 |
AboutOpen | 2021; 8: 81-87 ISSN 2465-2628 | DOI: 10.33393/ao.2021.2263 ORIGINAL RESEARCH ARTICLE |
 |
Brentuximab vedotin in adult patients with HL CD30+ at high risk of relapse or progression following ASCT: a cost-analysis in Italy
ABSTRACT
Introduction: In Hodgkin Lymphoma (HL), the early administration of brentuximab vedotin (BV) represents a highly effective treatment to consolidate patients after autologous stem cell transplantation (ASCT). This study aimed at assessing costs accrued by using BV in consolidation after ASCT and compare them with the ones associated with the main options today used in Italy for HL.
Methods and results: A cost-analysis based on patients at high risk of relapse after ASCT was developed by collecting data about drugs and monitoring. The model is described by two arms: “A,” where BV is used as consolidation therapy after ASCT, and “B”, where patients are treated only at the time of relapse. A 3-year time horizon and the Italian National Health System perspective were adopted. All data inputs were sourced from the available literature and official list prices. A sensitivity analysis was conducted. The introduction of BV as consolidation therapy would allow savings in terms of drug acquisition and resource consumption. Over a 3-year time frame, arm A overall expenditure was 137,059€ vs. 225,418€ in arm B. Early after the ASCT, BV administration guarantees a long period free from relapses (5-year PFS is not reached), thus reducing the clinical and economic burden of the subsequent therapies needed to treat further relapses.
Conclusions: The present pharmacoeconomic analysis shows that the introduction of BV as consolidation therapy after ASCT represents a sustainable expenditure for the National Healthcare System (NHS) and a cost-saving paradigm when compared with the drug mainly used for treating the relapses.
Keywords: Brentuximab vedotin, Cost-analysis, Economic evaluation, Hodgkin lymphoma, Italian NHS
Received: March 31, 2021
Accepted: May 6, 2021
Published online: September 2, 2021
AboutOpen - ISSN 2465-2628 - www.aboutscience.eu/aboutopen
© 2021 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Classic Hodgkin lymphoma (HL) is a relatively rare hematological malignancy and one of the lymphoproliferative disorders characterized by strong histological expression of the CD30 antigen on the pathognomonic Reed-Sternberg cells (CD30+) (1). In Italy, in the period 2000-2010, the observed incidence for men and women was 3.84 (95% confidence interval [CI], 3.73-3.96) and 3.18 (95% CI, 3.08-3.29) cases every 100,000 residents per year, respectively (2). The observed overall survival (OS) 5 years after the diagnosis is over 90% in the 15-44 years range, but for patients older than 65 years, HL still represents a relatively aggressive malignancy, with an overall survival of 34% and 52%, for men and women, respectively (3). Recent therapeutic advances in HL are expected to lead to an enduring remission in 70% to 90% of the patients treated with standard chemotherapy with or without consolidation radiotherapy (4); still, 10% to 15% of patients with early HL and up to 30% with advanced HL fail to respond or relapse after primary therapy (5). Second-line regimens suggested by guidelines (6), consisting of salvage chemotherapy followed by autologous stem cell transplantation (ASCT), have even lower responses, with OS probability of 47% (95% CI, 37%-57%) (7) at 10 years, with overall survival (OS) probability of reaching 61% (95% CI, 52-72%) (7) at 4 years, and a dismal median post-progression survival of about 1-2 years (7,8). Before brentuximab vedotin (BV) becomes available, third-line options for HL treatment were: palliation with single drug chemotherapy or radiotherapy for patients ineligible for transplant; intensive chemotherapy regimens to induce complete response (CR) or good partial response (PR) for patients eligible for transplant (6). BV is an antibody-drug conjugate combining an anti-CD-30+ antibody with the microtubule-dissolving agent, monomethyl auristatin E (9). Recently the European Medicines Agency issued an additional approval for BV for the treatment of adult CD30+ HL patients at increased risk of relapse or progression following ASCT based on results of the global, phase III, randomized controlled trial AETHERA (10). The early post-transplant administration of BV provided a consistent improvement in the median progression-free survival (mPFS) vs. Placebo, which was subsequently confirmed by long-term results (11), with the median PFS not yet reached in the experimental study arm after 5 years. The extended follow-up demonstrated sustained benefit and long-term tolerability and safety (11). In Italy, BV is not yet reimbursed for this indication. The present simulation aims to calculate the impact of BV consolidation therapy, thus introducing a novel paradigm not yet codified on a national level. The analysis estimates treatment and management costs for patients at high risk of lymphoma relapse (HL CD30+ HR). To this objective, a cost model was developed to compare the expenditure between two possible arms, BV in consolidation vs. no consolidation therapy (NCT) after ASCT, from the Italian National Health System’s perspective. The model’s input parameters were obtained from the AETHERA (12) trial and other published sources.
Material and methods
Model structure
The present cost model, developed in Excel® (Microsoft Corporation, WA, USA), compares an arm where BV is administered as consolidation treatment early after ASCT (arm A), with an arm representing the main treatment pathway adopted in the Italian clinical practice for HL CD30+ patients treated for relapse after ASCT (arm B). The population of the analysis is aligned with the indication of BV approved for post-transplant consolidation therapy. It is represented by adult patients with HL CD30+ at increased risk of relapse or progression following ASCT. The time horizon of the analysis is 3 years, which is perceived as a sufficiently long time for the most significant costs to be accrued in this patient population (13). The Italian National Healthcare System (NHS) perspective was adopted.
Clinical inputs
For both arms, the length of treatment was assumed to be equal to the median time on treatment (ToT, Fig. 1) derived from the related studies, while time to next relapse (time to next treatment) was calculated using the median PFS as a proxy (11,12,14-16). In arm A, the only active treatment is represented by BV, which is administered for 10.4 months (12) (Fig. 1), ensuring, on average, no relapses over a time horizon of 3 years. Indeed, the 5-year median PFS is not reached (11) and so considered not reached for the remaining 25.6 months after BV treatment for arm A of this analysis. Arm B is assumed to be composed of the most representative and innovative sequence reimbursed by the Italian NHS in post-ASCT relapse (IQVIA MAT 12/13). The treatment pathway includes: BV administered for 6.2 months as a rescue therapy after a first relapse post-ASCT (11,17) (Fig. 1), and nivolumab used at the second relapse (18) for 14.7 months (95% CI, 11.3-18.5; Fig. 1) (16). The median PFS after ASCT (see NCT in Fig. 1) is 6.3 months (95% CI, 3.3-11.9) (11) and after BV rescue (Fig. 1) is 9.3 months (95% CI, 7.1-12.2) (15). After the third relapse (see “Other” in Fig. 1), in line with the work of Radford et al (19) and as confirmed by Italian opinion leaders, patients could follow four different clinical interventions (“Other”): chemotherapy (47.5%), chemotherapy followed by allogenic stem cell transplantation (Allo-SCT; 35.0%), chemotherapy followed by ASCT (2.5%), and finally palliative care in the remaining 15.0% of the patients (Tab. II). Treatment with “Other” was assumed to cover the remaining 5.7 months of the analysis. This assumption is in line with the publications of different authors (16,20-22). To calculate the weighted cost of “Other,” the estimated monthly cost of chemotherapy or PC was multiplied by 5.7 months, while the cost of Allo-SCT and ASCT was considered as a one-off cost (given the fact they are employed only once per patient) (see Tab. II). For a detailed overview of the costs calculated for each regimen, please refer to Tab. A in the supplementary materials.
Cost inputs
The cost-analysis for both arms included drug acquisition, drug administration, and health care resource utilization (HCRU). The treatment costs were estimated according to the dosage reported for each regimen in the respective SPC and applied for the ToT reported in Fig. 1. The ex-factory price published in the Official Journals was used for BV and nivolumab (17,18), and the mandatory discounts (5%+5%) (23,24) were applied. Similarly, for drugs used in the fourth segment of Fig. 1, the prices were collected from codifa.it (Tabs. I, II; Supplementary Tables A and B). The dosage was based on the average body weight of the intent to treat (ITT) population in the AETHERA study (76.3 ± 20.6 kg) (12), assuming this was valid for the three or more risk factors population as well. Wherever required, the body surface area (BSA; 1.91 ± 0.10) was estimated by applying the mean body weight and height (171.9 ± 0.10 cm) (12) of the AETHERA trial ITT population to the Mosteller equation (25). Tables I and II have summarized the total costs associated with the two arms compared in the present analysis.
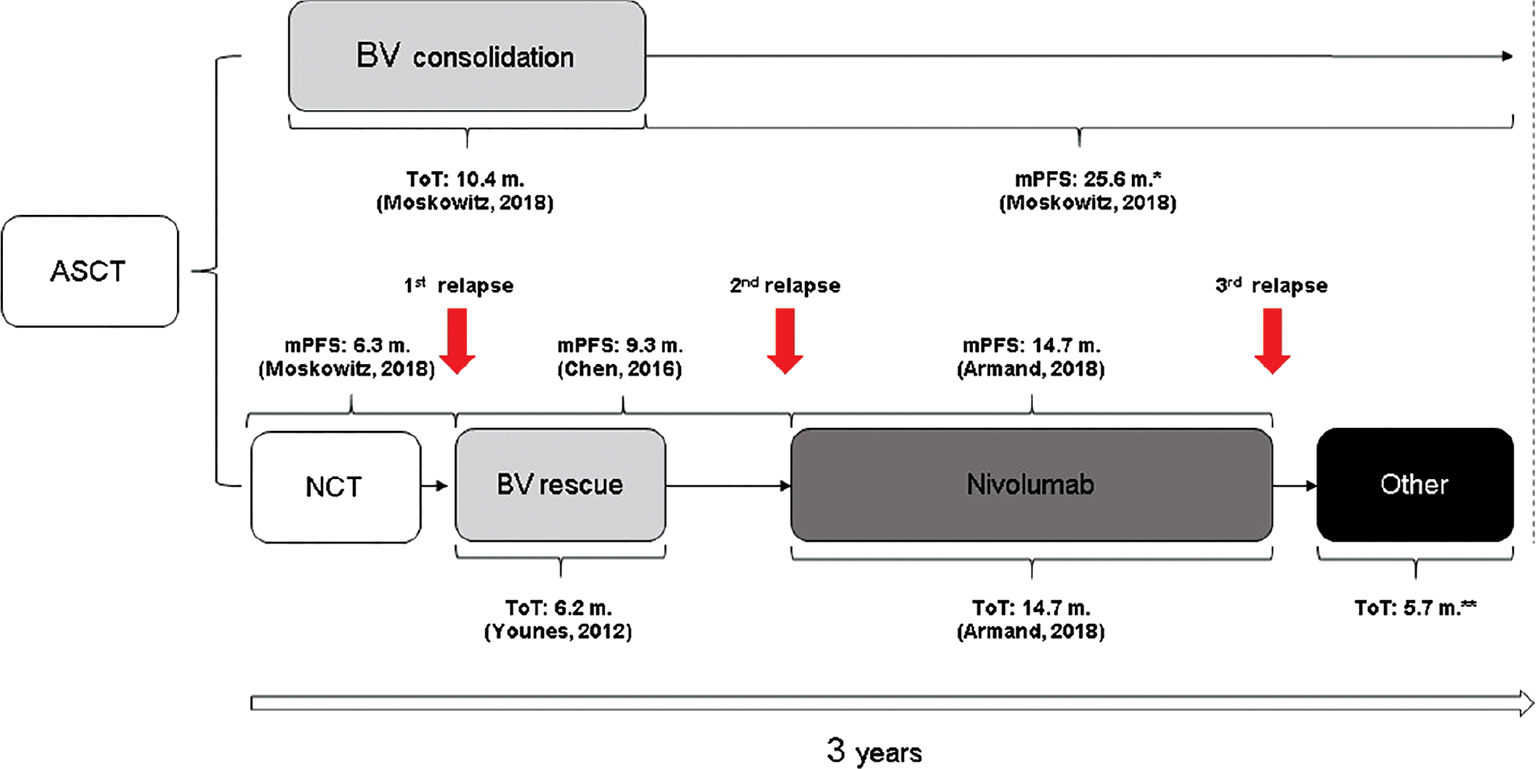
With regard to “Other” (Tab. II):
– the chemotherapies included represent multiagent salvage regimens recommended in different guidelines or reported in the literature for the management of patients relapsing after ASCT (6,21,22,26). These consisted of the following regimens administered to different percentages of patients: gemcitabine, cisplatin, methylprednisolone (GEM-P, 15%) (27); gemcitabine, oxaliplatin (GEM-Ox, 15%) (28); chlorambucil, vinblastine, procarbazine, prednisolone (ChlVPP, 25%) (29); bendamustine monotherapy 20% (30); bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone (BEACOPP, 10%) (31), and dexamethasone, cisplatin, cytarabine (DHAP, 10%) (32). We also considered that 5% of the patients could have been enrolled in a clinical trial without additional costs.
– the PC includes the most common (>15%) concomitant therapies reported for all patients in the AETHERA study (12,33): pantoprazole, Bactrim®, acyclovir, paracetamol, lorazepam, and psychological support (Tab. II and Supplementary table B).
– for ASCT and Allo-SCT, the costs (Tab. II) were obtained from Diagnosis Related Group (DRG) 481, considering specific codes (code 41.01, 41.04, 41.07, and 41.09 for ASCT; code 41.02, 41.03, 41.05, 41.06, and 41.08 for Allo-SCT) based on a document issued by the Region of Emilia Romagna (34). The costs include the whole procedure package (consultation fees, bone marrow transplant, stem cell apheresis, pharmacological treatment). The unit cost for ASCT was €37,197.79 and for Allo-SCT was €85,761.58.
| Mg per unit | Posology | Dose/administration | Unit/administration | Adm/month | Cost per unit (€) | Cost month (€) | ToT (months) | Cost per ToT (€) | |
|---|---|---|---|---|---|---|---|---|---|
| Arm A | |||||||||
| BV | 50 | 1.8 mg/kg/21 days | 137.34 mg | 2.7468 | 1.4494 | 3,008.03 | 11,975.61 | 10.4 | 124,546 |
| Total | 124,546 | ||||||||
| Arm B | |||||||||
| BV | 50 | 1.8 mg/kg/21 days | 137.34 mg | 2.7468 | 1.4494 | 3,008.03 | 11,975.61 | 6.2 | 74,249 |
| Nivolumab | 240 | 3 mg/kg/14 days | 228.90 mg | 0.95375 | 2.1741 | 3,226.01 | 6,689.31 | 14.7 | 98,333 |
| “Other” | (see Tab. II) | 5,644.66 | 5.7 | 32,174 | |||||
| Total | 204,756 | ||||||||
BV = brentuximab vedotin; ToT = time on treatment.
| Alternatives included in “Other” | % use | Cost per ToT per patient (€) |
|---|---|---|
| Chemotherapy* | 47.50 | 5.7 months of chemotherapy = 1,390.62
Total per patient = 1,390.62 |
| Chemotherapy* + Allo-SCT | 35.00 | 5.7 months of chemotherapy = 1,390.62
Allo-SCT = 85,761.58 Total per patient = 87,152.20 |
| Chemotherapy* + ASCT | 2.50 | 5.7 months of chemotherapy = 1,390.62
ASCT = 37,197.79 Total per patient = 38,588.41 |
| Palliative Care** | 15.00 | 5.7 months of PC = 306.95 |
| Weighted cost per ToT per patient | 32,174.57 |
(*) For the details about how the monthly costs were calculated, refer to Tabs. A and (**) B in Supplementary materials.
Allo-SCT = allogenic stem cell transplantation; ASCT = autologous stem cell transplantation; ToT = time on treatment.
The administration cost of €45.34 was considered for all intravenous medicines based on the average outpatient tariffs applied in different Italian regions to assess the variability in how this is reimbursed (34-38; Tab. III). For the other drugs, the administration costs were not included.
For each resource considered in the model (Tab. IV), the frequency of use was collected 1) from the AETHERA trial (12) for arm A (Fig. 1); 2) from the AETHERA trial and various published sources for arm B (39,40). The annual frequencies reported in Table IV were applied according to each segment of the two arms’ duration compared (Fig. 1). The resource consumption in the off-treatment period was assumed to equal the one considered for the previous treatment period. In line with clinical reports (6,41-43), the health care resources included routine monitoring (computed tomography [CT] scans, physician counseling fees, laboratory test costs), emergency room (ER) visits, and hospitalizations. All the DRGs and outpatient service tariffs reported in the article were obtained from the Official Journal (44) if not stated differently. When two or more relevant DRGs were available, the number of hospitalizations was used to calculate a weighted average (45).
Sensitivity analysis
A one-way sensitivity analysis (OWSA) was conducted by varying each parameter’s value to test the assumptions’ robustness. Except for BV and nivolumab prices, all model parameters were changed around their estimated range when available from the literature or around an arbitrary range of ±10% of the base-case value.
| Single regimen | Freq. (%) of use | Aver. admin/month | Cost per month (€) | Average nr. admin./ToT | Cost (€) cycle/ToT |
|---|---|---|---|---|---|
| BV consolidation | 100% | 1.45 | 65.76 | 15.05 | 683.60 |
| BV rescue | 100% | 1.45 | 65.76 | 8.97 | 407.53 |
| Nivolumab | 100% | 2.17 | 98.41 | 31.902 | 1,449.36 |
| Regimen “Other” | Freq. (%) of use* | Aver. admin/month† | Aver. cycles/ToT (5.7 months) | Average nr. admin/ToT | Cost (€) cycle/ToT |
| GEM-P | 15% | 3.26 | 1.56 | 5.11 | 231.82 |
| GEM-Ox | 15% | 2.17 | 3.23 | 7.02 | 318.46 |
| ChlVPP | 25% | 2.17 | 2.85 | 6.20 | 281.00 |
| Bendamustine | 20% | 2.17 | 2.32 | 5.06 | 229.48 |
| BEACOPP | 10% | 4.35 | 3.04 | 13.22 | 599.46 |
| DHAP | 10% | 1.45 | 1.42 | 2.07 | 93.67 |
| CT | 5% | 0 | |||
| Weighted average cost/ToT (5.7 months) | 282.11 | ||||
* = to calculate the weighted cost, percentages were scaled down to 100%, excluding CT.
† = the nr. of administrations per month is defined accordingly to the SPC of the drugs reported in Tab. A (Supplementary material) and sourced from the literature.
CT = computed tomography; ToT = time on treatment.
| Frequency event/patient/year | Cost per event (€) | Service code (44) | |||||
|---|---|---|---|---|---|---|---|
| Arm A | Arm B | ||||||
| BV | NCT | BV rescue | Nivolumab | “Other” | |||
| CT scan | 5.72(12) | 3.00(39) | 3.00(39) | 3.00(39) | 3.00(39) | 124.10 | 87.4.1 |
| WBCC | 5.72(12) | 8.09(12) | 8.09(12) | 10.40(39) | 10.40(39) | 6.50 | 90.70.4; 91.49.2 |
| RBCC | 5.72(12) | 8.09(12) | 8.09(12) | 10.40(39) | 10.40(39) | 5.80 | 90.62.2; 91.49.2 |
| Consultation | 4.84(12) | 6.84(12) | 6.84(12) | 10.40(39) | 10.40(39) | 20.70 | 88.7 |
| ER visit | 0.08(12) | 0.12(12) | 0.12(12) | 0.12(12) | 0.12(12) | 241.05 | (46) |
| Hospitalization | 0.61(12) | 0.83(12) | 0.83(12) | 1.30(40) | 1.30(40) | 4,989.12(*) | DRG 403; 404 |
| Duration of monitoring | 3 years | 6.3 months | 9.3 months | 14.7 months | 5.7 months | ||
| Cost per duration (€) | 11,829 | 2,516 | 3,715 | 8,857 | 3,434 | ||
| Total cost per arm (€) | 11,829 | 18,522 | |||||
BV = brentuximab vedotin; CT = computed tomography; ER = emergency room; NCT = no consolidation therapy; WBCC = White Blood Cell Count; RBCC = Red Blood Cell Count.
(*) = Weighted average cost.
Results
The early administration of BV consolidation therapy has demonstrated a significant and long-lasting improvement in the PFS vs. no consolidation for patients at high risk of relapse or progression after ASCT (10,11). Indeed, after 3 years: 1) in arm A, the median PFS was not reached, so the cost evaluated was the one of BV for consolidation, only; 2) in arm B, further relapses occurred, so the estimated treatment cost resulted in the sum of all therapies considered to treat them. The median pharmaceutical cost per patient in 3 years was higher in patients without consolidation therapy as shown in Table V: €204,756 per patient not receiving consolidation therapy and treated only at the time of the relapse (arm B) compared to €124,546 per patient treated with BV consolidation therapy (arm A). Considering the drug acquisition cost alone, the savings due to the administration of BV consolidation therapy is around 40% (€80,210) in 3 years. Moreover, if other direct costs were considered (administration and resource consumption), the total expenditure would be €225,418 in arm B and €137,059 in arm A. Considering all the direct costs, the use of BV consolidation therapy allowed to save around €88,359, in 3 years (about 40%).
The NHS savings calculated with this model could be potentially underestimated because all the other direct costs associated with relapses were not considered (i.e., possible multiple transplants). Moreover, as reported in the SPC, the adoption of the BV consolidation therapy is associated not only with a reduction in the rate of hospitalizations and outpatient visits but also with a lower number of missed working days for both patients (5.62 for BV vs. 11.70 for no consolidation) (12) and their caregivers (0.11 for BV vs. 1.80 for no consolidation) (12). For this reason, when also considering indirect costs, the saving associated with the adoption of BV in consolidation would be even more significant than the one estimated in the present analysis focused on direct costs only. If we adopted a time horizon of 5 years, the results would still remain in favor of BV: 1) in arm A, the expenditure would remain stable due to a 5-year median PFS still not reached (11); 2) in arm B, the spending could be ≥€225,418 per patient, due to the possibility of further relapses and the eventual need of subsequent therapies and/or transplant procedures.
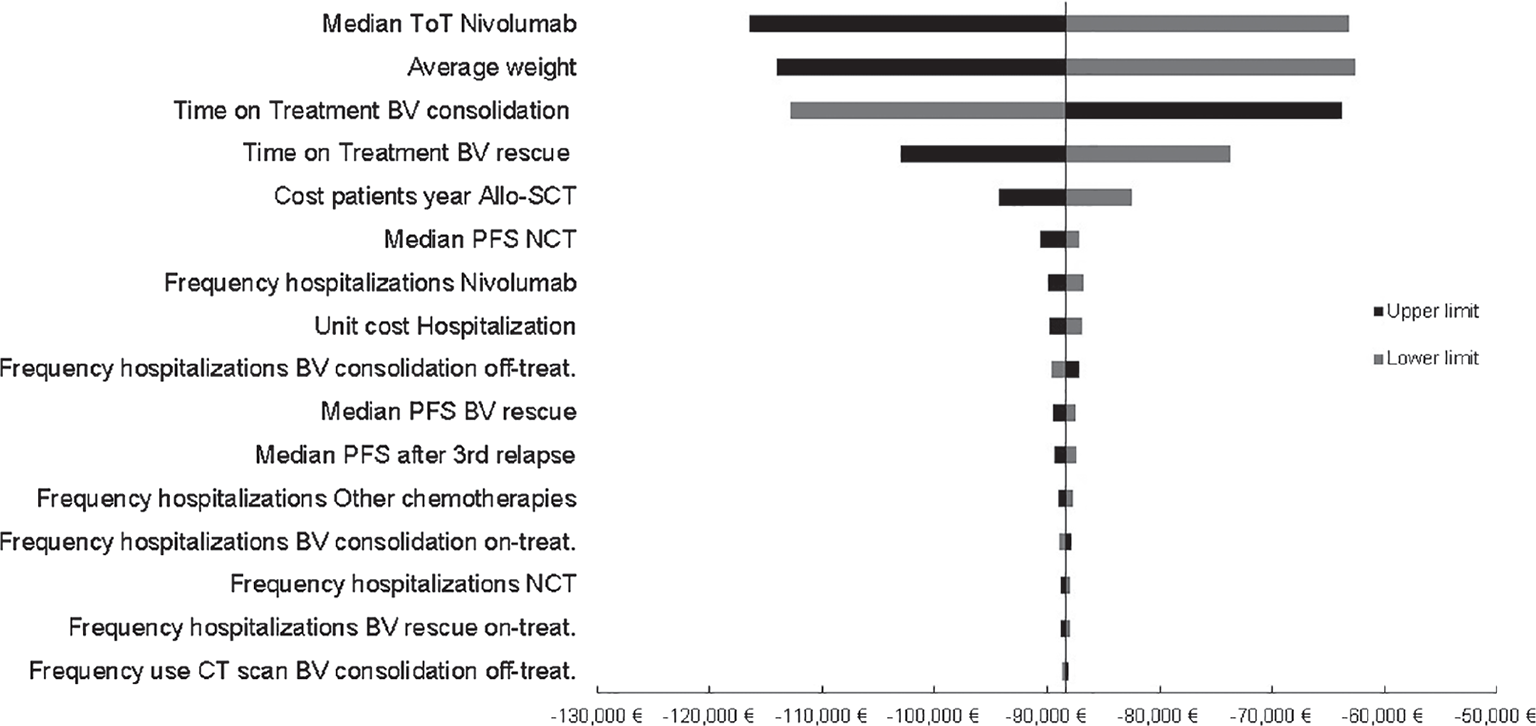
One-way sensitivity analysis
The results of the OWSA are depicted in the tornado diagram (Fig. 2) around the overall saving calculated for the base case (€−88,359). The parameters with the highest potential to influence the result are those related to the cost of treatments, as the treatment duration of nivolumab in arm B (+28.4%, −31.8%), the average body weight of the cohort (±29.1%), and the duration of the consolidative treatment with BV (±27.8%).
Discussion
BV is an anti-CD-30+-based antibody-drug conjugate recently approved by the European Medicines Agency to treat adult CD30+ HL patients at increased risk of relapse or progression following ASCT. This indication is not yet reimbursed in Italy, as the post-ASCT consolidation is a strategy that remains unrecognized in the lymphoma space. The objective of this analysis was to evaluate the advantages of introducing BV as consolidation therapy (arm A) vs. no-consolidation strategy and the administration of subsequent treatments at the time of relapse (arm B). The prescription of brentuximab as consolidative monotherapy demonstrated enhanced clinical outcomes vs. the choice of observation (10,11,14,15,47). Moskowitz and colleagues (11) confirmed that the median PFS for patients at high risk of HL relapse or progression receiving BV consolidation was not reached after 5 years compared to the progression rate for placebo (41%) through the long-term follow-up analysis (hazard ratio [HR] 0.52; 95% CI, 0.379-0.7171). Moreover, the risk associated with only one treatment (BV consolidation), which guarantees a long period free from relapses, is presumed to be lower than the one resulting from the sum of the risks associated with all the therapies needed to treat further relapses.
| Resource | Drug acquisition (€) | Other costs (€) | Tot. (€) | ||||
|---|---|---|---|---|---|---|---|
| BV consolidation | BV rescue | Nivolumab | “Other” | Administration | Resource consumption | ||
| Arm A | 124,546 | 0 | 0 | 0 | 683 | 11,829 | 137,059 |
| Arm B | 0 | 74,248 | 98,333 | 32,174 | 2,139 | 18,522 | 225,418 |
| Difference | +124,546 | −74,248 | −98,333 | −34,835 | −1,456 | −6,694 | −88,359 (−39.20%) |
BV = brentuximab vedotin.
The modeling of the present analysis is based on median PFS values. As confirmed in the recent article from Sureda and colleagues (48), PFS is considered a clinically relevant endpoint in evaluating HL (12,49). The direct comparison of the two arms modeled highlighted a saving of about 40% generated from the administration of BV as consolidation therapy vs. the current paradigm, which consists of treating HL patients at the time of relapses. This saving could be even more significant if: 1) other direct costs would be considered; 2) indirect costs would be accounted for; 3) pembrolizumab (recently reimbursed for the same indication as nivolumab) (50) would be considered as an alternative to pre/post-nivolumab (its treatment cost is higher than the one estimated for nivolumab). Yasenchak and collaborators, in their work, assessed the burden of the disease after the first relapse and found that second and third lines of therapy were 2.7-3.5 times more expensive than the first-line therapy (51). Evidence from another study suggested that relapsed patients accrued $401,529 compared to $89,709 in non-relapsed patients (52). In line with observations reported in articles published in the pre-BV era, we also observed an increase in the year total costs after the first relapse (€79,449) to the second relapse (€106,281) in arm B (no consolidation). While the impact of the BV consolidation is estimated with appreciable precision, it appears clear that the economic burden for subsequent therapies is variable. This burden depends on many factors such as the number of relapses, the drugs administered at each relapse, the treatment durations, and the need for a transplant. All this highlights the importance to prevent relapses not only from the patients’ point of view but also from an economic perspective. The present analysis has clearly pointed out a preeminence of one arm concerning the other, despite several limitations like the duration of therapies in the real practice, the mix of therapies/transplants adopted, and the uncertainty on the PFS duration after the third relapse. Hence, real-world evidence would be opportune to validate the outcomes of the present analysis.
Acknowledgments
All authors have materially participated in the research and article preparation. Maria De Francesco designed the research project and supervised data collection. Gian Luca Breschi collected, edited, and analyzed data and performed statistical analyses, and also produced the manuscript. Paolo Morelli, Maria De Francesco, Federica Demma contributed to data interpretation and revising of the document. All authors have approved the final article.
Disclosures
Conflict of interest: Breschi GL and De Francesco M declare that they have no conflicts of interest in this research. Demma F and Morelli P are full-time employees of Takeda Italia S.p.A.
Financial support: Takeda sponsored the research.
References
- 1. de Leval L, Gaulard P. CD30+ lymphoproliferative disorders. Haematologica. 2010;95(10):1627-1630. CrossRef PubMed
- 2. AIRTUM. The burden of rare cancers in Italy. Riv. dell’Associazione Ital. di Epidemiol. (2016).
- 3. Coviello V, Buzzoni C, Fusco M, et al; AIRTUM Working Group. Survival of cancer patients in Italy. Epidemiol Prev. 2017;41(2)(suppl 1):1-244. PubMed
- 4. Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183-1194. CrossRef PubMed
- 5. Lapuz C, Enjeti AK, O’Brien PC, Capp AL, Holliday EG, Gupta SA. Outcomes and relapse patterns following chemotherapy in advanced Hodgkin lymphoma in the positron emission tomography era. Blood Lymphat Cancer. 2018;8:13-20. CrossRef PubMed
- 6. Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19-iv29. CrossRef
- 7. Bazarbachi A, Boumendil A, Finel H et al, Evolution of outcome over time for relapsed Hodgkin lymphoma after autologous stem cell transplant: Improved survival for early relapse in recent years. Blood. 2020;136(Supplement 1):9-10. CrossRef
- 8. Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54(11): 2531-2533. CrossRef PubMed
- 9. EMA, E. Allegato I Riassunto delle caratteristiche del prodotto, Adcetris. (2018). Online
- 10. Moskowitz CH, Nademanee A, Masszi T, et al; AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853-1862. CrossRef PubMed
- 11. Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639-2642. CrossRef PubMed
- 12. AETHERA CSR, data on file.
- 13. Caro JJ, Briggs AH, Siebert U, Kuntz KM; ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—1. Value Health. 2012;15(6):796-803. CrossRef PubMed
- 14. Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18): 2183-2189. CrossRef PubMed
- 15. Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12): 1562-1566. CrossRef PubMed
- 16. Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II checkmate 205 trial. J Clin Oncol. 2018;36(14):1428-1439. CrossRef PubMed
- 17. AIFA. Agenzia Italiana del Farmaco. Regime di rimborsabilità e prezzo di vendita del medicinale per uso umano «Adcetris». Gazzetta n. 143 del 23 giugno 2014. Determina n. 607/2014.Determina 2018. Online
- 18. AIFA. Agenzia Italiana del Farmaco. Regime di rimborsabilità e prezzo di vendita del medicinale per uso umano «Opdivo». Gazzetta n. 295 del. 17 dicembre 2019 Determina n. 1799/2019. (2019). Online
- 19. Radford J, McKay P, Malladi R, et al. Treatment pathways and resource use associated with recurrent Hodgkin lymphoma after autologous stem cell transplantation. Bone Marrow Transplant. 2017;52(3):452-454. CrossRef PubMed
- 20. Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin lymphoma after autologous stem-cell transplantation and brentuximab vedotin failure: a prospective phase 2 multi-cohort study. Lancet Oncol. 2016; 17(9): 1283-94.
- 21. Cheah CY, Chihara D, Horowitz S, et al. Patients with classical Hodgkin lymphoma experiencing disease progression after treatment with brentuximab vedotin have poor outcomes. Ann Oncol. 2016;27(7):1317-1323. CrossRef PubMed
- 22. Alinari L, Blum KA. How I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplant. Blood. 2016; 127(3):287-295. CrossRef PubMed
- 23. Gazzetta Ufficiale numero 156 del 7 Luglio 2006. Supplemento Ordinario n.161. Online
- 24. Gazzetta Ufficiale numero 227 del 29 Settembre 2006. Online
- 25. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. CrossRef PubMed
- 26. Collins GP, Parker AN, Pocock C, et al; British Committee for Standards in Haematology; British Society of Blood and Marrow Transplantation. Guideline on the management of primary resistant and relapsed classical Hodgkin lymphoma. Br J Haematol. 2014;164(1):39-52. CrossRef PubMed
- 27. Ng M, Waters J, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone (GEM-P) is an effective salvage regimen in patients with relapsed and refractory lymphoma. Br J Cancer. 2005;92(8):1352-1357. CrossRef PubMed
- 28. El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18(8):1363-1368. CrossRef PubMed
- 29. Selby P, Patel P, Milan S, et al. ChlVPP combination chemotherapy for Hodgkin’s disease: long-term results. Br J Cancer. 1990;62(2):279-285. CrossRef PubMed
- 30. Heider A, Niederle N. Efficacy and toxicity of bendamustine in patients with relapsed low-grade non-Hodgkin’s lymphomas. Anticancer Drugs. 2001;12(9):725-729. CrossRef PubMed
- 31. Diehl V, Sieber M, Rüffer U et al; German Hodgkin’s Lymphoma Study Group. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin’s disease. The German Hodgkin’s Lymphoma Study Group. Ann Oncol. 1997;8(2):143-148. CrossRef
- 32. Josting A, Rudolph C, Reiser M, et al; Participating Centers. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13(10): 1628-1635. CrossRef PubMed
- 33. NICE. Single Technology Appraisal Brentuximab vedotin for treating CD30-positive Hodgkin’s lymphoma. Committee Papers [ID722]. (2016).
- 34. Regione Emilia Romagna. Tariffa unica convenzionale per le prestazioni di assistenza ospedaliera regole e tariffe valide per l’anno 2009 secondo cms-drg versione 24. (2009). Online
- 35. Regione Emilia Romagna. Specifiche tecniche per l’applicazione delle tariffe n.317 del 30 ottobre 2014. Online
- 36. Regione Lombardia. Deliberazione N° IX/2946, Seduta del 25 gennaio 2012. Tariffario prestazioni ambulatoriali. (2012). Online
- 37. Regione Piemonte. Nomenclatore giugno 2013. (2013). Online
- 38. Regione Puglia. Bollettino Ufficiale della Regione Puglia n. 94 del 16 luglio 2014. Online
- 39. Nivolumab for treating relapsed or refractory classical Hodgkin lymphoma (ID972). Second appraisal committee meeting Chair’s presentation. 12 April 2017 Online
- 40. Lalibertè F, Raut M, Duh M, et al. Real-world healthcare resource utilization (HRU) of classical Hodgkin Lymphoma (cHL) patients (pts) treated with anti-PD1 checkpoint inhibitors in the United States (US). Hematol Oncol. 2019;37:495-496. CrossRef
- 41. Hoppe RT, Advani RH, Ai WZ, et al. NCCN guidelines® insights Hodgkin lymphoma, version 1.2018 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2018;16(3):245-254. CrossRef
- 42. Bonafede M, Feliciano J, Cai Q, et al. Real-world analysis of cost, health care resource utilization, and supportive care in Hodgkin lymphoma patients with frontline failure. Clinicoecon Outcomes Res. 2018;10:629-641. CrossRef PubMed
- 43. Shao C, Liu J, Zhou W, et al. Treatment patterns, health care resource utilization, and costs in patients with relapsed/refractory Hodgkin lymphoma treated with brentuximab vedotin. Leuk Lymphoma. 2019;60(4):947-954. CrossRef PubMed
- 44. Gazzetta Ufficiale, numero 23; 28 gennaio 2013; Supplemento n.8; Ministero della Salute. Tariffario prestazioni ambulatoriali. (2013). Online
- 45. Ministero della salute. Rapporto annuale sull’attività di ricovero ospedaliero, Dati SDO 2017. Online
- 46. Ministero della Salute 2007. Progetto Mattoni SSN. Pronto Soccorso e sistema 118. Proposta metodologica per la valutazione dei costi dell’emergenza. Online (2007).
- 47. Vassilakopoulos TP, Chatzidimitriou C, Asimakopoulos JV, et al. Immunotherapy in Hodgkin lymphoma: present status and future strategies. Cancers (Basel). 2019;11(8):E1071. CrossRef PubMed
- 48. Sureda A, André M, Borchmann P, et al. Improving outcomes after autologous transplantation in relapsed/refractory Hodgkin lymphoma: a European expert perspective. BMC Cancer. 2020;20(1):1088. CrossRef PubMed
- 49. Brentuximab (Adcetris) for Hodgkin Lymphoma—Resubmission, pan-Canadian Oncology Drug Review Final Economic Guidance Report, Feb. 2018, Online (2018).
- 50. AIFA. Agenzia Italiana del Farmaco Regime di rimborsabilità e prezzo a seguito di nuove indicazioni terapeutiche del medicinale per uso umano «Keytruda». Gazzetta n. 290 del 27 novembre 2019. Determina n. 1763/2019.Determina 2019. (2019).
- 51. Yasenchak CA, Tseng WY, Yap M, Rembert D, Patt DA. Economic impact of disease progression following front-line therapy in classical Hodgkin lymphoma. Leuk Lymphoma. 2015;56(11):3143-3149. CrossRef PubMed
- 52. Hansen RN, Ramsey SD, Slejko J, Carlson JJ. The cost of relapse in Hodgkin lymphoma. J Clin Oncol. 2014;32(15 suppl): e17537.