. Robotic-assisted laparoscopy is an innovative mini-invasive surgical technique. Its application in gynecological surgery and in endometriosis has increased in the last decade. Our purpose is to offer an overview of the role of robotic-assisted laparoscopy in the surgical treatment of endometriosis.
Methods: We evaluated studies dealing with the new technique in surgery for endometriosis with a focus on robotic surgery. We performed a compressive literature research on PubMed and the Cochrane Library in December 2022.
Expert opinion: Robotic-assisted surgery is a feasible and safe approach to endometriosis surgery and is superimposable to laparoscopy in terms of complication rate, blood loss, hospitalization, and long-term improvement of symptoms.
The effect of robotic-assisted surgery on operative time is still contradictory and needs to be further investigated. Robotic-assisted laparoscopic surgery can provide particular benefit in the management of women with severe endometriosis secondary to its advantage in surgical precision and ergonomics.
Indocyanine green fluorescence angiography could be useful to assist in the vascularization of ureters and bowel anastomosis, to prevent postoperative complication and leakage.
Keywords: Deep endometriosis, Endometriosis, Indocyanine green, Laparoscopy, Robotic surgery, Surgical treatment
Received: December 12, 2022
Accepted: February 21, 2023
Published online: March 17, 2023
AboutOpen - ISSN 2465-2628 - www.aboutscience.eu/aboutopen
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Endometriosis is a chronic disease characterized by functional endometrial-like tissue located outside the uterus (1,2). Endometriosis manifestations could range from asymptomatic cases to severe chronic diseases characterized by pelvic pain, dysmenorrhea, dyspareunia, neurologic pain, dyschezia, dysuria, and infertility (1,2,3). Despite its high prevalence, the severity of symptoms, and its high socioeconomic impact, the real incidence of endometriosis is unknown (1,2). It is estimated that this condition affects 2%-10% of reproductive age women and up to 50% of infertile women. Moreover, a significant delay between the onset of first symptoms and a reliable diagnosis has been demonstrated (2).
Endometriosis can be divided into superficial peritoneal implants, ovarian endometriomas, and deep infiltrating endometriosis (DIE), in which ectopic implants infiltrate the peritoneum >5 mm (4). Endometriotic cells preserve the capability of response to sex hormones, causing cyclic bleedings, chronic inflammation, adhesion formation, and anatomic distortion (1).
Treatment for endometriosis should be customized according to symptoms, the stage of the disease, and the desire of pregnancy. In asymptomatic patients with an incidental diagnosis, periodic follow-up with ultrasound monitoring can be considered (2).
Medical therapy should always be used as the first line in endometriosis treatment. The current recommendation includes combined hormonal contraceptive and progestogen; meanwhile gonadotropin-releasing hormone (GnRH) agonist, GnRH antagonist, and aromatase inhibitors are considered as a second-line medical treatment. Danazol is no longer described as a medical treatment for endometriosis-associated pain (2).
Surgery for endometriosis may include complex procedures that can involve significant complications (3,5). Surgical approach should be considered only in select cases, as in refractory pain or symptoms that don’t respond to medical therapy and significantly impact the quality of life (QoL) of patients, in case of organ dysfunction or obstruction, or risk of malignancy (2). In these patients, surgery results in a significant improvement in pain and QoL (6). The complete excision of endometriosis offers good long-term symptomatic relief, especially in cases with severe or debilitating symptoms (7,8).
The impact of surgical treatment on infertility is still debated (2,9). Surgery may have a beneficial impact on the chance of spontaneous conception (10), although surgery should be considered cautiously, secondary to the risk of damage to ovarian reserve, in particular among patients with endometrioma. In the absence of other contraindications to surgery, in case of infertility, assisted reproductive technology is generally preferred as the first-line treatment (2,10,11).
Mini-invasive surgery represents the standard surgical treatment in endometriosis, secondary to the advantages in visualization, shorter hospital stays, faster recovery, and better cosmetic results compared to laparotomy (2,3).
Robotic-assisted laparoscopy and endometriosis
Robotic-assisted laparoscopy (RAL) is an emerging innovation in mini-invasive technique, developed in order to overcome some limitation of standard laparoscopy (LPS) (12). RAL guarantees better surgical field visualization through high-resolution 3D view, better mobility thanks to the wrist-like motion of the robotic arms, a tremor-free handling, direct control of surgeons’ three or four arms, and an improved ergonomics. This technique increases the abilities in LPS suturing, knot-tying, lysis of adhesions, and retroperitoneal exploration (12,13,14,15,16). Moreover, the higher degree of freedom in motion and the possibility of working in a parallel console facilitate training of the less expert surgeons and reduce the learning curve of RAL compared to LPS (12,13,17). Practically, robots make LPS easy.
Recently, the use of RAL in gynecological surgery has increased. Nevertheless, RAL presents significant limitations in the lack of tactile feedback (14). One of the biggest limitations of RAL is the significantly higher cost compared to LPS, which restricts its application, in particular, easy procedure (12,15,18). Regarding endometriosis surgery, there are no data comparing RAL to LPS in ovarian endometriomas. The procedure is easily performed by LPS surgery; therefore, the use of RAL would not be a cost-effective option and it should not be recommended (13).
Surgery for DIE may include complex procedures such as extensive adhesiolysis, ureterolysis, partial bladder or bowel resection, ureteral resection, and reanastomosis (Fig. 1) (3). DIE treatment needs high surgical skills and wide experience to achieve the radicality and to preserve, as much as possible, the vascularization and the neurovegetative function of the pelvic organs. Major complications from surgery for deep endometriosis can occur in 3.9% of cases (3).
Many studies have shown that RAL is a feasible and safe alternative to LPS for the treatment of endometriosis (5,18,19,20,21). A systematic review from Restaino et al (5) and the prospective randomized trial LAROSE (19) confirmed that RAL and LPS are superimposable in terms of blood loss, complication rate, hospital stay, and both significantly improved pain and QoL after intervention. One of the major concerns about RAL is the possible increase in operative time compared to LPS (5,20,22). Docking time represents critical factors in determining the operation time (17). Restaino et al (5) reported an increase of operative time in RAL, despite the elimination of docking time, although the authors highlight that none of the study analyzed mentioned the learning curve for RAL procedures. The authors hypothesized that surgeon expertise with RAL could explain the increase in operative time (5).
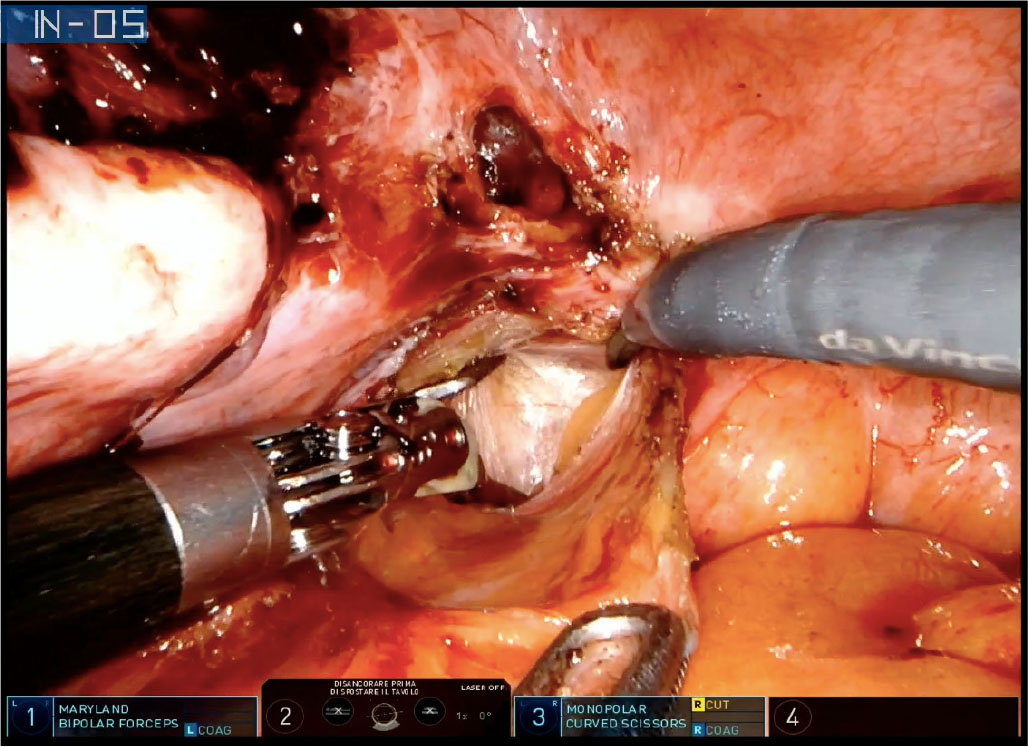
Fig. 1 - Robotic-assisted left ureterolysis with DaVinci® Xi in a patient with left uterosacral deep infiltrating endometriosis nodule.
Raimondo et al (23) compared perioperative outcome in a multicentric prospective study of 44 cases with stage III to IV endometriosis eradication performed by two surgeons proficient in both LPS and RAL. Data showed no significant difference between the two groups regarding operative time, confirming the importance of an adequate training of the operator approach to this innovative surgical technique.
Moreover, in a large retrospective study by Magrina et al (24) of patients affected by severe endometriosis, the authors observed that RAL results in shorter mean operative time, after adjusting the findings for age, blood loss, and number of procedures per patient.
It is worth to notice that the most encouraging result has been observed in advanced stage endometriosis, a procedure that requires demanding surgical effort (5,23,24,25). In previous published case series (26,27,28), RAL has demonstrated a high success rate in patients with stage IV endometriosis with colorectal involvement. Moreover, the postoperative follow-up showed a high pregnancy rate, significant decrease in pain symptoms, and a significant improvement of QoL (26,27,28).
In the case study from Ercoli et al. (29) on 33 women with retro-cervical endometriosis not involving rectal mucosa treated with RAL surgery, all nodules were shaved completely, independent by size, without major complications and with a low rate of segmental resection. Despite the large variety of procedures performed in this case series (ureterolysis, ureteral and rectovaginal nodule excision, bowel resection, uterosacral ligament resection, excision of posterior vaginal fornix and of peritoneal nodules), operative times were superimposable to those reported for LPS (Fig. 2) (29,30). The authors attributed the results to the highly precise surgery obtainable by RAL.
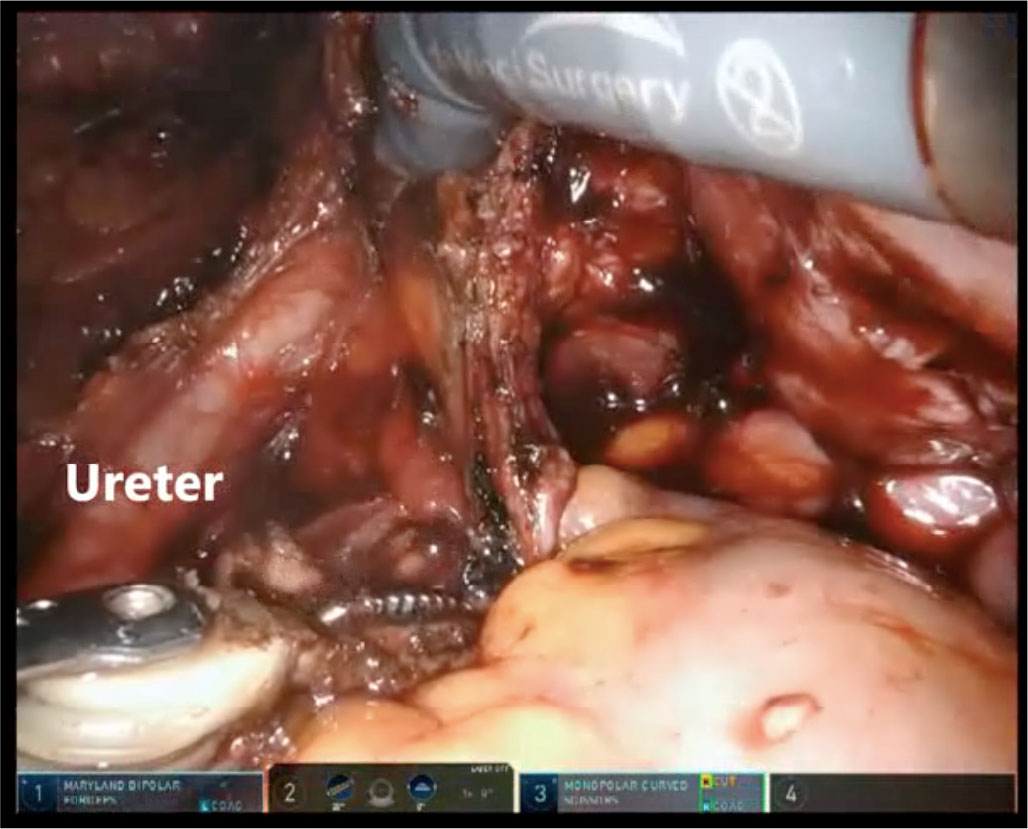
Fig. 2 - Opening of the right pararectal fossa with lateromedial approach to isolate a right uterosacral deep infiltrating endometriosis nodule with DaVinci® Xi.
In two recent case studies (31,32), RAL has been successfully applied to nerve-sparing eradication of DIE, confirming the possible benefit of RAL in nerve-sparing surgery.
Indocyanine green in endometriosis
Endometriosis has a pleiomorphic appearance and it is not always easily recognizable (33). The use of indocyanine green (ICG) dye, as a support in surgery for endometriosis, has been spreading over the last few years. After its intravenous administration, using near-infrared (NIR) cameras, ICG can be perceived as the emission of fluorescent light (34). ICG may improve the diagnosis of endometriosis and allow an accurate intraoperative real-time assessment of tissue vascularization thanks to its ability to bind plasma proteins (33,34,35).
ICG fluorescence may be considered as a good diagnostic and screening test for DIE and peritoneal endometriosis. In a previous clinical trial, the use of NIR-ICG had high positive predictive value, specificity, negative predictive value, and sensitivity (36,37).
The Firefly™ technology is incorporated in the main robotic platforms and used for NIR imaging to detect injected ICG dye.
Levey was the first to use DaVinci® Si’s fluorescent technology with ICG for increase in the detection and improvement of the surgical management of endometriosis (33). The use of ICG for detecting endometriosis in RAL has subsequently spread (38,39).
The ability of the 3D robotic Firefly™ imaging of DaVinci® Si Surgical System compared with 2D LPS on the detection of non-visible endometriosis has been investigated by Vizzielli et al (37). The authors observed higher sensibility and specificity in ICG fluorescence imaging in detecting endometriosis compared to simple white light imaging. The differences did not reach statistical significance. However, in the trial by Jayakumaran et al (35), the performance of 3D robotic ICG fluorescence imaging in detecting endometriotic lesions had overcome the one of white light 3D robotic imaging and 2D LPS imaging.
ICG fluorescence is helpful in separating the endometriotic nodules from the healthy tissue. It has been used to guide shaving of bowel DIE nodules (40,41,42). In case of bowel involvement, ICG could be used to evaluate shaving feasibility and to better define the limits between nodule and healthy tissue (Fig. 3) (42).
Moreover, intravenous administration of ICG can be used to intraoperatively investigate ureteral perfusion to identify local ischemia (43). In case of bowel resection, it allows to assess the perfusion of the bowel, select the transaction line as well as evaluate the adequacy of the blood supply to anastomosis (Fig. 4) (44,45).
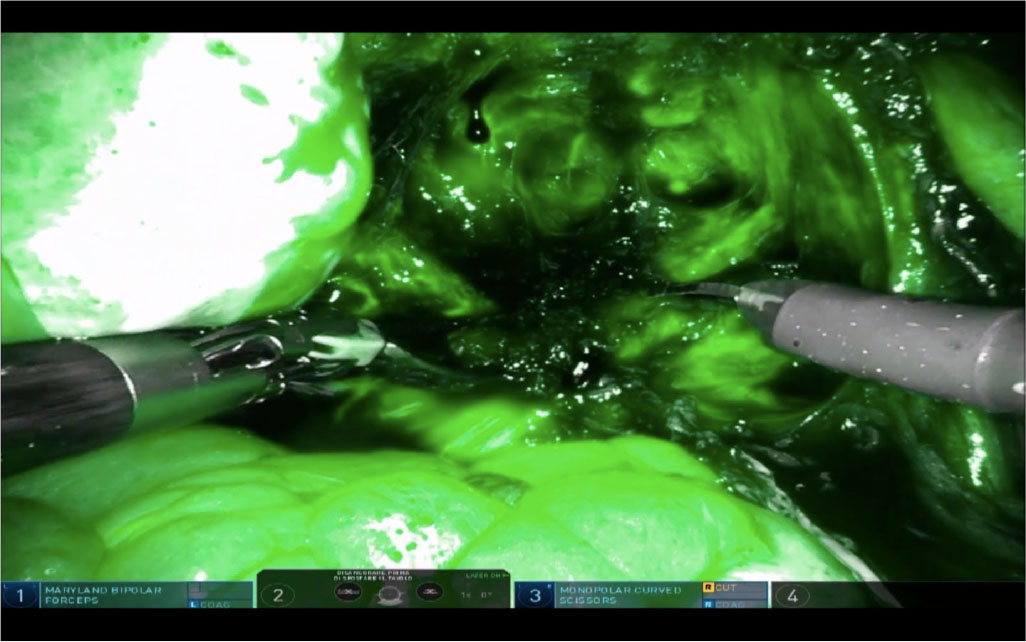
Fig. 3 - View of a rectal deep infiltrating endometriosis nodule (dark) after intravascular administration of indocyanine green dye with robotic Firefly™ imaging of DaVinci® Xi.
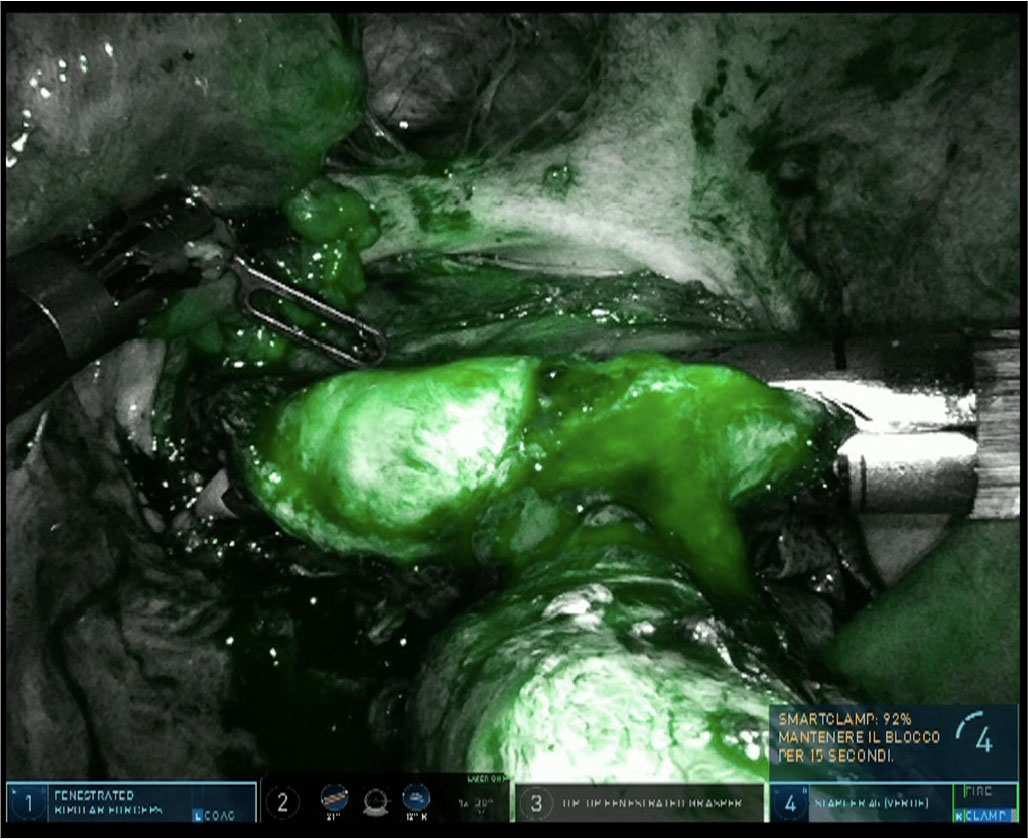
Fig. 4 - Use of indocyanine green dye to study the adequacy of vascularization prior to rectal resection with robotic Firefly™ imaging of DaVinci® Xi.
Previous retroperitoneal surgery, fibrosis, and the reduction of neoangiogenesis related to the use of estrogen-progestin or GnRH agonist may alter microcirculation of endometriotic lesions and could influence the success of this method (16).
Conclusion
RAL is a safe and feasible option and it might be considered an alternative to LPS in the surgical treatment of endometriosis. Some studies suggest that the use of RAL could cause an increase in operative use, although other trials demonstrate that operative time in RAL is superimposable to that of LPS if the procedure is performed by a trained and skilled surgeon.
Moreover, several studies had demonstrated the non-inferiority of RAL to LPS in terms of intraoperative and postoperative complication rate, blood loss, and hospital stay. Both the techniques significantly improve pain symptoms and QoL.
The advantages of the robotic platform are more pronounced in patients with severe endometriosis. In fact, the procedures required in these patients are more complex and the surgeon can benefit from the high surgical precision and decreasing fatigue of surgeons related to robotic platforms.
ICG fluorescence seems to be a good diagnostic test for guiding the surgeons to the approach to endometriosis. The data of the scientific literature are still not of adequate quality to recommend its systematic use. The use of ICG fluorescence for vascularization is fundamental for the choice of the correct site of transection to avoid failure of anastomotic site and leakages.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors contribution: All authors contributed equally to this manuscript.
References
- 1. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. CrossRef PubMed
- 2. Becker CM, Bokor A, Heikinheimo O, et al; ESHRE Endometriosis Guideline Group. ESHRE guidelines: endometriosis. Hum Reprod Open. 2022;2022(2):hoac009. CrossRef PubMed
- 3. Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM. Laparoscopic surgery for endometriosis. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2020 Oct 23 [cited 2022 Nov 14];10(10): CD011031. Available from: CrossRef PubMed
- 4. Revised American Fertility Society Classification of Endometriosis. Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril. 1985;43(3):351-352. CrossRef PubMed
- 5. Restaino S, Mereu L, Finelli A, et al. Robotic surgery vs laparoscopic surgery in patients with diagnosis of endometriosis: a systematic review and meta-analysis. J Robot Surg. 2020;14(5):687-694. CrossRef PubMed
- 6. Arcoverde FVL, Andres MP, Borrelli GM, Barbosa PA, Abrão MS, Kho RM. Surgery for endometriosis improves major domains of quality of life: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2019;26(2):266-278. CrossRef PubMed
- 7. Angioni S, Peiretti M, Zirone M, et al. Laparoscopic excision of posterior vaginal fornix in the treatment of patients with deep endometriosis without rectum involvement: surgical treatment and long-term follow-up. Hum Reprod. 2006;21(6):1629-1634. CrossRef PubMed
- 8. Minelli L, Fanfani F, Fagotti A, et al. Laparoscopic colorectal resection for bowel endometriosis: feasibility, complications, and clinical outcome. Arch Surg. 2009;144(3):234-239. CrossRef PubMed
- 9. Vercellini P, Somigliana E, Viganò P, Abbiati A, Barbara G, Crosignani PG. Surgery for endometriosis-associated infertility: a pragmatic approach. Hum Reprod. 2009;24(2):254-269. CrossRef PubMed
- 10. Gale J, Singh SS. A practical approach to fertility considerations in endometriosis surgery. Obstet Gynecol Clin North Am. 2022;49(2):241-256. CrossRef PubMed
- 11. Vercellini P, Barbara G, Somigliana E. Which treatments are effective for endometriosis-related infertility? Fertil Steril. 2020;113(2):328-329. CrossRef PubMed
- 12. Moon AS, Garofalo J, Koirala P, Vu MT, Chuang L. Robotic surgery in gynecology. Surg Clin North Am. 2020;100(2):445-460. CrossRef PubMed
- 13. Cela V, Obino ME, Sergiampietri C, et al. The role of robotics in the management of endometriosis. Minerva Ginecol. 2017;69(5):504-516. Accessed November 14, 2022. Online PubMed
- 14. Lawrie TA, Liu H, Lu D, et al. Robot-assisted surgery in gynaecology. Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2019 Apr 15 [cited 2022 Nov 14]; 4(4):CD011422. Available from: CrossRef PubMed
- 15. Nezhat C, Saberi NS, Shahmohamady B, Nezhat F. Robotic-assisted laparoscopy in gynecological surgery. JSLS. 2006;10(3):317-320. PubMed
- 16. Mosbrucker C, Somani A, Dulemba J. Visualization of endometriosis: comparative study of 3-dimensional robotic and 2-dimensional laparoscopic endoscopes. J Robot Surg. 2018;12(1):59-66. CrossRef PubMed
- 17. Nagendran M, Gurusamy KS, Aggarwal R, Loizidou M, Davidson BR. Virtual reality training for surgical trainees in laparoscopic surgery. Cochrane Hepato-Biliary Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2013 Aug 27 [cited 2022 Nov 13]; 2013(8): CD006575. Available from: CrossRef PubMed
- 18. Carvalho L, Abrão MS, Deshpande A, Falcone T. Robotics as a new surgical minimally invasive approach to treatment of endometriosis: a systematic review. Int J Med Robot. 2012;8(2):160-165. CrossRef PubMed
- 19. Soto E, Luu TH, Liu X, et al. Laparoscopy vs. Robotic Surgery for Endometriosis (LAROSE): a multicenter, randomized, controlled trial. Fertil Steril. 2017;107(4):996-1002.e3. CrossRef PubMed
- 20. Chen SH, Li ZA, Du XP. Robot-assisted versus conventional laparoscopic surgery in the treatment of advanced stage endometriosis: a meta-analysis. Clin Exp Obstet Gynecol. 2016;43(3):422-426. CrossRef PubMed
- 21. Siesto G, Ieda N, Rosati R, Vitobello D. Robotic surgery for deep endometriosis: a paradigm shift. Int J Med Robot. 2014;10(2):140-146. CrossRef PubMed
- 22. Liu C, Perisic D, Samadi D, Nezhat F. Robotic-assisted laparoscopic partial bladder resection for the treatment of infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15(6):745-748. CrossRef PubMed
- 23. Raimondo D, Alboni C, Orsini B, et al. Comparison of perioperative outcomes between standard laparoscopic and robot-assisted approach in patients with rectosigmoid endometriosis. Acta Obstet Gynecol Scand. 2021;100(9):1740-1746. CrossRef PubMed
- 24. Magrina JF, Espada M, Kho RM, Cetta R, Chang YHH, Magtibay PM. Surgical excision of advanced endometriosis: perioperative outcomes and impacting factors. J Minim Invasive Gynecol. 2015;22(6):944-950. CrossRef PubMed
- 25. Bedaiwy MA, Rahman MY, Chapman M, et al. Robotic-assisted hysterectomy for the management of severe endometriosis: a retrospective review of short-term surgical outcomes. JSLS. 2013;17(1):95-99. CrossRef PubMed
- 26. Pellegrino A, Damiani GR, Trio C, et al. Robotic shaving technique in 25 patients affected by deep infiltrating endometriosis of the rectovaginal space. J Minim Invasive Gynecol. 2015;22(7):1287-1292. CrossRef PubMed
- 27. Morelli L, Perutelli A, Palmeri M, et al. Robot-assisted surgery for the radical treatment of deep infiltrating endometriosis with colorectal involvement: short- and mid-term surgical and functional outcomes. Int J Colorectal Dis. 2016;31(3):643-652. CrossRef PubMed
- 28. Abo C, Roman H, Bridoux V, et al. Management of deep infiltrating endometriosis by laparoscopic route with robotic assistance: 3-year experience. J Gynecol Obstet Hum Reprod. 2017;46(1):9-18. CrossRef PubMed
- 29. Ercoli A, Bassi E, Ferrari S, et al. Robotic-assisted conservative excision of retrocervical-rectal deep infiltrating endometriosis: a case series. J Minim Invasive Gynecol. 2017;24(5):863-868. CrossRef PubMed
- 30. Collinet P, Leguevaque P, Neme RM, et al. Robot-assisted laparoscopy for deep infiltrating endometriosis: international multicentric retrospective study. Surg Endosc. 2014;28(8):2474-2479. CrossRef PubMed
- 31. Alboni C, Farulla A, Facchinetti F, Ercoli A. Robot-assisted nerve-sparing resection of bilateral parametrial deep infiltrating endometriosis. J Minim Invasive Gynecol. 2021;28(1):18-19. CrossRef PubMed
- 32. Kanno K, Andou M, Aiko K, et al. Robot-assisted nerve plane-sparing eradication of deep endometriosis with double-bipolar method. J Minim Invasive Gynecol. 2021;28(4):757-758. CrossRef PubMed
- 33. Levey KA. Use of fluorescence imaging technology to identify peritoneal endometriosis: a case report of new technology. Surg Laparosc Endosc Percutan Tech. 2014;24(2):e63-e65. CrossRef PubMed
- 34. Ianieri MM, Della Corte L, Campolo F, et al. Indocyanine green in the surgical management of endometriosis: a systematic review. Acta Obstet Gynecol Scand. 2021;100(2):189-199. CrossRef PubMed
- 35. Jayakumaran J, Pavlovic Z, Fuhrich D, Wiercinski K, Buffington C, Caceres A. Robotic single-site endometriosis resection using near-infrared fluorescence imaging with indocyanine green: a prospective case series and review of literature. J Robot Surg. 2020;14(1):145-154. CrossRef PubMed
- 36. Cosentino F, Vizzielli G, Turco LC, et al. Near-infrared imaging with indocyanine green for detection of endometriosis lesions (Gre-Endo Trial): a pilot study. J Minim Invasive Gynecol. 2018;25(7):1249-1254. CrossRef PubMed
- 37. Vizzielli G, Cosentino F, Raimondo D, et al. Real three-dimensional approach vs two-dimensional camera with and without real-time near-infrared imaging with indocyanine green for detection of endometriosis: a case-control study. Acta Obstet Gynecol Scand. 2020;99(10):1330-1338. CrossRef PubMed
- 38. Tang NZ, Goldman TL, Prabakar C. Robotically-assisted laparoscopic resection of endometriosis using firefly technology. J Minim Invasive Gynecol. 2015;22(6S):S151. CrossRef PubMed
- 39. Guan X, Nguyen MTA, Walsh TM, Kelly B. Robotic single-site endometriosis resection using firefly technology. J Minim Invasive Gynecol. 2016;23(1):10-11. CrossRef PubMed
- 40. Bar-Shavit Y, Jaillet L, Chauvet P, Canis M, Bourdel N. Use of indocyanine green in endometriosis surgery. Fertil Steril. 2018;109(6):1136-1137. CrossRef PubMed
- 41. De Neef A, Cadière GB, Bourgeois P, Barbieux R, Dapri G, Fastrez M. Fluorescence of deep infiltrating endometriosis during laparoscopic surgery: a preliminary report on 6 cases. Surg Innov. 2018;25(5):450-454. CrossRef PubMed
- 42. Cela V, Papini F, Vacca C, et al. Clinical use of indocyanine green in bowel endometriosis surgery. J Minim Invasive Gynecol. 2021;28(7):1275-1276. CrossRef PubMed
- 43. Raimondo D, Borghese G, Mabrouk M, et al. Use of indocyanine green for intraoperative perfusion assessment in women with ureteral endometriosis: a preliminary study. J Minim Invasive Gynecol. 2021;28(1):42-49. CrossRef PubMed
- 44. Seracchioli R, Raimondo D, Arena A, Zanello M, Mabrouk M. Clinical use of endovenous indocyanine green during rectosigmoid segmental resection for endometriosis. Fertil Steril. 2018;109(6):1135. CrossRef PubMed
- 45. Malzoni M, Iuzzolino D, Rasile M, et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258. CrossRef PubMed