 |
AboutOpen | 2020; 7(1): 95-102 ISSN 2465-2628 | DOI: 10.33393/abtpn.2020.2213 ORIGINAL RESEARCH ARTICLE |
 |
Model for estimating the healthcare costs and capacity of intensive care units in Italy in the treatment of patients with COVID-19: remdesivir impact assessment
Introduction: Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome–associated coronavirus 2, which is a human coronavirus responsible for a pandemic. Direct interventions, that is, physical distancing and use of protective devices, can prevent or limit contagions; however, it is also required to evaluate the optimization of limited resources, such as the intensive care unit (ICU). For this purpose, it is relevant to estimate the impact of therapeutic solutions that reduce the probability that the symptomatic patient needs to transit to ICU and in need of hospitalization. The therapeutic solutions allow a more rapid recovery of the patient and save scarce resources that can be used in the treatment of other patients.
Methods: A forecasting model is designed to estimate the impact of one therapeutic solution, that is, the antiretroviral remdesivir, on both the capacity of intensive care and the healthcare costs for hospitals when managing the current emergency. A base case is presented as well as a best and a worst case scenario deriving from the sensitivity analyses.
Results: The introduction of remdesivir in patients receiving low-flow oxygen therapy with the purpose of reducing ICU accesses and deaths leads to 431 million euros cost savings and avoids 17,150 hospitalizations in intensive care and 6,923 deaths. In the best case, 294 million euros savings are estimated, while in the worst case the model estimates a saving of 512 million euros.
Conclusions: Remdesivir has the potential to reduce the negative effects of the Coronavirus disease, improving patient conditions and reducing death tolls and can also save scarce healthcare resources during this pandemic, resulting in a shorter hospital stay and fewer ICU admissions.
Keywords: COVID-19, Intensive care units, Remdesivir
Received: November 24, 2020
Accepted: December 4, 2020
Published online: December 17, 2020
© 2020 The Authors. This article is published by AboutScience and licensed under Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). Any commercial use is not permitted and is subject to Publisher’s permissions. Full information is available at www.aboutscience.eu
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), a human coronavirus that has spread rapidly worldwide, leading the World Health Organization to declare this a pandemic.
Since the beginning of the COVID-19 epidemic in Europe, Italy has been among the first countries affected, and the number of reported cases is still among the highest on the continent. In the early months of 2020, Italy was the country most affected by infections, deaths and hospitalizations. To date, more than 1,600,000 cases have been detected since the beginning of the pandemic and, unfortunately, there have been more than 55,000 deaths (data as of 2 December) (1).
Irrespective of the inevitable impact that the pandemic has had and is having on the health of citizens, particularly the elderly and frail, let alone the significant economic impact, with a contraction of gross domestic product (GDP) estimated, at the end of the year, to be around 14.8% (2), the epidemic has had a significant impact on the acute pathology sector of the national healthcare system (SSN (Servizio Sanitario Nazionale [National Healthcare Service])), particularly with regard to emergency departments and intensive care units (ICUs), which have experienced increasing pressure.
The ICUs, which are dedicated to treating the most serious patients requiring automatic ventilation, are, on the one hand, traditionally considered the most complex of services, of which there is a very limited number in Italy and, on the other, they are extremely expensive. The daily cost of a patient in the ICU is on average approximately €1600 (3), and this is heavily influenced by the treatments that are administered.
With regard to the economic burden of the disease, and very conservatively taking into account only hospital services, in the period from 1 March to 21 July 2020, the cost for the SSN was quantified, at Diagnosis Related Groups (DRG) rates, at approximately 657 million euros and the cost of ICU admission days at more than 265 million euros (4).
It is clear that, in addition to a series of interventions already in place and aimed essentially at preventing or, at least, limiting the contagion (physical distancing, use of protective equipment), it is necessary to reason in terms of optimizing the use of limited resources such as ICUs, especially due to new waves of the pandemic which, in confirming the forecasts already made, are affecting Italy very badly. As confirmed by the Agenzia Nazionale per i Servizi Sanitari Regionali [National Agency for Regional Healthcare Services] (AGENAS), it is estimated, as of 13 November 2020, that there were 3,230 patients hospitalized in ICUs, out of a total of 8,423 beds (38.3% of total beds, considering an excess burden for ICUs that exceeds the threshold of 30%, as identified by the Minister for Health Decree of 30/04/2020) (5).
In this sense, it is particularly important to estimate the impact of any therapeutic solutions that, in symptomatic subjects who need to be hospitalized, reduce the likelihood that the patient will be admitted to the ICU, on the one hand allowing the patient to recover more quickly and, on the other, saving limited resources that could be used to treat other patients who are not necessarily suffering from COVID-19.
Remdesivir, the first medicinal product approved in the United States for the treatment of patients with COVID-19 and from July 2020 also approved in the European Union for adult and adolescent patients (aged 12 years or older and weighing at least 40 kg) with pneumonia requiring supplemental oxygen therapy, is available today in Italy as part of a European Joint Procurement Agreement (JPA), meaning the drug can be accessed through the Agenzia Italiana del Farmaco (Italian Medicines Agency) (AIFA) register (6). The register allows the medicinal product to be accessed only by patients with a clinical condition that corresponds to the indication authorized by the European Medicines Agency (EMA) and compliant with the criteria defined by AIFA’s Commissione Tecnico Scientifica (CTS) (Technical Scientific Commission), for a treatment cycle of 5 days (7). These criteria are:
- diagnosis of radiologically documented pneumonia;
- appearance of symptoms for less than 10 days;
- receiving oxygen therapy;
- not receiving non-invasive mechanical ventilation, high-flow oxygen therapy, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO);
- renal function: estimated glomerular filtration rate (eGFR), from 30 to 90 mL/min inclusive;
- liver function: alanine aminotransferase (ALT), normal or less than five times the upper limit of normal at baseline.
Clinical data from an independent randomized, double-blind, placebo-controlled trial on more than 1,000 patients hospitalized for COVID-19 demonstrated that remdesivir significantly improves patient recovery time by 5 days (7 days in patients on oxygen therapy at baseline) and reduces the duration of hospital stays by a median of 5 days (8). The same clinical study showed that therapy with remdesivir reduces disease progression, measured as a lower incidence of new mechanical ventilation or ECMO (compared to placebo, 43% fewer patients were started on mechanical ventilation); this step typically coincides with the patient’s admission to an ICU and is, therefore, of particular importance in terms of the organization of hospital facilities. In the low-flow oxygen therapy population at baseline, remdesivir is also able to reduce the risk of mortality by 70% compared to placebo (8). It is, therefore, clear that treatment with remdesivir can generate significant value for patients as well as savings for the SSN and, more generally, can benefit society as a whole. The clinical benefits of remdesivir, in fact, translate to a reduction in the patient’s time in hospital and may also result in reducing the need for intensive care.
From this perspective, a forecasting model was built to estimate the impact of a therapeutic solution such as remdesivir on the capacity of ICUs and, ultimately, on the direct healthcare costs for hospitals in the management of this current emergency.
Methods
Model structure
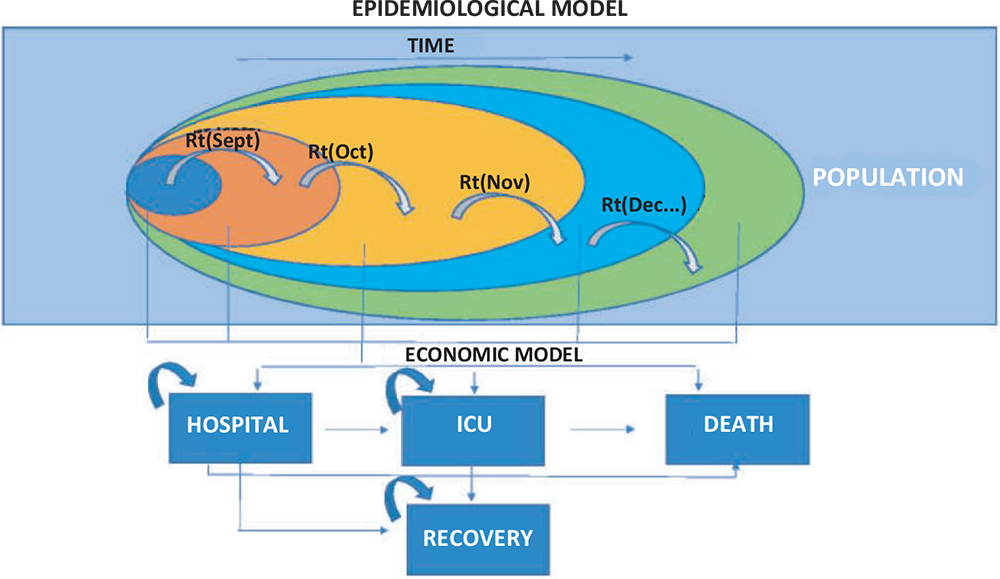
The model is structured into two phases (Fig. 1) with the following objectives:
- to estimate the total number of infected patients based on the Italian population;
- to estimate the direct healthcare costs associated with the treatment of hospitalized patients and those in intensive care, treated both without remdesivir therapy and with remdesivir used in low-flow oxygen therapy patients.
In the first estimate phase – starting from reported epidemiological data relative to contagiousness (the Rt index) as well as data updated daily in relation to hospitalized patients, those admitted to intensive care and deaths – the trends of the pandemic in terms of infected patients, admissions to intensive care and deaths were simulated over a 20-week time period. In view of the epidemiological and clinical peculiarity of the coronavirus infection and the uncertainty of being able to form hypotheses on its evolution in terms of medium- to long-term infections and virulence, it was decided to limit the time period to a single pandemic wave.
The simulation that leads to the first phase of estimation hypothesized an evolution of contagiousness based on the variations in the Rt observed week after week in October. The reduction in the Rt resulting from further restrictive measures could be linked to the Prime Ministerial Decree (DPCM – Decreto del Presidente del Consiglio dei Ministri [Decree of the President of the Council of Ministers]) issued on 25 October 2020. It was hypothesized that the effects of these measures on contagiousness could be observed starting from the third week (i.e. week 5 of the model) and that they had an impact that could be quantified as a reduction in the Rt index, according to the trend described in Table I. Further scenarios were simulated subsequently (see scenario analysis below).
This simulation was used to feed the second estimation phase consisting of an open-cohort Markov model that allowed for the reconstruction of the course of a hospitalized patient, thus considering both the probability of direct admission to intensive care and hospitalization and the probability of subsequent evolution in terms of death or intensification of the treatments required (Fig. 1).
In accordance with the epidemiological model structured in the first estimate phase, the Markov model consists of 20 cycles, each lasting 1 week.
This has made it possible to estimate hospital costs by quantifying the number of days of hospitalization, characterized according to the level of intensity of the healthcare.
According to the data currently available and published daily, the frequency of hospitalized patients is 6.6%, while that of patients admitted to intensive care is 0.6% of the total number of infected patients, while mortality ranges from 0.6% to 2.4% (9,10) (Tab. I).
Efficacy data
To evaluate a scenario where remdesivir is adopted, the randomized, controlled, double-blind trial conducted by Beigel et al (8) was considered, which found that the adoption of remdesivir in patients on low-flow oxygen therapy, that is, in patients eligible for treatment with remdesivir in Italy, reduces the time-to-recovery of hospitalized patients (hazard ratio: 1.45; 95% confidence interval [CI], 0.18-1.79) and mortality (hazard ratio: 0.3; 95% CI, 0.14-0.64). The reduction in the probability of admission to intensive care following treatment was extrapolated by calculating the relative risk reduction (RRR) resulting from the clinical study data that reported a lower frequency of new mechanical ventilation or ECMO in the group treated with remdesivir (13%) vs placebo (23%) (Tab. II).
It was hypothesized that a percentage of between 50% and 60% of all patients hospitalized for COVID-19 would require low-flow oxygen therapy and would, therefore, be eligible for treatment with remdesivir. The use of this therapeutic strategy has not been hypothesized for patients admitted to intensive care, which is consistent with the eligibility of patients for that treatment according to AIFA criteria.
Table I reports the clinical data used together with the Rt values used for each week.
Economic data
Given that the perspective under analysis is that of the hospital, the model considers the duration of the hospital stay as the main driver of the cost estimate. Consequently, based on the time to recovery indicated in the clinical study, the reduction in duration of the hospital stay following treatment with remdesivir was estimated, in relation to an average of 19 days of hospitalization, as indicated by the Ministero della Salute (Italian Ministry of Health) data.
The data relating to the duration of hospitalizations and intensive care admissions, both for patients who recovered and for patients who died, were obtained from the study conducted by Grasselli et al (11) and from the “Instant reports” analysing the organizational models of response to COVID-19, published on the Altems website of the Università Cattolica di Roma (Catholic University of Rome) (4).
With regard to the value given to days of hospitalization, the estimate of the Ragioneria Generale dello Stato (General National Accounting Department) was used, which reports an average value per day of hospitalization of €674 (12).
To evaluate intensive care, data were used from the Mattoni project of the Ministry of Health, which estimates a minimum rate per day of hospitalization in intensive care of €1654 (13).
With regard to the value given to the therapeutic alternative of reference, the price was considered to be identical in all developed countries and equal to $390 per vial. Consistent with the AIFA criteria, a duration of therapy of 5 days (six vials) was hypothesized.
| Parameters | Value | Source |
|---|---|---|
| Mortality in Italian population | 0.6%-2.4% | Bassi et al (9) |
| Time-to-recovery hazard ratio in patients on low-flow oxygen therapy | 1.45 | Beigel et al (8) |
| Relative reduction in the risk of progression to intensive care with remdesivir | 36% | Beigel et al (8) |
| Mortality hazard ratio in patients on low-flow oxygen therapy | Beigel et al (8) | |
| Rt wk 1 | 1.1 | ISS (Istituto Superiore di Sanità [Higher Institute of Health]) (average national datum 5-11 October 2020) |
| Rt wk 2 | 1.3 | ISS (average national datum 12-18 October 2020) |
| Rt wk 3 | 1.7 | ISS (average national datum 19-26 October 2020) |
| Rt wk 4 | 1.7 | ISS (average national datum 19-26 October 2020) |
| Rt wk 5 | 1.5 | |
| Rt wk 6 | 1.3 | |
| Rt wk 7 | 1.2 | |
| Rt wk 8 | 1.1 | Hypotheses on data |
| Rt wk 9 | 1.1 | ISS |
| Rt wk 10 | 1 | |
| Rt wk 11 | 0.8 | |
| Rt wk 12 | 0.8 | |
| Rt wk 13 | 0.8 | |
| Rt wk 14 | 0.8 | |
| Rt wk 15 | 0.8 | |
| Rt wk 16 | 0.8 | |
| Rt wk 17 | 0.8 | |
| Rt wk 18-20 | 0.75 |
| Parameter | Value | Source |
|---|---|---|
| Cost per day of hospital stay | €674.00 | Gen. Nat. Acc. Dep. (12) |
| Duration of hospital stay | 19 days | Extrapolated from Grasselli et al‡ (11) |
| Cost per day in ICU | €1,654.00 | ALTEMS (13) |
| Duration of stay in ICU | 9 days | Grasselli et al‡ (11) |
| Duration of hospital stay (deceased) | 8 days | Grasselli et al‡ (11) |
| Duration of hospital stay (RDV patients) | 14 days | Hypothesis from Beigel et al* (8) |
| Cost per day of hospital stay (RDV patients) | €807.00 | Hypothesis† |
| Duration of therapy with RDV | 5 days | Beigel et al (8) |
ICU = intensive care unit; RDV = remdesivir.
*The results of Beigel et al estimate a reduction in recovery time of 5 days (p < 0.001). Therefore, a reduction in the duration of hospital stays was hypothesized to be of the same number of days, starting from the Italian average value of 19 days. The sensitivity analysis also considered the hypothesis that the reduction in recovery time did not have an impact on the total duration of hospital stays. †The cost of 1 day of hospitalization in patients treated with remdesivir was calculated by multiplying the price per vial of the drug by the number of vials (6). The result was distributed over the expected duration of stay (15.58 days) and added to the cost per day of hospitalization without remdesivir (€674).
‡Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted for ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581.
Sensitivity analysis
In order to test the model’s sensitivity to variations in the parameters included, probabilistic sensitivity analyses were conducted on all parameters included both in the first estimation phase and in the second estimation phase envisaged by the model.
The Rt values were varied by applying the same variation to the expected values as that observed by the confidence intervals of the Rt of the previous months. Random beta distributions were applied to these values.
The values relating to the reduction in the probability of access to intensive care and the reduction in mortality were varied consistently with a beta distribution, hypothesizing a standard deviation consistent with the published clinical data (8). It should be noted that the choice of using this datum was dictated by the limited availability of other information that would allow a probabilistic approach to be adopted in the sensitivity analysis.
The values relating to the number of days of hospitalization in an ordinary regimen and in intensive care, as well as the time to recovery following treatment, were varied consistently with a gamma-type distribution, hypothesizing a standard deviation of 20% of the baseline value.
Scenario analysis
Two alternative scenarios to the baseline scenario were also hypothesized, depending on the evolution of the epidemiological curve. More specifically, different rates of Rt reduction were simulated following more or less restrictive measures.
The first alternative scenario, defined as “optimistic”, hypothesized an Rt reduction of 0.2 points per week starting from the fourth week, to then stop at 0.9 from the eighth week onwards.
The second alternative scenario, defined as “pessimistic”, instead assumed that the Rt value remained at 1.1 until week 12 and that it then subsequently dropped to 1 from week 12 to week 20.
Results
Table III shows the results of the first estimation phase. Over a time period of 20 weeks, more than 57,000 admissions to intensive care and 16,586 deaths are estimated, in relation to approximately 2,750,000 infected patients. The adoption of remdesivir in patients requiring low-flow oxygen therapy would be able to reduce admissions to intensive care by more than 17,000 units and avoid nearly 7,000 deaths.
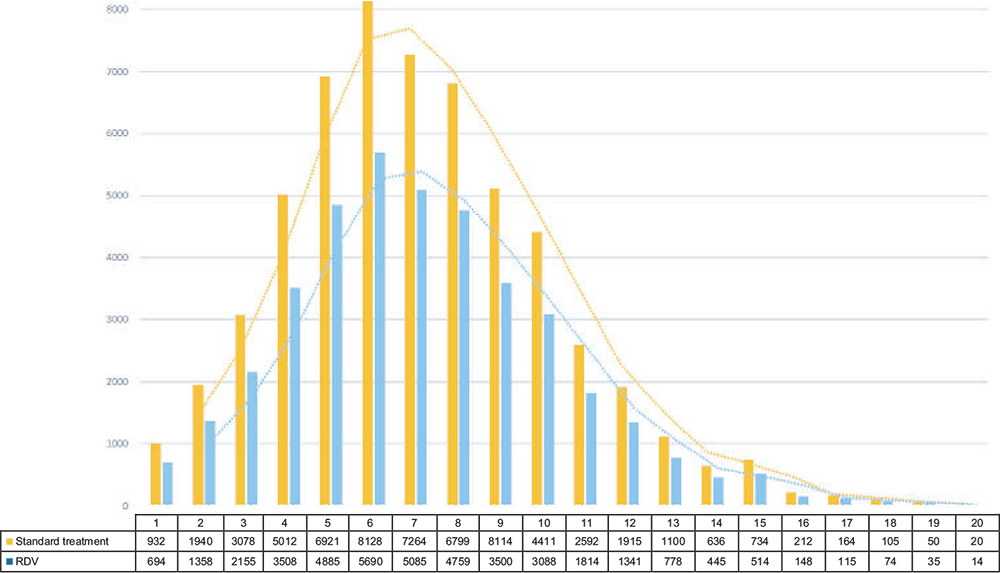
Figure 2 shows the distribution of the number of admissions to intensive care per week with and without remdesivir-based therapy and highlights how remdesivir therapy can help reduce the saturation rate of ICUs.
Table IV shows the costs of the two comparative alternatives divided between hospitalized patients, those hospitalized in intensive care and deceased patients.
The model shows that treatment with remdesivir can lead to a saving for the SSN of more than 430 million euros, mainly thanks to the effect of the reduction in the use of intensive care.
| Standard treatment | With remdesivir | ||
|---|---|---|---|
| Infected patients | 2,748,950 | 2,748,950 | |
| Admissions to intensive care | 57,166 | 40,016 | −17,150 |
| Deaths | 16,586 | 9,663 | −6,923 |
| Standard treatment | With remdesivir | ||
|---|---|---|---|
| Hospitalized patient cost | €865,641,240 | €733,050,312 | –€132,590,928 |
| Intensive care cost | €915,698,493 | €668,643,983 | –€247,054,509 |
| Deaths cost | €151,527,522 | €100,323,867 | –€51,203,654 |
| Total costs | €1,932,867,255 | €1,502,018,162 | –€430,849,093 |
Sensitivity analysis
Table V shows the results of the sensitivity analyses which were conducted. The parameters to which the estimates are most sensitive are as follows, in decreasing order of importance:
– the Rt value;
– the rate of reduction of admissions to intensive care;
– the mortality reduction rate;
– the duration of hospital stays;
– the number of days in intensive care;
– the time to recovery.
The multivariate sensitivity analysis shows that, with the simultaneous variation of all the parameters of the model, the results, ordered from the 25th to 75th percentile of joint distribution, return dominant values with variable savings between 251 and 534 million euros. The reduction in admissions to intensive care varies between 9,400 and 24,100, while the reduction of deaths varies between 4,430 and 12,101.
Scenario analysis
Best case scenario
The optimistic scenario, which hypothesized a Rt reduction of 0.2 points per week starting from the fifth week, estimates 1.3 million infected patients, about 25,000 admissions to intensive care and 8,670 deaths; in this scenario, the introduction of remdesivir would result in a reduction of 12,500 admissions to intensive care and 4,800 deaths. From an economic point of view, this would result in savings of approximately 294 million euros.
Worst case scenario
The pessimistic scenario hypothesizes the Rt index value constantly staying at 1.1 until week 12 and then falling to 1 from week 12 to week 20. The results show an estimate of approximately 5.4 million infected patients, 62,500 admissions to intensive care and 27,159 deaths. In view of this, the introduction of remdesivir would result in a reduction of 25,750 admissions to intensive care and 15,047 deaths. From an economic point of view, this would result in savings of approximately 512 million euros.
Discussion
The results of this study show that the introduction of remdesivir in patients on low-flow oxygen therapy may result in savings of 431 million euros over a 20-week pandemic wave in relation to 17,150 admissions to ICU and 6,923 deaths prevented. In other words, treatment with remdesivir may not only reduce the burden of the disease – improving the health of patients and reducing deaths – but it may also enable savings on healthcare resources during this pandemic, leading to a shorter duration of hospitalization and a lower rate of intensive care admissions. From a hospital point of view, the use of remdesivir in patients on low-flow oxygen therapy would, therefore, free up both hospital beds, thus allowing more patients to be treated, and intensive care beds, with relative savings from not using invasive respiratory devices and optimizing the management of ICU – units that, during this pandemic, are exceedingly congested.
Several studies, in fact, highlight the growing concern for the capacity of the Italian national health system to effectively respond to the needs of patients who require intensive care for COVID-19 (11,14). Hospital resource rationalization measures must be implemented urgently, otherwise a considerable increase in deaths will become inevitable. The SSN should prepare for a massive increase in the demand for intensive care during uncontrolled COVID-19 outbreaks, guaranteeing a bed for each patient who requires admission to the ICU. The Comitato Nazionale per la Bioetica (National Bioethics Committee) itself also expressed its view on the issue of access to healthcare under conditions of limited resources during the current healthcare emergency, defining the clinical criterion as the only ethically acceptable criterion for making clinical decisions and stating that the Constitution recognizes the right of each individual to receive all necessary healthcare.
| Parameter | Difference in costs | Difference in intensive care | Difference in deaths | |||
|---|---|---|---|---|---|---|
| 25th percentile | 75th percentile | 25th percentile | 75th percentile | 25th percentile | 75th percentile | |
| Rt | −€152,498,724 | −€677,516,201 | −10,594 | −22,358 | −965 | −12,780 |
| Reduction in admissions to intensive care | −€178,264,513 | −€598,415,678 | −9,851 | −20,561 | −5,831 | −12,250 |
| Reduction in mortality | −€152,321,489 | −€554,897,632 | −9,542 | −21,549 | −4,247 | −12,364 |
| Duration of hospital stays | −€148,326,124 | −€535,497,801 | −12,564 | −21,006 | −4,111 | −12,201 |
| Number of days in intensive care | −€195,231,476 | −€512,990,223 | −13,428 | −22,056 | −3,964 | −12,165 |
| Time to recovery | −€192,316,473 | −€539,248,100 | −13,894 | −22,561 | −3,221 | −12,327 |
The data used to feed the model described in this article have been taken from the study by Beigel et al (8), a randomized, double-blind, placebo-controlled, unsponsored study conducted by the United States National Institute for Allergies and Infectious Diseases. To date, the Beigel study is the only study that provides usable data in a model that estimates the effects deriving from the use of remdesivir in the population under analysis. The results of this study show that the use of remdesivir can significantly reduce the clinical time to recovery of the patient and the probability of progressing to more serious stages of the disease, requiring admission to ICUs; particularly for patients on low-flow oxygen therapy, that is, patients eligible for treatment with remdesivir in Italy according to AIFA indications, the study has shown that remdesivir is able to reduce the risk of mortality by 70%.
Further studies have been conducted on remdesivir with the aim of evaluating treatment on patients with COVID-19 (15-18); however, they are not suitable for inclusion in this model due to certain characteristics, such as, for example, early discontinuation of the study due to difficulty in enrolment, the choice to conduct the study as an open-label study and without having standard of care or placebo as a comparison, or the lack of specific data available on patients on low-flow oxygen therapy.
Strengths and weaknesses of the study
The model has both strengths and weaknesses. Generally speaking, its strength is represented by the fact that, to date, this is the only model, adaptable to different scenarios, that allows a link to be made between an epidemiological trend and the introduction of therapeutic strategies, such as remdesivir, and organizational variables, such as, for example, the use of ICUs, which represent the most critical point associated with the management of this current crisis. For this reason, this tool could be useful for decision-makers who are also responsible for managing later waves or, with the appropriate adaptations, pandemics caused by different viruses.
The model can also make regional/local adaptations (e.g. using the relative Rt or even real-world data on recovery times coming from different realities), thus allowing us to make estimates that could be useful for decision-making at various levels.
Its main weakness is the fact that the epidemiological basis is represented by Rt index data observed until the end of October and, subsequently, hypothesized taking into account the restrictive measures implemented by the government following the worsening of the pandemic wave. The model, therefore, may not be representative of the real epidemiological conditions, even though, in its form, it can still be readjusted and populated with new real data as and when that become available.
The second weakness is the fact that the data are based on the results of the only study (8) for which it is possible to perform this type of analysis and may change depending on the new evidence available from other active studies.
Conclusions
In conclusion, our analysis shows that in addition to the measures aimed at reducing viral transmission, the use of remdesivir in hospitalized patients on low-flow oxygen therapy, based on the data currently available, would be able to guarantee both improved management of the emergency and better clinical outcomes for patients as well as significant savings for the SSN. This analysis can help to support political leaders and health authorities to make the most appropriate decisions to ensure that there are sufficient resources, including staff, hospital beds and intensive care facilities, to address any further pandemic waves.
Acknowledgements
The authors thank Claudio Ripellino for medical writing assistance on behalf of Health Publishing & Services Sri. The authors would like to extend their gratitude also to Dr. Elisa Martelli (Gilead Sciences) for the precious clarifications concerning the trial design and the population involved.
Disclosures
Conflict of interest: The authors declare no conflict of interest.
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The view expressed by authors does not represent – and is not to be interpreted – as the view of the Institutions they are affiliated to.
References
- 1. Istituto Superiore di Sanità, Epidemia COVID-19, aggiornamento nazionale, 2 dicembre 2020. Online
- 2. Istituto Nazionale di Statistica, Conti Economici Trimestrali, 2 ottobre 2020. (Accessed November 2020). Online
- 3. Healthcare Datascience Lab, Centro sull’Economia e sul Management nella Sanità e nel Sociale, assorbimento di risorse economiche correlato alla gestione ospedaliera dei pazienti COVID-19 (Accessed November 2020). Online
- 4. ALTEMS, Alta Scuola di Economia e Management dei Sistemi Sanitari, COVID-19, Instant REPORT#18: 03 settembre 2020. Online
- 5. Agenzia Nazionale per i Servizi Sanitari Regionali (AGENAS), Rapporto COVID-19 (Accessed November 2020. Online
- 6. Summary of product characteristics (SMPC), Remdesivir. (Accessed November 2020). Online
- 7. Agenzia Italiana del Farmaco (AIFA). Emergenza COVID-19, Procedura di richiesta per il farmaco Veklury® (remdesivir). (Accessed November 2020). Online
- 8. Beigel JH, Tomashek KM, Dodd LE, et al; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 – Final Report. N Engl J Med. 2020;383(19):1813-1826. CrossRef Medline
- 9. Bassi F, Arbia G, Falorsi PD. Observed and estimated prevalence of COVID-19 in Italy: how to estimate the total cases from medical swabs data. Sci Total Environ. 2020;142799:142799; Epub ahead of print. CrossRef Medline
- 10. ISTAT. Indagine di siero prevalenza sul SARS-CoV-2. (Accessed November 2020). Online
- 11. Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted for ICUs of the Lombardy Region, Italy. JAMA. 2020; 323(16):1574-1581. CrossRef Medline
- 12. Ministero dell’Economia e delle Finanze – Commissione Tecnica per la Finanza Pubblica. Libro verde sulla spesa pubblica (2007). Online
- 13. ALTEMS, Alta Scuola di Economia e Management dei Sistemi Sanitari, COVID-19, Instant REPORT#28: 12 novembre 2020. Online
- 14. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225-1228. CrossRef Medline
- 15. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [published correction appears in Lancet. 2020 May 30;395(10238):1694]. Lancet. 2020;395(10236):1569-1578. CrossRef Medline
- 16. Spinner CD, Gottlieb RL, Criner GJ, et al; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate covid-19: a randomized clinical trial. JAMA. 2020;324(11):1048-1057. CrossRef Medline
- 17. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827-1837. CrossRef Medline
- 18. World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. (Accessed November 2020). Online