 |
AboutOpen | 2023; 10: 78-81 ISSN 2465-2628 | DOI: 10.33393/ao.2023.2511 BRIEF REPORT |
 |
Possible role of glucose-6-phosphatase 3 in the pathogenesis of uterine leiomyomas
ABSTRACT
Background and aim: Glucose-6-phosphatase catalytic subunit 3 (G6PC3) has been recently described as a metabolite repair enzyme involved in the disposal of the phosphorylated glucose analog 1,5-anhydroglucitol-6-phosphate (1,5AG6P). This function is especially relevant in neutrophils; indeed, G6PC3 deficiency leads to neutropenia as the accumulated metabolite 1,5AG6P inhibits the first step of glycolysis. Like neutrophils, tumoral metabolism also mainly relies on glycolysis, and we wondered if G6PC3 is expressed in uterine leiomyoma samples and if it can eventually have a role in the pathogenesis of these tumors. Understanding the complex pathophysiology of leiomyomas is a prerequisite to develop new therapeutic strategies.
Methods: We used human uterine leiomyoma and matched myometrial samples. Immunohistochemistry and quantitative polymerase chain reaction (qPCR) were performed.
Results: Immunohistochemical analysis has not evidenced appreciable differences between pathologic versus normal tissue samples. Indeed, qPCR analysis suggests a higher expression of G6PC3 in human uterine leiomyoma than in matched myometrial samples.
Conclusion: A targeted therapeutic inhibition of G6PC3 in uterine leiomyoma samples is a potential strategy to slow down tumor growth.
Keywords: G6PC3, G6PT, 1,5-Anhydroglucitol-6-phosphate, Glycolysis, Uterine leiomyomas
Received: October 26, 2022
Accepted: May 9, 2023
Published online: June 5, 2023
AboutOpen - ISSN 2465-2628 - www.aboutscience.eu/aboutopen
© 2023 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Glucose-6-phosphatase catalytic subunit 3 (G6PC3) is an almost ubiquitous enzyme whereas its homologous glucose-6-phosphatase catalytic subunit 1 (G6PC1) counterpart is mainly expressed in the liver and kidney (1), playing a key role in regulating blood glucose homeostasis.
Both G6PC3 and G6PC1 are phosphatases that catalyze the last reaction of glycogenolysis and gluconeogenesis, namely the dephosphorylation of glucose-6-phosphate (G6P) to glucose and inorganic phosphate. Even though both pathways occur in the cytoplasm, the G6PC enzymes are associated with the endoplasmic reticulum (ER) membranes and the active site is located in the lumen of ER. Because of that, G6P has to be transported into the ER by the glucose-6-phosphate transporter (G6PT) (1) in order to allow enzymatic activity.
Mutations in the G6PC and in the G6PT genes cause glycogen storage disease type 1a and type 1b, respectively. The second form mentioned has the worst symptoms: it is a metabolic disorder, but it is also characterized by severe infections due to neutropenia and neutrophil dysfunctions (1). Mutations in the G6PC3 gene cause congenital neutropenia type 4 that is characterized by recurrent infections but lacks metabolic derangement (1,2).
The common trait linking these genetic diseases has been recently evidenced since G6PC3, in cooperation with its transporter G6PT, has emerged as a metabolite repair enzyme involved in the disposal of the phosphorylated glucose analog 1,5-anhydroglucitol-6-phosphate (1,5AG6P) (3). In physiologic conditions, 1,5-anhydroglucitol (1,5AG) is phosphorylated to 1,5AG6P thanks to the enzyme hexokinase (EK). The latter metabolite is toxic for the organism and it is usually detoxified by G6PC3. G6PC3 recognizes 1,5AG6P and dephosphorylates the molecule to 1,5AG (Fig. 1A). In patients with a deficit of either G6PC3 or G6PT, 1,5AG6P remains in the cytosol as a toxic molecule since it is a strong inhibitor of EK (Fig. 1B). This has been demonstrated to lead to neutropenia: G6P concentration diminishes in the cytosol and consequently, the neutrophils do not get the proper amount of energy through glycolysis. Therefore, the process results in the apoptosis of the cells (3).
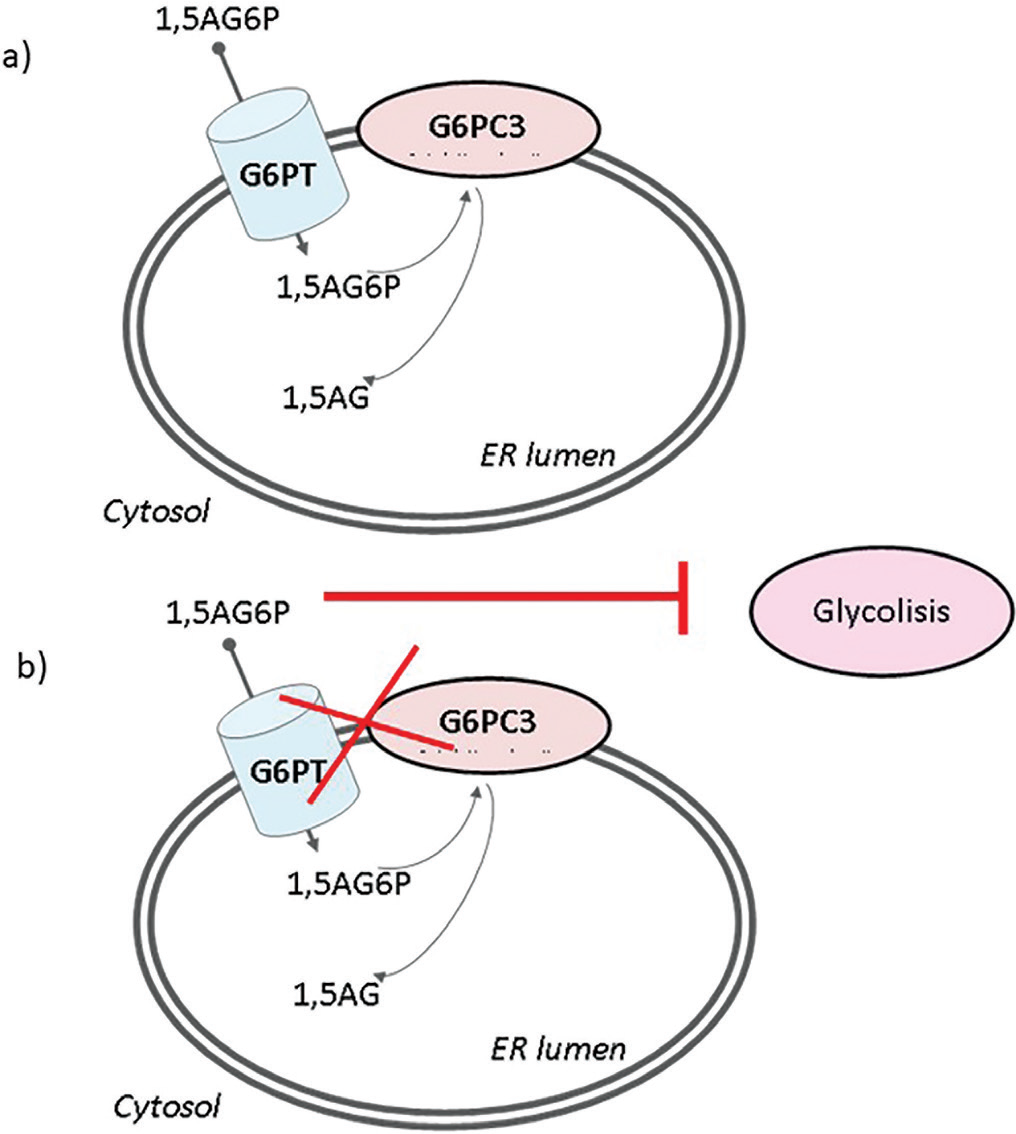
Fig. 1 - Role of the enzymatic couple G6PC3-G6PT in detoxifying 1,5AG. 1,5AG6P is transported into the ER by G6PT and dephosphorylated by G6PC3 (A). When G6PC3 or G6PT does not work properly, 1,5AG6P accumulates in the cytosol and inhibits glycolysis (B). 1,5AG = 1,5-anhydroglucitol; 1,5AG6P = 1,5-anhydroglucitol-6-phosphate; ER = endoplasmic reticulum; G6PC3 = glucose-6-phosphatase catalytic subunit 3; G6PT = glucose-6-phosphatase transporter.
It is well known that glycolysis is a predominant metabolic pathway in tumors. Nonetheless, the role of G6PC3 in cells other than neutrophils is largely undefined.
We decided to focus our attention on leiomyomas, a common type of benign neoplasms of female also known as fibroids and extremely frequent in the reproductive age. Uterine leiomyomas originate from the smooth muscle layer of the myometrium and are characterized by disordered accumulation of extracellular matrix. Leiomyomas are a gynecological condition that is a serious health problem due to the associated chronic pelvic pain, uterine bleeding and infertility that represent a heavy burden for the public health care system due to the medical management and surgery (i.e., hysterectomy) (4).
On these assumptions, the aim of the present study was to evaluate the expression of G6PC3 in human leiomyomas and matched myometrial tissues to envisage a possible role in the leiomyoma’s metabolism that could be a potential target to hamper tumoral cell proliferation.
Materials and methods
Sample collection
Uterine leiomyoma and adjacent normal myometrial tissue samples were collected from six premenopausal, nonpregnant patients undergoing myomectomy or hysterectomy for symptomatic fibroids, at the University Hospital of Siena. Leiomyomas were previously diagnosed by ultrasonography, and surgical specimens were confirmed as leiomyomas by histopathological evaluation. The median age of the patients was 46.5 years (age range, 44–48 years). The location of fibroids (single or multiple) was predominantly intramural, and their size range was 1–10 cm in diameter. Patients included in the study didn’t receive any hormonal treatment in the 3 months before surgery. All participants gave written, informed consent before entering this study, which was approved by the local Human Investigation Committee.
Immunohistochemistry
Tissues were cut into pieces and fixed in 10% buffered formalin for 48 hours. The samples were then dehydrated in graded ethanol, clarified in xylene, embedded in paraffin wax and cut into 7 μm serial sections with a microtome (Leica Microsystems, Milan, Italy).
For immunohistochemistry, sections were deparaffinized in xylene using serial alcohol solutions and rinsed with distilled water. Sections were subjected to antigen retrieval in 0.01 M sodium citrate buffer, pH 6.0 in a microwave oven for 15 min and then incubated in 3% hydrogen peroxide for 10 min at room temperature.
Immunoreaction was performed overnight at +4°C with G6PC3 and G6PT antibodies: 5 μg/mL rabbit polyclonal antihuman G6PC3 antibody (Aviva System Biology, USA) and rabbit polyclonal anti-G6PT antibody solution 1:250, as previously reported (5). The reaction products were visualized using 0.33% hydrogen peroxide and 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB), as chromogen (Sigma, St. Louis, MO, USA). The sections were finally counterstained with Meyer’s hematoxylin, dehydrated and mounted with Eukitt for light microscopy detection. The immunohistochemical controls were performed by omitting the primary antibody. The images were acquired by a digital camera DS-U1 (Nikon, Japan) mounted on a Nikon Eclipse E600 optical microscope.
RNA Isolation and qPCR
Tissue samples were immediately frozen in liquid nitrogen after surgery and stored at −80°C until use. The ribonucleic acid (RNA) was extracted from frozen tissue and the complementary deoxyribonucleic acid (cDNA) was reverse transcribed as previously reported (6). Analyses were performed in triplicate in a 20 µL reaction mixture. The cDNA (1 µL) was amplified with PowerUp SYBR Green Master Mix (Applied Biosystems) and used as a template for the target genes G6PC3 and G6PT. The quantitative polymerase chain reaction (qPCR) was performed in 10 µL using Luna® Universal qPCR Master Mix (NEB New England BioLabs). The sequence of G6PC3 and G6PT primers was the following. For human G6PC3, the oligonucleotide primers were: sense, 5ʹ CACCTTCCTTTTGGCGGTTG 3ʹ; and antisense, 5ʹ GCTTAGCTCCCGCTCCATAG 3ʹ and for human G6PT, the oligonucleotide primers were: sense, 5ʹ TGTCCCCTTACCTGTGGGTGCTCTC 3ʹ; and antisense, 5ʹ CCAGGAGAAAGGACAGTCCAGCCCTT 3ʹ. Melting curve analysis was used to confirm the specificity of the amplified products and absence of primer dimers. Gene expression values of leiomyoma samples were expressed as fold-change versus normal myometrium samples, by using the 2-∆∆Ct method with HPRT1 (hypoxanthine phosphoribosyltransferase) as endogenous reference gene.
Statistical analysis
Results are reported as means ± standard error of the mean (SEM). The unpaired t-test was performed using the GraphPad Prism Program, version 5 (GraphPad Software, USA).
Results and discussion
We evaluated the expression of G6PC3 and G6PT myometrium and leiomyomas (Fig. 2). Tissue samples were reacted with anti-G6PC3 and anti-G6PT antibodies to evaluate, respectively, the expression of the G6PC3 enzyme and the G6PT.
The reaction with the anti-G6PC3 antibody was found to be predominantly perinuclear in accordance with the fact that G6PC3 is a reticular enzyme; in fact, the outer nuclear membrane is continuous with the ER (7). Obvious differences in the expression of G6PC3 and G6PT are not clearly appreciable in the tissue samples analyzed. We decided to investigate using an alternative technique such as qPCR. G6PC and G6PT messenger RNA (mRNA) expressions were assessed by real-time PCR (Fig. 3). The G6PC3 mRNA levels in the leiomyoma samples were calculated using the myometrium for comparison. Our results showed that both G6PT and G6PC3 mRNA expression in the leiomyoma samples showed a higher expression with respect to myometrium, although not statistically significant.
In conclusion, while it is certainly necessary to increase the number of samples, the data obtained show a good expression of the enzymatic couple G6PC3-G6PT in uterine leiomyomas. Thus, it’s possible to speculate that a pharmacological inhibition of this system through the use, for example, of highly specific inhibitors for G6PC3 or G6PT could limit cell proliferation of this benign tumor.
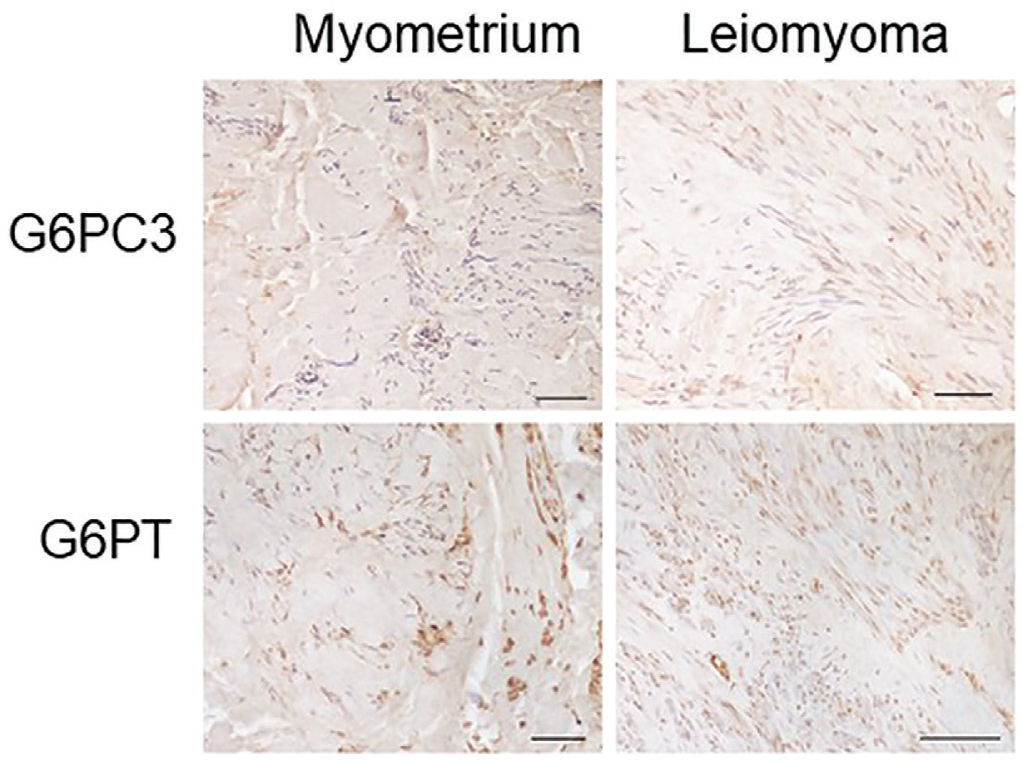
Fig. 2 - Representative images of G3PC3 and G6PT immunostaining of myometrium and leiomyoma of the same patient. The immunohistochemical signal is marked in brown and nuclei are colored in blue with hematoxylin. Scale bar 100 µm. G6PC3 = glucose-6-phosphatase catalytic subunit 3; G6PT = glucose-6-phosphatase transporter.
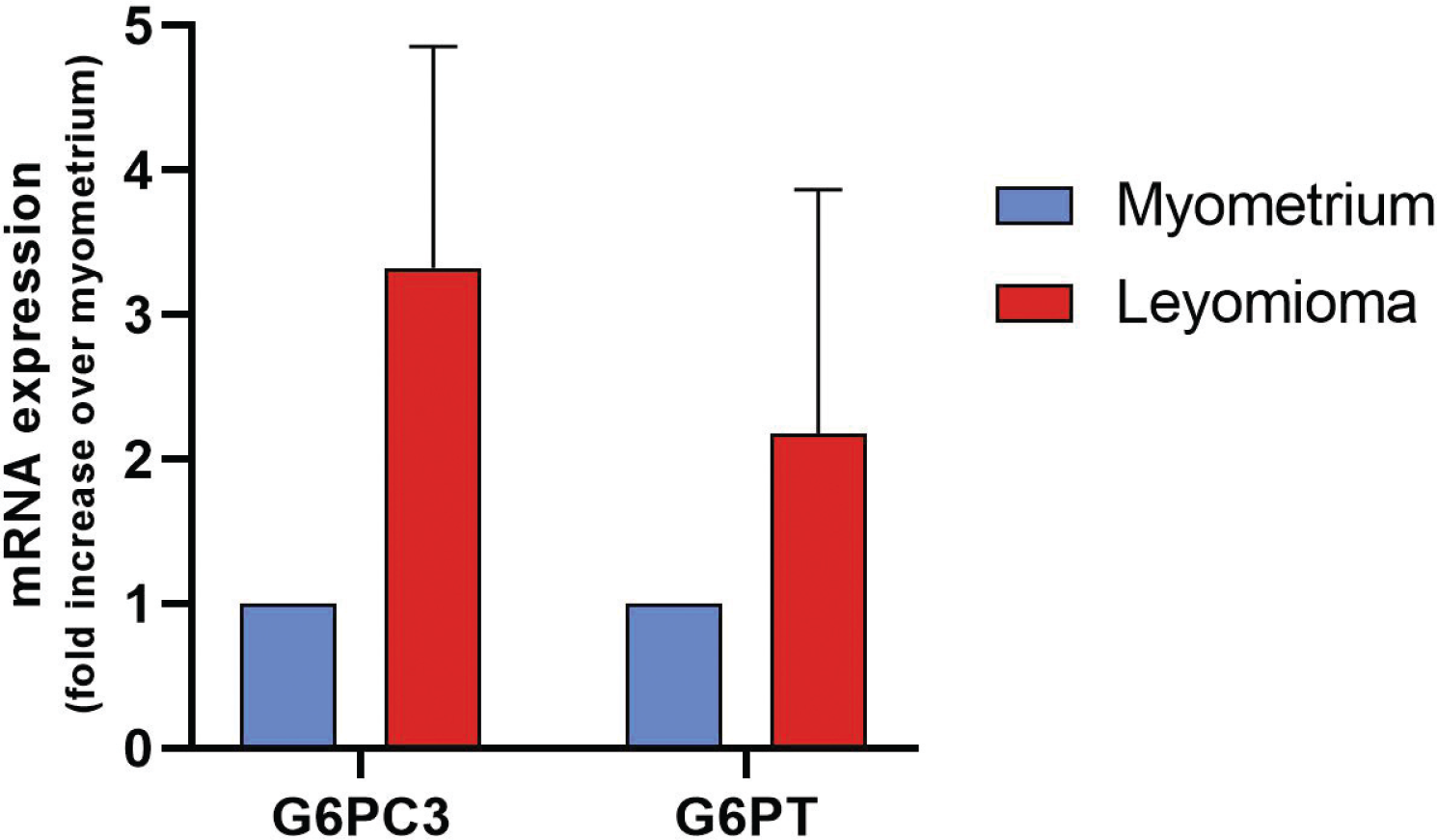
Fig. 3 - Real-time PCR analysis for the G6PC3 and G6PT mRNA expression levels. Each assay was run in triplicate, and negative controls were included. The levels of leiomyoma mRNA were expressed relative to the myometrium, which was considered 1. Bars display mean ± SEM. PCR = polymerase chain reaction; G6PC3 = glucose-6-phosphatase catalytic subunit 3; G6PT = glucose-6-phosphatase transporter; mRNA = messenger ribonucleic acid; SEM = standard error of the mean.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This work was supported by grants from the University of Siena PSR 2022 (grant 2267-2022-MP-PAR_001 to P.M.).
References
- 1. Marcolongo P, Fulceri R, Gamberucci A, Czegle I, Banhegyi G, Benedetti A. Multiple roles of glucose-6-phosphatases in pathophysiology: state of the art and future trends. Biochim Biophys Acta. 2013;1830(3):2608-2618. CrossRef PubMed
- 2. Boztug K, Appaswamy G, Ashikov A, et al. A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med. 2009;360(1):32-43. CrossRef PubMed
- 3. Veiga-da-Cunha M, Chevalier N, Stephenne X, et al. Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency. Proc Natl Acad Sci USA. 2019;116(4):1241-1250. CrossRef PubMed
- 4. Marcolongo P, Maellaro E, Luisi S. Regulation of autophagy in the uterus: from physiological processes to endometriosis and uterine fibroids. F S Rev. 2022;3(1):69-75. CrossRef
- 5. Senesi S, Marcolongo P, Kardon T, et al. Immunodetection of the expression of microsomal proteins encoded by the glucose 6-phosphate transporter gene. Biochem J. 2005;389(Pt 1):57-62. CrossRef PubMed
- 6. Del Bello B, Marcolongo P, Ciarmela P, et al. Autophagy up-regulation by ulipristal acetate as a novel target mechanism in the treatment of uterine leiomyoma: an in vitro study. Fertil Steril. 2019;112(6):1150-1159. CrossRef PubMed
- 7. De Magistris P, Antonin W. The dynamic nature of the nuclear envelope. Curr Biol. 2018;28(8):R487-R497. PMID:29689232. CrossRef PubMed